Abstract
Corticotropin-releasing hormone (CRH) is widely recognized as the primary mediator of the neuroendocrine and behavioral responses to stress, including stress-induced anxiety. The biological activity of CRH and other mammalian CRH-like peptides, such as urocortin, may be modulated by CRH-binding protein (CRH-BP). To assess directly the CRH-BP function, we created a mouse model of CRH-BP deficiency by gene targeting. Basal adrenocorticotropic hormone and corticosterone levels are unchanged in the CRH-BP-deficient mice, and the animals demonstrate a normal increase in adrenocorticotropic hormone and corticosterone after restraint stress. In contrast, adult male CRH-BP-deficient mice show significantly reduced body weight when compared with wild-type controls. CRH-BP-deficient mice also exhibit a significant increase in anxiogenic-like behavior as assessed by the elevated plus maze and defensive withdrawal tests. The increased anorectic and anxiogenic-like behavior most likely is caused by increased “free” CRH and/or urocortin levels in the brain of CRH-BP-deficient animals, suggesting an important role for CRH-BP in maintaining appropriate levels of these peptides in the central nervous system.
Corticotropin-releasing hormone (CRH) is the key mediator of the mammalian endocrine, behavioral, autonomic, and immune responses to stress (1). Within the hypothalamic-pituitary-adrenal (HPA) axis, CRH is the principal regulator of pituitary adrenocorticotropic hormone (ACTH) and adrenal glucocorticoid secretion in response to stressful stimuli. Within the central nervous system (CNS), CRH acts as a neurotransmitter to elicit anxiogenic-like effects, decrease food intake, reduce weight gain, modulate locomotor activity, and improve arousal and learning (2–4). Aberrant CRH activity has been implicated in the pathophysiology of a variety of human psychiatric disorders, including major depression, anxiety disorders, and anorexia (1, 5).
The actions of CRH in the CNS and pituitary are mediated via binding to two distinct CRH receptors, CRH-R1 and CRH-R2 (6). CRH also is bound by CRH-binding protein (CRH-BP), a 37-kDa protein that binds CRH with an affinity equal to or greater than the CRH-Rs (7–9). CRH-BP mRNA is expressed exclusively in the brain and pituitary of rodents, whereas it is detected in human brain, pituitary, liver, and placenta (9–11). Major CNS sites of CRH-BP mRNA expression in rodents are cerebral cortex, amygdala, bed nucleus of the stria terminalis, raphe nuclei, brainstem reticular formation, and olfactory, auditory, trigeminal and vestibular sensory relay systems (10). These sites of CRH-BP expression include several examples of cellular colocalization with CRH-producing neurons (i.e., lateral septal nucleus, bed nucleus of the stria terminalis, medial preoptic area, central nucleus of the amygdala) or CRH target cells, including anterior pituitary corticotropes that express CRH-R1 (10).
Both CRH-BP and CRH-R2 mRNA are present in lateral septum, a site of expression of both CRH and urocortin (12). Urocortin shares 43% amino acid identity with CRH and is bound by CRH-BP, CRH-R1, and CRH-R2 with affinities equal to or greater than CRH (12). Intracerebroventricular (i.c.v.) injection of urocortin causes anorectic and anxiogenic-like effects (13, 14). The sites of overlapping expression of CRH-BP with CRH, urocortin, and/or the CRH-Rs suggest a role for CRH-BP as a modulator of the synaptic or endocrine actions of CRH and/or urocortin in the CNS and the pituitary.
Although the biochemical properties of CRH-BP are well characterized, the function of CRH-BP in normal and disease states remains unknown. CRH-BP binds 40–90% of the total CRH, and CRH-BP levels are approximately 10-fold higher than total CRH levels in most human brain regions (11). CRH-BP blocks CRH-mediated ACTH secretion from anterior pituitary cultures and AtT-20 cells (9, 15). Together, these results support the hypothesis that endogenous CRH-BP acts as a negative regulator of CRH in vivo, playing a role in CRH clearance or degradation. This hypothesis is supported by studies on transgenic mice that overexpress CRH-BP in the pituitary. These mice respond to constitutively elevated pituitary CRH-BP levels by compensatory elevation of hypothalamic CRH and vasopressin, thus maintaining homeostasis in the HPA axis (16). To address the in vivo role of CRH-BP in the CNS and the HPA axis, we have created CRH-BP-deficient mice by gene targeting.
MATERIALS AND METHODS
Targeting Vector Construction.
A 129SVJ mouse lambda genomic DNA library (Stratagene) was screened with a mouse CRH-BP cDNA PvuII fragment (15, 17). The 17-kb DNA insert from hybridization-positive lambda clone 11C contained exons 1–6 of the mCRH-BP gene and was subcloned into pGEM-3Z (Promega). A 1.2-kb BglII fragment of the CRH-BP gene containing 5′ flanking sequences and 94 bp of the untranslated portion of exon 1 was inserted between the XhoI–NotI sites of the pPNT vector (18) as the 5′ region of homology. A 3.6-kb BamHI fragment of the CRH-BP gene containing exon 6 was inserted into the BamHI site of pPNT as the 3′ region of homology. The resulting 7.1-kb targeting vector contained the phosphoglycerate kinase (PGK)-neomycin resistance cassette (PGK-neo) in place of the translated portion of CRH-BP exon 1 and exons 2–5, and included the herpes simplex virus thymidine kinase cassette. The targeting vector was linearized with HindIII for transfection.
