Abstract
The mitochondrial replication machinery in human cells includes the DNA polymerase γ holoenzyme and the TWINKLE helicase. Together, these two factors form a processive replication machinery, a replisome, which can use duplex DNA as template to synthesize long stretches of single-stranded DNA. We here address the importance of the smaller, accessory B subunit of DNA polymerase γ and demonstrate that this subunit is absolutely required for replisome function. The duplex DNA binding activity of the B subunit is needed for coordination of POLγ holoenzyme and TWINKLE helicase activities at the mtDNA replication fork. In the absence of proof for direct physical interactions between the components of the mitochondrial replisome, these functional interactions may explain the strict interdependence of TWINKLE and DNA polymerase γ for mitochondrial DNA synthesis. Furthermore, mutations in TWINKLE as well as in the catalytic A and accessory B subunits of the POLγ holoenzyme, may cause autosomal dominant progressive external ophthalmoplegia, a disorder associated with deletions in mitochondrial DNA. The crucial importance of the B subunit for replisome function may help to explain why mutations in these three proteins cause an identical syndrome.
INTRODUCTION
The molecular mechanisms by which mitochondrial DNA (mtDNA) is replicated in mammalian cells are of fundamental biological interest. Saccharomyces cerevisiae has served as a model system for studies of mammalian mtDNA replication, but there are significant differences between yeast and mammalian cells (1). Replication of the S. cerevisiae mtDNA is initiated from multiple sites of the ∼86 kb genome and the mtDNA molecules frequently undergo recombination. In contrast, the smaller mammalian mtDNA (∼16 kb) initiates DNA replication from two specific origins of replication, oriH and oriL, and recombination is a rare or possibly even non-existent phenomenon (2). The replicative DNA polymerase γ (POLγ) has been characterized in many species. In animal cells, the POLγ holoenzyme consists of a catalytic subunit (POLγA) and an accessory subunit (POLγB). POLγA has a molecular weight of 140 kDa, and harbors polymerase and 3′-5′ exonuclease activities. POLγB is present in Homo sapiens, Mus musculus, Drosophila melanogaster and Xenopus laevis (3,4), but is notably absent in S. cerevisiae. POLγB acts as a processivity factor (5), which increases the affinity of the polymerase for DNA and promotes tighter nucleotide binding, thereby increasing the polymerization rate (5,6). The crystal structures of both mouse and human POLγB have revealed the protein as a dimer with high similarities to aminoacyl tRNA synthetases (7,8). Molecular modeling using the POLγB structure and the bacteriophage T7 DNA polymerase ternary complex, has shown that POLγB together with POLγA may encircle the double-stranded DNA (dsDNA), thereby tethering the enzyme to the DNA substrate and increasing processivity (8). POLγB also binds non-specifically to stretches of duplex DNA longer than 45 bp, but the functional importance of this activity remains unclear (6).
The mitochondrial DNA helicase TWINKLE displays sequence similarity to the bacteriophage T7 gene 4 protein, a helicase-primase required at the phage DNA replication fork (9). TWINKLE displays distinct substrate requirements and can only unwind short stretches (<20 bp) of dsDNA in the 5′ to 3′ direction (10). In a related way, the POLγ holoenzyme is unable to use dsDNA as template for DNA synthesis. However, when the POLγ holoenzyme and TWINKLE are combined, they form a processive replication machinery, a replisome, which can utilize duplex DNA as template to synthesize ssDNA molecules of about 2 kb. Addition of the mitochondrial single-stranded DNA-binding protein (mtSSB) stimulates the reaction further, generating DNA products of about 16 kb, the size of the mammalian mtDNA molecule (11). A close functional interaction between the mitochondrial replication factors is also demonstrated by the many different mutations in both TWINKLE and POLγA, which cause autosomal dominant progressive external ophthalmoplegia (adPEO) (9), a human disorder associated with deletions in mitochondrial DNA. Recently a mutation in POLγB was also shown to cause the same disorder (12).