Tissue Culture and Transfection.
R1 embryonic stem cells (3.2 × 107) were electroporated with 80 μg linearized targeting vector at 300 V and 250 mF (Bio-Rad). Electroporated cells were plated on Mitomycin-C treated neomycin-resistant mouse embryonic fibroblast (MEF) feeder cells. All plates were selected with medium containing 300 μg/ml G418 (Life Technologies, Gaithersburg, MD), and half of the plates also were selected with medium containing 2 μM gancyclovir (Cytovene, Roche, Nutley, NJ) from day 5 until day 8 or 9 (19, 20). A total of 450 individual colonies were picked from the plates and transferred to individual wells of 96-well plates containing Mitomycin-C-treated MEF feeder cells. Cells were trypsinized on day 14 and split. Two-thirds of each colony was frozen at −80°C on 96-well plates in 10% DMSO (Sigma), and one-third was plated onto gelatin-coated 96-well plates and grown for 1 week. Genomic DNA was prepared from the individual clones and analyzed by PCR (21). PCR-positive clones were thawed from the frozen 96-well plates and expanded for Southern analysis, chromosome count, and blastocyst injection.
Screening of G418-Resistant Colonies.
PCR analysis was performed on the potential homologous recombinant clones by using a forward primer, primer e, (5′-GTC TAA GGA CTG TTT ATA GCA TTA GAT CAG-3′) and a reverse primer, primer f, (5′-AGA CTA GTG AGA CGT GCT ACT TCC ATT TGT-3′) (Fig. 1.) Cycling conditions were 94°C, 30 sec.; 68°C, 30 sec.; 72°C, 2 min; 30 cycles. For Southern analysis, 10 μg of genomic DNA was digested overnight with PvuII (probe A) or HindIII (probe B) (19). Probes for the 5′ and 3′ ends of the mouse CRH-BP gene were prepared from 0.9-kb NcoI (probe A) and 1.9-kb EcoRI (probe B) fragments, respectively.
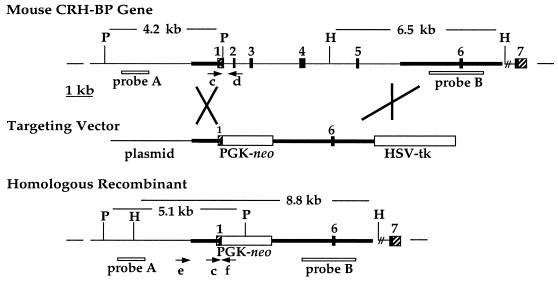
Figure 1.
Targeted disruption of the CRH-BP gene. The endogenous mouse CRH-BP gene is shown with exons numbered. Hatched portions of exons indicate untranslated regions. The targeting vector contained 1.2 kb of 5′ homologous sequence and 3.6 kb of 3′ homologous sequence (bold) inserted into unique restriction sites of the pPNT vector that contains the PGK-neo and herpes simplex virus thymidine kinase (HSV-tk) cassettes. The structure of the disrupted gene after homologous recombination is depicted. The probes (A, B, boxes) and PCR primers (arrows) used to screen for homologous recombinant clones (e, f) and to screen mouse litters (c, d, f) are indicated. P, PvuII; H, HindIII.
Generation and Maintenance of CRH-BP-Deficient Mice.
Confirmed homologous recombinant embryonic stem cells were injected into blastocysts from C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) to obtain chimeras (22). Male chimeras were bred to C57BL/6J females. Progeny heterozygous for the disrupted allele were intercrossed to produce homozygous-deficient animals and wild-type control littermates for all studies. Mouse husbandry conditions were as described (16). Mice were individually housed for at least 3 days before stress, feeding, or behavior experiments. All procedures were approved by the University of Michigan Committee on Use and Care of Animals. All experiments were conducted in accordance with the principles and procedures outlined in the National Research Council Guide for the Care and Use of Laboratory Animals.
Genotyping.
Offspring of heterozygous matings were genotyped by detection of the disrupted allele in genomic DNA prepared from tail biopsies. Multiplex PCR analysis was performed with three primers: primer c, (5′-TGG ACC CTC GTC ATT GCC AGG-3′), primer f, (sequence above), and primer d, (5′-CCC GTC GGT ACG GCT GCT CCT CTG CCA GGT-3′). Cycling conditions were as above. Southern analysis was performed to confirm genotypes on some animals.
In Situ Hybridization.
In situ hybridization of brain cerebral cortex sections for CRH-BP mRNA was performed as described (16, 17). Antisense and sense riboprobes for CRH-BP were labeled with [35S]uridine triphosphate (Amersham Pharmacia). Slides were dipped in nuclear emulsion NTB-2 (Eastman Kodak) and developed after 4 weeks. There was no signal above background in any sections hybridized with the sense riboprobe.