Here we demonstrate that POLγB and its dsDNA-binding activity are absolutely required for the function of the mitochondrial DNA replisome. The dsDNA-binding activity of POLγB is not required to stimulate the DNA synthesis rate or the processivity of the POLγA, but is instead needed for functional interactions between the POLγ holoenzyme and TWINKLE at the mtDNA replication fork. In the absence of proof for direct physical interactions between the components of the mtDNA replisome, this functional interaction may explain the strict interdependence of TWINKLE and the POLγ holoenzyme for DNA synthesis on a duplex DNA template.
MATERIALS AND METHODS
Recombinant proteins
TWINKLE, mtSSB, POLγA and POLγB were expressed and purified as described previously (11), with the following modifications. The peak fractions of POLγA from the Hi-trap Heparin column was diluted to 100 mM NaCl with buffer B (20 mM Tris-HCl [pH 8.0], 0.5 mM EDTA [pH 8.0], 10% glycerol and 1 mM DTT) and loaded on a 1-ml Mono Q column (Amersham Biosciences) equilibrated in buffer B (0.1 M NaCl). The column was washed with three column volumes of buffer B (0.1 M NaCl) and then eluted with a linear gradient (10 ml) of buffer B (0.1 − 1.0 M NaCl). POLγA eluted at 400 mM NaCl and the yield from 800 ml of culture was about 1 mg POLγA protein. The peak fractions of POLγB eluted from the Hi-trap Heparin column was dialyzed against buffer C (50 mM NaPO4 [pH 7.0], 0.5 mM EDTA [pH 8.0], 10% glycerol and 1 mM DTT) containing 0.1 M NaCl. POLγB was further purified on a 1-ml Hi-Trap SP column (Amersham Biosciences) equilibrated in buffer C (0.1 M NaCl). After washing the column with three column volumes of buffer C (0.1 M NaCl), POLγB was eluted with a linear gradient (10 ml) of buffer C (0.1 − 1.0 M NaCl) and the peak of protein eluted at 300 mM NaCl. A 6xHis-tagged version of POLγB cloned into pBacPAK9 (11) was used for PCR-based mutagenesis, as previously described (13). Plasmids containing the RK, RKK, IF and VV mutations were sequenced and used to prepare Autographa California nuclear polyhedrosis virus recombinant for the proteins as described in the BacPAK manual (Clontech). The mutant versions of POLγB were purified following the same protocol used for the wt, with the exception that the RK and RKK mutant did not bind to the heparin and SP columns, but eluted in the flow through fractions from both these columns. The proteins were concentrated on centricon 30 (Amicon). We estimated the purity of the proteins to be at least 95% by SDS-PAGE with Coomassie blue staining (Figure 1A).
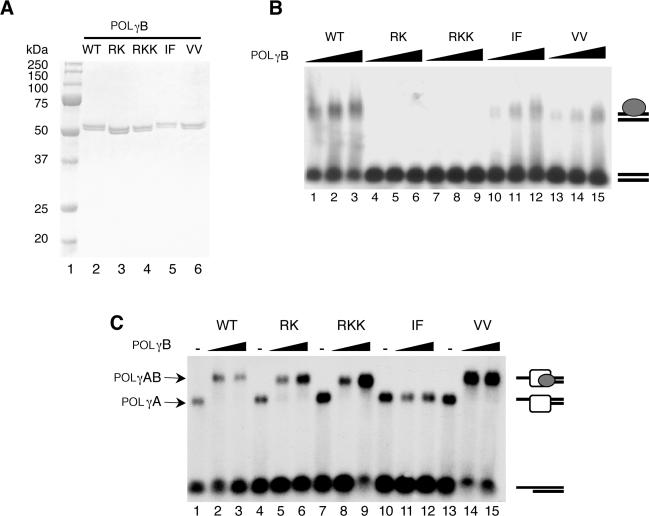
Figure 1.