CRH-BP Crosslinking.
The binding of CRH-BP to (2-[125I]iodohistidyl32) human CRH (Amersham Pharmacia) was analyzed by chemical crosslinking as described (15, 16).
Blood Collection, RIA, and Acute Stress Analysis.
Plasma for ACTH and corticosterone analysis was collected by rapid retroorbital phlebotomy at 09:30 or 19:00 (16). Plasma ACTH was determined by using the human ACTH 130T kit (Nichols Institute Diagnostics, San Juan Capistrano, CA). Plasma corticosterone was determined by using the Coat-A-Count Rat Corticosterone RIA kit (DPC, Los Angeles). Animals were stressed by restraint for 30 min (16).
Weight Gain Study.
Animals were weighed three times a week (3–8 weeks old) or once a week (8–15 weeks). Weight was measured between 12:00 and 15:00. CRH-BP-deficient and wild-type control animals were housed together.
Feeding Study.
Two pellets of food were placed in each cage lid (16). Mice and food pellets were weighed within an hour of the beginning (6:00) and the end (20:00) of the light period for 5 consecutive days. Food (g) eaten in each period was averaged over the 5-day period. Average total daily food intake per gram weight was determined for each mouse.
Food Deprivation Study.
Mice were food deprived with free access to water for 24 hr (14:00 to 14:00). Preweighed food then was provided, and remaining food including spillage was weighed 30, 60, 90, and 120 min later. Cumulative amount of food eaten was calculated.
Behavioral Tests.
For each behavioral test, mice were between 2 and 7 months of age. All tests were videotaped and scored. Elevated plus maze (16, 23) activity was measured for 5 min. Number of entries into closed and open arms was scored as well as time spent in the open arms. Defensive withdrawal testing was conducted in a novel environment using a clear 40 × 20 × 18-cm polycarbonate cage containing a small opaque chamber 12 cm deep and 8 cm in diameter (24–27). The chamber was situated in the middle of the cage, with the open end facing the corner. The cage was illuminated by a lamp (1,700 lux on the floor). The mice were introduced into the small chamber, the chamber was placed in the cage, and activity was measured for 10 min. Latency to first exit the chamber (all four paws in the open field), the number of exits, and the total time spent out of the chamber were scored. Zone monitoring in an open field (28) was measured for 5 min in a 40 × 20 × 18-cm polycarbonate cage with grids marked on the floor. The floor was divided into 32 squares (8 × 4). The center 12 squares were scored as the inner zone, and the remaining 20 squares were scored as the outer zone. Grids crossed in the inner and outer zones were scored as well as time spent in the inner zone. Numbers of rears and fecal boli deposited also were scored.
Statistical Analysis.
All statistical analysis was performed with statview 4.0, by using the unpaired t test and ANOVA. Data are presented as mean ± SE.
RESULTS
Targeted Disruption of the CRH-BP Gene.
The mouse CRH-BP gene consists of seven exons and spans approximately 18 kb (Fig. 1). This gene was disrupted by homologous recombination in embryonic stem cells. A targeting vector was constructed with a 1.2-kb 5′ homologous region that contained CRH-BP 5′ flanking sequences and 94 bp of the untranslated portion of exon 1. The 3.6-kb 3′ region of homology included exon 6. Homologous recombination resulted in deletion of the translation start site in exon 1 and exons 2–5 of the CRH-BP gene (Fig. 1).
Two homologous recombinant clones (3G12 and 4H9) were identified by PCR from 450 independently selected colonies. The PCR screen used a forward primer located immediately 5′ of the region of 5′ homology (primer e) and a reverse primer in the PGK-1 promoter region of the PGK-neo cassette (primer f). These primers amplify a 1.7-kb product from targeted clones but no product from nontargeted clones. The homologous recombinant clones were confirmed by Southern analysis of both the 5′ and 3′ ends of the locus and injected into C57BL/6J blastocysts (22). Male chimeras were bred to C57BL/6J females, and germ-line transmission of the disrupted CRH-BP allele was obtained from both clones. There was no difference in the phenotypes exhibited by mice derived from the two independent clones.
CRH-BP Gene Disruption Produces a Null Mutation.
Heterozygous progeny from chimera x C57BL/6J crosses were intercrossed and progeny were genotyped by multiplex PCR analysis (Figs. 1 and 2a). Litter sizes averaged eight pups, indicating normal fertility of both male and female heterozygotes. The ratio of genotypes represented at 2–3 weeks of age did not deviate significantly from the expected 1:2:1 distribution, and equal proportions of male and female deficient homozygotes were obtained. Homozygous deficient mice were mated and produced litters, which indicates that the lack of CRH-BP does not affect viability or fertility.
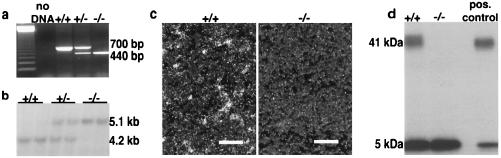
Figure 2.
Confirmation that the disruption of the CRH-BP gene created a null allele. (a) Mice were typed with a combination of three PCR primers, one forward primer, c, located in the untranslated region of exon 1, and two reverse primers, d and f. Primer d is located in exon 2 and produces a 700-bp amplification product with forward primer c in the endogenous allele. Primer f is located in the PGK-1 promoter region of the PGK-neo cassette and produces a 440-bp amplification product with forward primer c in the disrupted allele. The position of the primers is illustrated in Fig. 1. A 123-bp ladder served as a molecular weight marker. (b) PvuII digests of genomic DNA from +/+, +/−, and −/− mice hybridized with a 0.9-kb NcoI probe (probe A), located 5′ to the region of homology, revealed the expected 4.2-kb and 5.1-kb fragments from the endogenous and disrupted alleles, respectively. (c) Representative dark field photomicrographs of in situ hybridization histochemical analysis of cerebral cortex sections from a +/+ and a −/− mouse. Sections were hybridized with an antisense CRH-BP probe confirming the absence of the CRH-BP mRNA in the deficient mice. Magnification bars = 100 microns. (d) Crosslinking of 125I-labeled human CRH to pituitary protein extracts from a +/+ and a −/− mouse confirms the absence of CRH-BP activity in the deficient mouse. The pituitary extracts contain an equivalent amount of total protein. The crosslinked CRH:CRH-BP complex is 41 kDa, whereas unbound CRH is 5 kDa. The positive control is purified CRH-BP.
CRH-BP gene disruption was confirmed in mice by Southern analysis (Fig. 2b). CRH-BP in situ hybridization analysis in sections of cerebral cortex from wild-type and CRH-BP-deficient mice confirmed the absence of CRH-BP mRNA. CRH-BP normally is expressed abundantly in layers II, III, V, and VI of the cortex (10) (Fig. 2c). Deficiency of CRH-BP activity was confirmed by an 125I-hCRH crosslinking assay using total pituitary protein extract (Fig. 2d).
Normal HPA Axis Function in CRH-BP-Deficient Mice.
Male CRH-BP-deficient mice and wild-type mice were analyzed to determine the effects of CRH-BP deficiency on basal plasma levels of ACTH and corticosterone (16). No significant difference was observed between male CRH-BP-deficient and wild-type mice in basal morning ACTH levels, (−/− = 67.7 ± 16.7 pg/ml, n = 18; +/+ = 66.8 ± 19.9 pg/ml, n = 18), or basal morning corticosterone levels, (−/− = 37.2 ± 7.0 ng/ml, n = 12; +/+ = 36.2 ± 6.2 ng/ml, n = 14). Similarly, basal evening corticosterone levels were not significantly different between male CRH-BP-deficient and wild-type mice, (−/− = 67.1 ± 19.6 ng/ml, n = 7; +/+ = 79.8 ± 31.7 ng/ml, n = 6). The CRH-BP-deficient mice therefore exhibit the normal circadian pattern of corticosterone secretion. Consistent with these data, adrenal tissue from deficient mice exhibits normal morphology (data not shown).
The ability of male CRH-BP-deficient mice to appropriately increase and decrease plasma ACTH and corticosterone levels in response to acute stress was examined by using a 30-min restraint stress protocol. Blood samples were collected and analyzed for plasma levels of ACTH or corticosterone at various time points after initiation of stress (16). The CRH-BP-deficient mice exhibited normal ACTH (Fig. 3a) and corticosterone (Fig. 3b) profiles after acute restraint stress. To determine whether the 129/SvJ background contributed by the embryonic stem cells might confound stress test results, we compared C57BL/6J and 129/SvJ male mice. The two strains showed no significant difference in corticosterone response to the acute restraint stress (data not shown).
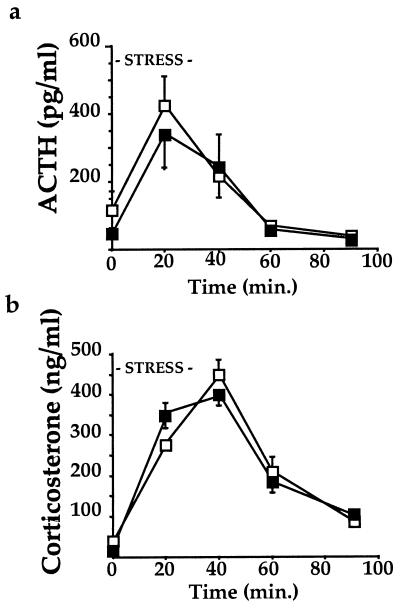
Figure 3.
Response of CRH-BP-deficient mice to acute restraint stress. (a) ACTH response to 30 min of restraint stress for male −/− mice and +/+ mice shows no significant difference (n = 6 of each genotype at each time point). ■, −/− mice; □, +/+ mice. (b) Corticosterone response to 30 min of restraint stress for male −/− mice and +/+ mice shows no significant difference (n = 6 of each genotype at each time point). ■, −/− mice; □, +/+ mice.