Purification and characterization of the recombinant POLγB proteins (A), Purified recombinant wt and mutant versions of POLγB (0.5 µg) were separated by SDS–PAGE (12.5%) and revealed with Coomassie brilliant blue staining. lane 1, size marker; lane 2, wt POLγB; lane 3, RK mutant; lane 4, RKK mutant; lane 5, IF mutant; lane 6, VV mutant. (B) DNA binding affinity of POLγB mutants was determined by EMSA using a P32-labeled 50-bp dsDNA probe (10 fmol/reaction). The reactions were performed as described in experimental procedures in the presence of increasing amount of the indicated POLγB versions (0.2, 0.4 and 0.8 pmol). After incubation for 10 min at RT the protein-DNA complexes were analyzed on a 0.8% native agarose gel. (C) Interactions between the POLγB mutants and the catalytic POLγA subunit were determined by EMSA using a P32-labeled primed DNA template (10 fmol). The template was incubated with POLγA (0.5 pmol) and increasing amounts of POLγB (0, 0.5 and 1 pmol) for 10 min at RT. The protein–DNA complexes were immediately analyzed on a native 0.8% agarose gel.
In vitro DNA replication
The mini-circle template for rolling-circle DNA replication was generated as previously described (14). The mini-circle template (10 fmol) was added to a reaction mixture (20 µl) containing 25 mM Tris-HCl (pH 7.5), 10 mM magnesium chloride, 1 mM DTT, 100 µg/ml BSA, 4 mM ATP, 10% glycerol, 250 µM dATP, 250 µM dTTP, 250 µM dGTP, 10 µM dCTP, 2 μCi [α-32P] dCTP, and the indicated amounts of the different replication factors. The reaction was incubated at 37°C and stopped at the times indicated by adding 200 µl of stop buffer (10 mM Tris-HCl [pH 8.0], 0.2 M NaCl, 1 mM EDTA and 0.1 mg/ml glycogen). The samples were then treated with 0.5% SDS and 100 µg/ml proteinase K for 45 min at 42°C, and precipitated by adding 0.6 ml of ice-cold 95% ethanol. The pellets were dissolved in 10 µl of gel-loading buffer (98% formamide, 10 mM EDTA [pH 8.0], 0.025% xylene cyanol FF, 0.025% bromophenol blue). The samples were heated at 95°C for 5 min and separated on a 10% denaturing polyacrylamide gel in 1 X TBE buffer. For analysis of longer replication products, the pellets were dissolved in denaturing agarose buffer and separated on a 0.75% denaturing agarose gel as described previously (11). The gels were dried onto DE81 (Whatman) and visualized by autoradiography overnight at −80°C with an intensifying screen.
Electrophoresis mobility shift assay
The dsDNA-binding affinity of POLγB was assayed by an electrophoresis mobility shift assay (EMSA) using a double-stranded probe (5′ GGCGTATATCCAAATTAAAAGCATTTTTGATTGCATATATATCATCAGCT 3′) labeled with [α-32P] dCTP using the Klenow fragment of DNA polymerase I. Reactions were carried out in 15 µl volumes containing 10 fmol DNA template, 20 mM Tris-HCl [pH 7.5], 1 mM DTT, 0.1 mg/ml bovine serum albumin, 10 mM MgCl2, 10% glycerol, 2 mM ATP, and the protein concentrations indicated in the figure legend. DNA binding affinity of POLγA to a primer-template was assayed using a 35-mer oligonucleotide (5′ TTTTTTTTTTATCCGGGCTCCTCTAGACTCGACCG 3′) labeled in the 5′ end with [γ-32P] ATP and annealed to a 20-mer complementary oligonucleotide (5′CGGTCGAGTCTAGAGGAGCC3′) to produce a primed-template with a 15 bases single-stranded 5′-tail. The reactions were carried out as with the dsDNA but 0.3 mM ddGTP and 3 mM dCTP were added to the reaction mixture. Proteins were added as indicated in the figure legends and reactions were incubated at RT for 10 min before separation on a 0.8% agarose gel in 1 X TBE for 2 h at 100 V.