Male CRH-BP-Deficient Mice Exhibit Reduced Weight Gain.
Elevated CRH has been associated with decreased weight gain and reduced food intake (2, 29). Recent studies also have demonstrated appetite-suppressing effects of urocortin (13). A CRH-BP deficiency therefore might be expected to alter feeding behavior because of increased “free” levels of CRH and/or urocortin in the brain. CRH-BP-deficient mice and wild-type controls were weighed during the 3-week rapid weight gain period after weaning and as adults up to 4 months of age. In the female mice, no significant differences in weight were found between the two groups from 3 to 9 weeks of age (Fig. 4). However, the male CRH-BP-deficient mice showed a tendency toward reduced weight gain at 7–9 weeks (Fig. 4). Extension of this study in males revealed continued significant weight reduction compared with wild-type controls at 10, 14, and 15 weeks of age. This difference in weight gain could be caused by decreased food intake and/or altered energy metabolism.
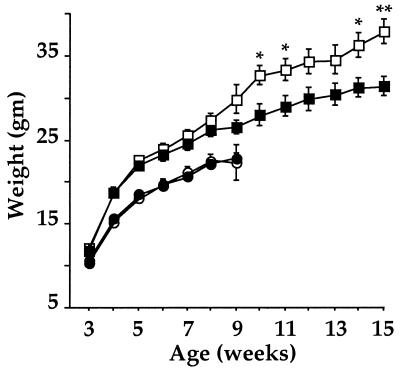
Figure 4.
CRH-BP-deficient male mice exhibit decreased weight gain. Weights of male and female −/− and +/+ mice at different ages are shown. The number of individuals for each point varied from 4–16. ∗, P = 0.04 for 10 weeks; ∗, P = 0.03 for 14 weeks, and ∗∗, P = 0.009 for 15 weeks. ■, male −/− mice; □, male +/+ mice; ●, female −/− mice; ○, female +/+ mice.
Animal weight and food intake were determined at the beginning and end of the light cycle for 5 consecutive days. In females, no significant differences in total daily food intake (g food/g weight) or feeding patterns (g food eaten in light vs. dark period) were detected between the two groups. In contrast, the male CRH-BP-deficient mice exhibited a trend toward reduced total daily food intake (g food/g weight) (−/− = 0.111 ± 0.009, n = 11; +/+ = 0.132 ± 0.005, n = 11, P = 0.06). The CRH-BP-deficient male mice showed a significant decrease in food intake (g food/g weight) during the light period (−/− = 0.032 ± 0.004, n = 11; +/+ = 0.047 ± 0.003, n = 11, ∗, P = 0.01) with a small, but not statistically significant, decrease in food intake during the dark period. These results suggest that decreased daily food intake may contribute to the reduced weight gain of the male CRH-BP-deficient mice.
Male mice also were tested for food intake after 24-hr food deprivation. CRH-BP-deficient mice showed no statistically significant difference in food intake over 120 min when compared with wild-type controls, suggesting that food intake in response to starvation is not altered in these mice (data not shown).
CRH-BP-Deficient Mice Exhibit an Increased Level of Anxiogenic-like Behavior.
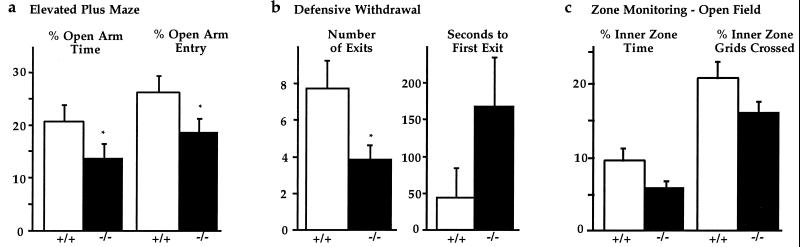
Administration (i.c.v.) of CRH or urocortin in rodents increases anxiogenic-like behavior as assessed by decreased investigation of open areas in a novel environment (2, 14). Anxiogenic-like responses have been demonstrated on the elevated plus maze, by defensive withdrawal tests, and by zone monitoring in an open field (2, 23–28, 30). To determine the behavioral consequences of deficiency of CRH-BP, a series of tests were performed on male and/or female CRH-BP-deficient and wild-type mice. When tested on an elevated plus maze, the male deficient mice spent significantly less time than male wild-type controls in the open arms of the maze, (−/− = 13.6 ± 2.3%, n = 48; +/+ = 20.9 ± 2.8%, n = 50, ∗, P = 0.05). They also entered the open arms significantly fewer times than wild-type mice, (−/− = 18.1 ± 2.2%, n = 48; +/+ = 26.7 ± 2.7%, n = 50, ∗, P = 0.02) (Fig. 5a). Analysis of total arm entries indicates that CRH-BP-deficient and wild-type mice were equally active, (−/− = 9.7 ± 0.6; +/+ = 9.3 ± 0.6). When females are tested on the elevated plus maze, similar anxiogenic-like effects are seen in CRH-BP-deficient mice. (Time: −/− = 22.5 ± 7.7%, n = 12; +/+ = 36.1 ± 6.7%, n = 12, P = 0.2. Entries: −/− = 24.6 ± 7.3%, n = 12; +/+ = 44.5 ± 8.7%, n = 12, P = 0.09.)
Figure 5.
CRH-BP-deficient mice exhibit increased anxiogenic-like behavior. (a) Elevated plus maze testing demonstrates that male −/− mice spend significantly less time in the open arms compared with +/+ mice. Percent of time spent in the open arms, ∗, P = 0.05. Percent of entries into the open arms, ∗∗, P = 0.02 (n = 50 wild-type male mice, 48 deficient male mice). (b) The defensive withdrawal test demonstrates that male −/− mice exit the small dark chamber significantly fewer times than the +/+ controls, ∗, P = 0.02. The latency period before first exit is longer for −/− mice than for +/+ controls, P = 0.2 (n = 5 wild-type male mice, 10 deficient male mice). (c) Zone monitoring in the open field demonstrates that the −/− mice spend less time in the inner zone of the open field as compared with +/+ mice. Percent of time spent in the inner zone, P = 0.06. Percent of grids crossed in the inner zone, P = 0.08 (n = 26 wild-type mice, 26 deficient mice).
In support, male CRH-BP-deficient mice made significantly fewer exits from the small dark chamber (protected area) into the open field (aversive environment) in the defensive withdrawal test, (−/− = 3.9 ± 0.8, n = 10; +/+ = 7.8 ± 1.5, n = 5, ∗, P = 0.02). They also showed a tendency toward a longer latency time to first exit the chamber, consistent with increased anxiogenic-like behavior, (−/− = 169.4 ± 65.5 sec, n = 10; +/+ = 46.6 ± 40.6 sec, n = 5, P = 0.2) (Fig. 5b). This test is based on the animal’s natural tendency to explore its environment in the absence of perceived threat and to retreat to an enclosed dark chamber when fearful (24).
Finally, zone monitoring in the open field showed a trend toward increased anxiogenic-like behavior in the CRH-BP-deficient mice, although the results did not reach statistical significance. The deficient mice spent less time than wild-type controls in the open inner zone, (−/− = 6.0 ± 0.8%, n = 26; +/+ = 9.5 ± 1.6%, n = 26, P = 0.06). They also crossed fewer grids in the inner zone than wild-type mice, (−/− = 16.2 ± 1.5%, n = 26; +/+ = 20.8 ± 2.2%, n = 26, P = 0.08) (Fig. 5c). Again, analysis of total grids crossed indicates that CRH-BP-deficient and wild-type mice are equally active (−/− = 162.3 ± 19.5, n = 26; +/+ = 137.5 ± 14.1, n = 26). When the zone monitoring data are separated by sex, females show a stronger anxiogenic-like effect in this assay. (Time: −/− females = 6.1 ± 1.5%, n = 12; +/+ females, = 12.1 ± 3.2%, n = 12, P = 0.1; −/− males = 5.9 ± 0.8%, n = 14; +/+ males = 7.2 ± 0.9%, n = 14, P = 0.3. Grids: −/− females = 14.8 ± 2.6%, n = 12; +/+ females = 23.7 ± 3.6%, n = 12, P = 0.06; −/− males = 17.3 ± 1.6%, n = 14; +/+ males = 18.4 ± 2.7%, n = 14, P = 0.7.) Other indicators of emotional reactivity such as rearing or deposits of fecal boli showed no significant differences (data not shown).
DISCUSSION
In this study, CRH-BP-deficient mice were generated by using targeted homologous recombination to examine the in vivo role of CRH-BP in modulation of CRH activity. In vitro studies suggest that the binding of CRH and/or urocortin by CRH-BP prevents activation of the CRH-Rs (9, 15). We hypothesized that the lack of CRH-BP in vivo would result in increased “free” CRH or urocortin levels, which could cause behavioral effects in the CNS and potentially activate the HPA axis. The CRH-BP-deficient mice exhibit increased anxiogenic-like behavior and decreased weight gain, consistent with the anxiogenic and anorectic effects of elevated CRH and/or urocortin. In contrast, the CRH-BP-deficient mice exhibit normal HPA axis function.
The HPA axis is required for the maintenance of body homeostasis. Disruptions of the HPA axis are associated with a number of disorders in humans, including major depression, anorexia nervosa, and Cushing’s syndrome (1, 5). Although the expression of CRH-BP in pituitary corticotropes might suggest that the HPA axis would be perturbed in the absence of CRH-BP, the CRH-BP-deficient mice show normal basal ACTH and corticosterone levels and respond normally to acute restraint stress. However, the HPA axis is tightly regulated to avoid hyper- and hypoactivity. Both ACTH and corticosterone act in negative feedback loops to control appropriate CRH production. Hypothalamic CRH and vasopressin mRNA levels are elevated in transgenic mice with constitutive overexpression of CRH-BP in the pituitary (16). Thus, a variety of compensatory mechanisms are likely to be acting to maintain homeostasis in the CRH-BP-deficient animals.