DNA synthesis assay
A 60-mer oligonucleotide (5′GGCCCCCTAGGTGATCAAGACACATAATTATTCTTATAAGAACATGTTCATGCCGAGGTT3′) was annealed to single-stranded pBluescript II KS+, which had been isolated according to the manufacturer's protocol (Stratagene). The template formed contains a 20-bp duplex region and a 40-bases single-stranded 5′-tail. Reactions were carried out in 20 µl volumes containing 10 fmol template DNA, 10 mM Tris-HCl [pH 7.5], 1 mM DTT, 0.1 mg/ml BSA, 10 mM MgCl2, 10% glycerol, 2 mM ATP, 10 µM dCTP, 100 µM dATP, 100 µM dTTP, 100 µM dGTP, 2 μCi [α-32P] dCTP. Reactions were incubated at 37°C for the indicated times and separated on a 0.8% agarose gel at 120 V for 2 h in 1 X TBE.
Polymerase/3′-5′ exonuclease coupled assay
A 35-mer oligonucleotide (5′TTTTTTTTTTATCCGGGCTCCTCTAGACTCGACCG3′) was annealed to a 20-mer complementary oligonucleotide (5′CGGTCGAGTCTAGAGGAGCC3′) labeled in 5′-end with [γ-32P] ATP to produce a primed-template that can be used as a substrate for both DNA polymerization and 3′-5′ exonuclease activity. The reaction mixture contained 10 fmol of the DNA template, 25 mM Tris-HCl [pH 7.5], 10% glycerol, 1 mM DTT, 10 mM MgCl2, 100 µg/ml BSA, 60 fmol of POLγA and 120 fmol of either wild-type or mutant POLγB and the indicated concentrations of the four dNTPs. The reaction was incubated at 37°C for 15 min and stopped by the addition of 10 µl of gel-loading buffer. The samples were analyzed on a 15% denaturing polyacrylamide gel in 1 X TBE buffer. Polymerization or 3′-5′ exonuclease activity are detected by, respectively, an increase or a decrease in the size (20-mer) of the 5′ labeled primer.
RESULTS
Purification and initial characterization of mutant POLγB proteins
We wanted to study the role of POLγB in mtDNA replication and therefore made a series of mutant constructs based on information from the crystal structure of POLγB (7). We constructed the POLγB-IF mutant by changing two conserved hydrophobic residues (I442F448 to A442D448) in a region required for interactions with POLγA. In the crystal structure of POLγB, two Na+ ions are buried in the dimer interface. The role of these metal-binding sites is not known, but the regular coordination geometry suggests that in vivo this could be a binding site for a divalent cation, e.g. magnesium. To address the functional importance of the metal-binding site for POLγB activity, we mutated two of the three amino acids involved in metal ion coordination (V119V125 to A119A125) to construct the POLγB-VV mutant. Finally, we made the POLγB-RK mutant by changing positions R328K329 into A328A329 and the POLγB-RKK mutant by changing positions R363K364K365 into A363A364A365. Others have shown that the RK and RKK mutations abolish the dsDNA-binding activity of POLγB (6).
We expressed the mutant versions of POLγB in insect cells and the proteins were purified to near homogeneity (Figure 1A). To verify the properties of our mutant POLγB proteins, we first investigated the dsDNA-binding activity (Figure 1B). Wild-type POLγB, as well as the IF and VV mutants bound duplex DNA in a gel-shift assay. In contrast, the RK and RKK mutants had lost the dsDNA-binding activity and failed to interact with the radioactively labeled probe.
We also investigated the ability of the mutant POLγB versions to interact with POLγA in a gel retardation assay. POLγA forms a distinct complex with a primed DNA substrate. POLγB alone does not bind the substrate, but can super-shift a POLγA–DNA complex, thereby revealing direct POLγA–POLγB interactions (15). All the mutants, except the IF mutant super-shifted the POLγA–DNA complex (Figure 1C). We could therefore conclude that the IF mutation impairs POLγB interaction with POLγA.
The dsDNA-binding activity of POLγB is not required to stabilize POLγA interactions with a primed DNA substrate
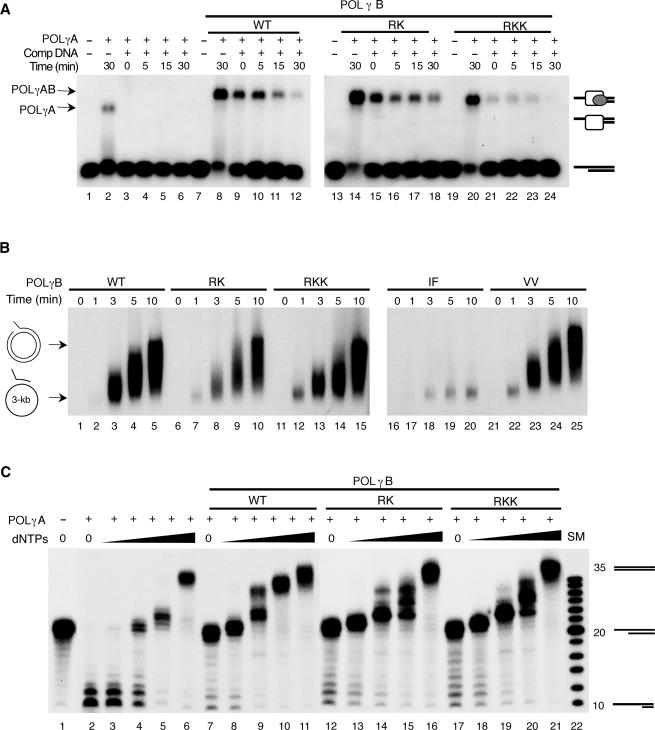
POLγB has been shown to stabilize POLγA interactions with a primed DNA substrate and we wanted to monitor the effects of our POLγB mutations on this activity (15). We formed the template by annealing a 35-mer oligonucleotide to a complementary 20-mer oligonucleotide, to produce a primed-template with a 15 bases single-stranded 5′-tail. We added POLγA in combination with POLγB to the radioactively labeled substrate (Figure 2A). Following 10 min of incubation, we added a 1000-fold excess of cold primed DNA substrate to the reactions and withdrew aliquots at the times indicated. POLγA alone dissociates from the template immediately after addition of competing primed DNA substrate (Figure 2A, lane 3). The addition of wt POLγB had a strong stabilizing effect on POLγA interactions with the primed DNA substrate (Figure 2A, lanes 8–12). To our surprise, the RK and RKK mutants behaved as the wt protein and stabilized POLγA binding to the primed DNA substrate (Figure 2A, lanes 14–24). Hence, the dsDNA-binding activity of POLγB is not required to stabilize POLγA interactions with a primed DNA substrate.
Figure 2.
POLγB mutations have distinct effects on POLγA activities. (A) The binding stability of POLγA alone or together with the different POLγB mutants was investigated by a challenging experiment as described in experimental procedures. POLγA (100 fmol) and the indicated POLγB mutants (300 fmol) were incubated with a P32-labeled primed DNA template (10 fmol). After 10 min of incubation an excess of the unlabeled primed DNA template (1000 X) was added and samples were taken at the times indicated. (B) DNA synthesis rate was measured using a primed circular single-stranded DNA template (∼3000 bases). POLγA (150 fmol) and the indicated POLγB mutants (300 fmol) were incubated with the primed template (10 fmol) at 37°C. Samples were taken at the times indicated, and analyzed on a 1% native agarose gel. (C) DNA polymerase/exonuclease coupled assay. The reaction was carried out as described in materials and methods using a primed DNA template, 60 fmol of POLγA, 120 fmol of the indicated POLγB versions and an increasing concentration of dNTPs (0, 1, 10, 100 and 1000 nM). The positions of the non-elongated primer (20-mer), the elongated primer (35-mer) and the degraded primer (10-mer) are indicated. lane 22 is a DNA size marker (SM).