Elevated CRH in the CNS elicits a variety of behavioral effects, including decreased food intake and reductions in weight gain (2, 29). In food-deprived animals, urocortin also suppressed food consumption, suggesting anorectic effects for both CRH and urocortin (13). Male, but not female, CRH-BP-deficient mice, show significant decreases in weight gain and daily food intake compared with wild-type controls. A similar sex-specific growth difference has been demonstrated in rats (29). Fourteen-day chronic infusion of CRH had no effect on the weight of female rats, but caused a small decrease in weight gain in males. In addition, total food intake over the 14 days was not significantly different in the female rats, but showed a small difference in males (29). Similarly, recent studies with a CRH-BP-overexpressing transgenic mouse revealed sexually dimorphic weight gain changes. Male transgenic CRH-BP-overexpressing mice exhibit a small, but significant, increase in weight gain from 3 to 12 months of age, whereas female transgenic mice show differences in weight gain only at 12 months of age (31). Together, these results demonstrate that chronic changes in free CRH levels may cause sexually dimorphic effects on weight gain and food intake.
Administration (i.c.v.) of urocortin produces anxiety-like effects in a number of behavioral paradigms (14). Central administration of CRH also produces a number of behavioral effects that correlate with an increased state of anxiety, including reduced investigation in novel environments, enhanced fear responses, decreased sleeping, and reduced sexual behavior (2). These changes are similar to many of the behavioral alterations seen in human affective disorders. Anxiogenic-like behavior can be examined by using tests sensitive to the natural aversion of rodents for open spaces where they are more vulnerable, including the defensive withdrawal test, locomotor activity in a novel environment, and activity on the elevated plus maze (2, 23–27, 30). In the elevated plus maze and open-field tests, total locomotor activity was the same in CRH-BP-deficient and wild-type control animals. In contrast, the CRH-BP-deficient male animals display a dramatic increase in anxiogenic-like behavior on the elevated plus maze with a greater than 32% decrease in both the time spent on the open arm and the percentage of entries into the open arm. In the defensive withdrawal test, the CRH-BP-deficient mice made significantly fewer exits from the dark chamber into the open field and showed a trend toward a longer latency time to emerge from the chamber. These results are consistent with previous reports of increased latency times in defensive withdrawal tests after i.c.v. administration of CRH, and decreased latency times and increased exits after i.c.v. administration of CRH-R antagonist (α helical CRH 9–41) (24). Finally, CRH-BP-deficient mice also spent less time in the inner zones of the open field, indicative of increased anxiety-like behavior. These results are consistent with increased “free” CRH or urocortin levels in the CNS of these animals, resulting in increased anxiogenic-like behavior.
This conclusion is supported by observations in three other mouse models. Transgenic mice that constitutively overexpress CRH exhibit increased anxiogenic-like behavior as measured by the elevated plus maze (32). CRH-R1 deficiency leads to a decrease in CRH and urocortin signaling and decreased anxiogenic-like behavior as assessed by increased exploratory behavior in both the elevated plus maze and defensive withdrawal test (25, 33). Overexpression of CRH-BP in transgenic mice produced a similar trend toward decreased anxiogenic-like behavior (16). Therefore, the results from several models support the hypothesis that increased levels of CRH and/or other CRH-like ligands in the CNS lead to increased anxiogenic-like behavior.
In summary, we have created CRH-BP-deficient mice by using targeted homologous recombination. These mice exhibit a significant increase in anxiogenic-like behavior and a reduced weight gain profile, most likely because of the anxiogenic and anorectic effects of increased “free” CRH or urocortin levels in the brain. These results are consistent with the hypothesis that the CRH-BP normally functions in vivo to bind CRH and/or urocortin and prevent its interaction with the receptor. In the absence of CRH-BP, CRH and other CRH-like ligands are no longer bound, creating increased CRH bioactivity. The CRH-BP-deficient mice, an animal model for anxiety-like behavior, may be useful for testing the efficacy of anxiolytic drugs.
Acknowledgments
We thank A. Nagy, R. Nagy, W. Abramov-Newerly, R. Mulligan, and T. Doetschman for reagents and T. Saunders, P. Gillespie, L. Samuelson, L. Gates, Z. Oraefa, and S. Coon for their contributions to the work. This work was supported by National Institutes of Health Grants R01-DK-42730 (to A.F.S.) and T32 GM-07863 (to H.L.B.), a University of Michigan Rackham Association of Fellows fellowship (to H.L.B.), and the University of Michigan transgenic animal model core (http://www.med.umich.edu/tamc/).