POLγB dsDNA-binding activity is not required for DNA synthesis on an ssDNA template
We examined how the individual POLγB mutants affected DNA synthesis rate on a primed ssDNA template. We hybridized a 60-nt oligonucleotide to a single-stranded pBluescript II KS+ plasmid and incubated this template with the POLγ holoenzyme. All the POLγB mutants, except the IF mutant (which does not interact with POLγA) were able to support the same rate of DNA synthesis as the wt POLγB protein (Figure 2B). We also monitored effects on POLγA processivity (data not shown). POLγA in isolation displays low processivity on a single-stranded DNA template, but becomes more processive when wt POLγB is added to the reaction (5). The RK, RKK and VV mutants stimulated the processivity of POLγA to the same extent as wt POLγB which is in agreement with previous reports that the dsDNA-binding activity is not required for this function (6). Therefore, the dsDNA-binding activity of POLγB is not required to stimulate POLγA processivity or DNA synthesis rate on an ssDNA template.
POLγB inhibits the POLγA exonuclease activity
Many DNA polymerases display a delicate balance between the 3′ to 5′ exonuclease and the polymerase activities. This balance is affected by the dNTP concentration. We monitored the ability of POLγB to influence this balance in a polymerase/exonuclease assay (Figure 2C). First, we added POLγA in isolation to a radioactively labeled primed DNA substrate in the presence of different dNTP concentrations. In the absence of dNTP, the exonuclease/polymerase balance shifted in favor of the exonuclease activity, but with increasing amounts of dNTP the balance changed in favor of polymerization (Figure 2C, lanes 2–6). Our experiments revealed that POLγA alone required a nucleotide concentration of about 1 µM for net polymerization (Figure 2C, lane 6). Interestingly, addition of POLγB blocked the exonuclease activity of POLγA (Figure 2C, compare lanes 2 and 7), but did not change the dNTP concentration required for net polymerization (Figure 2C, compare lanes 6 and 11). This observation is in agreement with previous reports that the excision rate of the POLγ holoenzyme is slower than for POLγA alone (16). We also monitored the ability of POLγB mutants RK and RKK to influence the exonuclease/polymerase balance. These mutants blocked the exonuclease activity of POLγA to the same extent as the wt POLγB protein (Figure 2C, lanes 12–22). Therefore, POLγB inhibits the POLγA exonuclease activity and the POLγB dsDNA-binding activity is not required for this inhibition.
POLγB is required for mtDNA replisome function
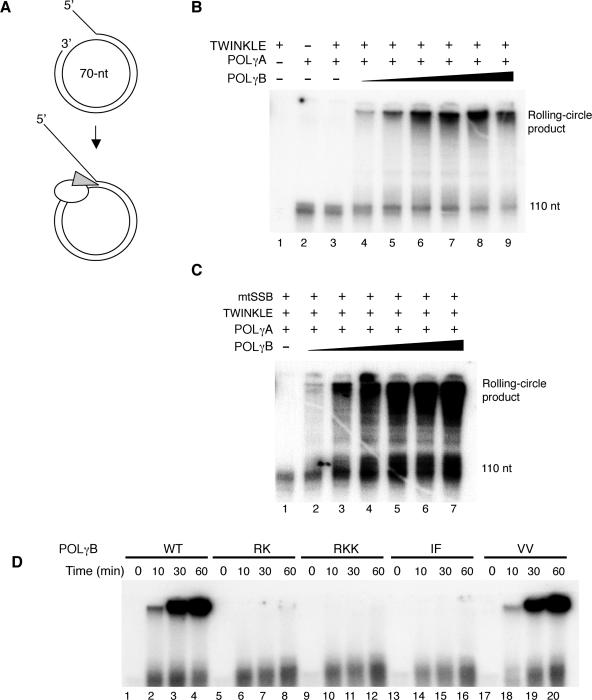
All our experiments so far had been performed on single-stranded DNA templates. Our assays had revealed some novel aspects of POLγB function, but we had not been able to identify a functional role for the dsDNA-binding activity. We decided to investigate the role of POLγB for DNA synthesis on a duplex DNA template. To this end, we formed a template for DNA replication by annealing a 90-nt oligonucleotide to a 70-nt ssDNA mini-circle (Figure 3A). The template contained a replication fork for loading the replication machinery, a 50-bp dsDNA region and a free 3′-hydroxyl terminus that could act as a primer for DNA synthesis. Once initiated, leading-strand DNA synthesis coupled to continuous unwinding of the double-stranded template could in principle progress indefinitely. We