ABBREVIATIONS
- CRH
corticotropin-releasing hormone
- CRH-BP
CRH-binding protein
- CRH-R
CRH receptor
- ACTH
adrenocorticotropic hormone
- HPA
hypothalamic-pituitary-adrenal
- CNS
central nervous system
- PGK
phosphoglycerate kinase
- i.c.v.
intracerebroventricular
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Owens M J, Nemeroff C B. Pharmacol Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- 2.Dunn A J, Berridge C W. Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 3.Koob G F, Bloom F E. Fed Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- 4.Behan D P, Heinrichs S C, Troncoso J C, Liu X-J, Kawas C H, Ling N, DeSouza E B. Nature (London) 1995;378:284–287. doi: 10.1038/378284a0. [DOI] [PubMed] [Google Scholar]
- 5.Tsigos C, Chrousos G P. Endocrinol Metab Clin North Am. 1994;23:451–467. [PubMed] [Google Scholar]
- 6.Chalmers D T, Lovenberg T W, Grigoriadis D E, Behan D P, De Souza E B. Trends Phamacol Sci. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 7.Behan D P, Linton E A, Lowry P J. J Endocrinol. 1989;122:23–31. doi: 10.1677/joe.0.1220023. [DOI] [PubMed] [Google Scholar]
- 8.Orth D N, Mount C D. Biochem Biophys Res Commun. 1987;143:411–417. doi: 10.1016/0006-291x(87)91369-6. [DOI] [PubMed] [Google Scholar]
- 9.Potter E, Behan D P, Fischer W H, Linton E A, Lowry P J, Vale W W. Nature (London) 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- 10.Potter E, Behan D P, Linton E A, Lowry P J, Sawchenko P E, Vale W W. Proc Natl Acad Sci USA. 1992;89:4192–4196. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behan D P, Khongsaly O, Owens M J, Chung H D, Nemeroff C B, De Souza E B. J Neurochem. 1997;68:2053–2060. doi: 10.1046/j.1471-4159.1997.68052053.x. [DOI] [PubMed] [Google Scholar]
- 12.Vaughan J, Donaldson C, Bittencourt J, Perrin M H, Lewis K, Sutton S, Chan R, Turnbull A V, Lovejoy D, Rivier C, et al. Nature (London) 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 13.Spina M, Merlo-Pich E, Chan R K W, Basso A M, Rivier J, Vale W W, Koob G F. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 14.Moreau J, Kilpatrick G, Jenck F. NeuroReport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- 15.Cortright D N, Nicoletti A, Seasholtz A F. Mol Cell Endocrinol. 1995;111:147–157. doi: 10.1016/0303-7207(95)03558-o. [DOI] [PubMed] [Google Scholar]
- 16.Burrows H L, Nakajima M, Lesh J S, Goosens K A, Samuelson L C, Inui A, Camper S A, Seasholtz A F. J Clin Invest. 1998;101:1439–1447. doi: 10.1172/JCI1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seasholtz A F, Bourbonais F J, Harnden C E, Camper S A. Mol Cell Neurosci. 1991;2:266–273. doi: 10.1016/1044-7431(91)90054-r. [DOI] [PubMed] [Google Scholar]
- 18.Tybulewicz V L, Crawford C E, Jackson P K, Bronson R T, Mulligan R C. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 19.Kendall S K, Saunders T L, Jin L, Lloyd R V, Glode L M, Nett T M, Keri R A, Nilson J H, Camper S A. Mol Endocrinol. 1991;5:2025–2036. doi: 10.1210/mend-5-12-2025. [DOI] [PubMed] [Google Scholar]
- 20.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saiki R K, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 22.Bradley A. In: Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. Robertson E J, editor. Oxford: Oxford Univ. Press; 1987. pp. 113–152. [Google Scholar]
- 23.Nakajima M, Inui A, Asakawa A, Momose K, Ueno N, Teranishi A, Baba S, Kasuga M. Peptides. 1998;19:359–363. doi: 10.1016/s0196-9781(97)00298-2. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi L K, Kalin N H, Vanden Burgt J A, Sherman J E. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- 25.Smith G W, Aubry J M, Dellu F, Contarino A, Bilezikjian L M, Gold L H, Chen R, Marchuk Y, Hauser C, Bentley C A, et al. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 26.Heinrichs S C, Lapansky J, Lovenberg T W, De Souza E B, Chalmers D T. Regul Pept. 1997;71:15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- 27.Yang X-M, Gorman A L, Dunn A J. J Pharmacol Exp Ther. 1990;255:1064–1070. [PubMed] [Google Scholar]
- 28.Harro J. Methods Neurosci. 1993;14:359–377. [Google Scholar]
- 29.Rivest S, Deshaies Y, Richard D. Am J Physiol. 1989;257:R1417–R1422. doi: 10.1152/ajpregu.1989.257.6.R1417. [DOI] [PubMed] [Google Scholar]
- 30.Montkowski A, Poettig M, Mederer A, Holsboer F. Brain Res. 1997;762:12–18. doi: 10.1016/s0006-8993(97)00370-3. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy D A, Aubry J M, Turnbull A, Sutton S, Potter E, Yehling J, Rivier C, Vale W W. J Neuroendocrinol. 1998;10:483–491. doi: 10.1046/j.1365-2826.1998.00206.x. [DOI] [PubMed] [Google Scholar]
- 32.Stenzel-Poore M P, Heinrichs S C, Rivest S, Koob G F, Vale W W. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul J M H M, Stalla G K, Blanquet V, Steckler T, Holsboer F, Wurst W. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]