have previously reported that the POLγ holoenzyme can utilize the 3′-hydroxyl terminus on this mini-circle template and initiate DNA synthesis, but that the enzyme fails to elongate through double-stranded regions and only forms a 110-nt product. To elongate through the dsDNA region, the POLγ holoenzyme requires the DNA strand unwinding activity of the TWINKLE helicase (11). In agreement with these previous studies of the POLγ holoenzyme, we found that the POLγA subunit in isolation could initiate DNA synthesis on the mini-circle template, but failed to elongate through double-stranded regions (Figure 3B, lane 2). In contrast to our previous observations with the POLγ holoenzyme, the TWINKLE helicase was unable to stimulate POLγA elongation through dsDNA (Figure 3B, lane 3). Addition of increasing amounts of POLγB to the reaction restored TWINKLE-dependent stimulation of POLγA and allowed for rolling-circle DNA synthesis on the dsDNA template. Our experiments therefore revealed that POLγB is absolutely required for mtDNA replisome function. In the absence of POLγB, TWINKLE cannot stimulate POLγA dependent DNA synthesis on a duplex template. Addition of mtSSB did not overcome this absolute requirement for POLγB (Figure 3C, lane 1).
Figure 3.
POLγB is required for rolling-circle DNA synthesis by POLγA and TWINKLE. (A) The mini-circle template was prepared as described in experimental procedures. DNA synthesis is initiated at the 3′-hydroxy terminus and proceeds 20 nt before it encounters the dsDNA region of the template. The template can be efficiently replicated by the POLγ holoenzyme (white) and TWINKLE helicase (grey). (B) Increasing amounts of POLγB together with constant amounts of TWINKLE (200 fmol) and POLγA (150 fmol) were incubated with the mini-circle template (10 fmol) for 90 min at 37°C as indicated. lane 4, 10 fmol POLγB; lane 5, 25 fmol POLγB; lane 6, 50 fmol POLγB; lane 7, 100 fmol POLγB; lane 8, 150 fmol POLγB; lane 9, 300 fmol POLγB. The products were analyzed on a 10% denaturing polyacrylamide gel as described in materials and methods. (C) The same experiment as in panel B was performed in the presence of mtSSB (5 pmol). lane 2, 10 fmol POLγB; lane 3, 25 fmol POLγB; lane 4, 50 fmol POLγB; lane 5, 100 fmol POLγB; lane 6, 150 fmol POLγB; lane 7, 300 fmol POLγB. (D) Rolling-circle DNA synthesis in the presence of different POLγB mutants. Constant amounts of TWINKLE (200 fmol), POLγA (100 fmol), and the indicated version of POLγB (200 fmol) were added to the mini-circle template (10 fmol). The reaction was allowed to proceed at 37°C for the indicated times. The products were analyzed on a 10% denaturing polyacrylamide gel.
POLγB dsDNA-binding activity is needed for mtDNA replisome function
We next investigated the ability of the different POLγB mutants to support rolling circle DNA replication (Figure 3D). Only wt POLγB and the VV mutant protein could support DNA synthesis on the dsDNA template. The RK, RKK and IF mutants did not affect the ability of POLγA to initiate DNA synthesis from the 3′-hydroxyl terminus, but failed to support elongation through double-stranded regions and only allowed formation of the 110-nt product. The outcome of the experiment was not affected by addition of mtSSB to the reactions (data not shown). We could conclude that both the ability of POLγB to interact with POLγA and the dsDNA-binding activity are essential for mtDNA replisome function.
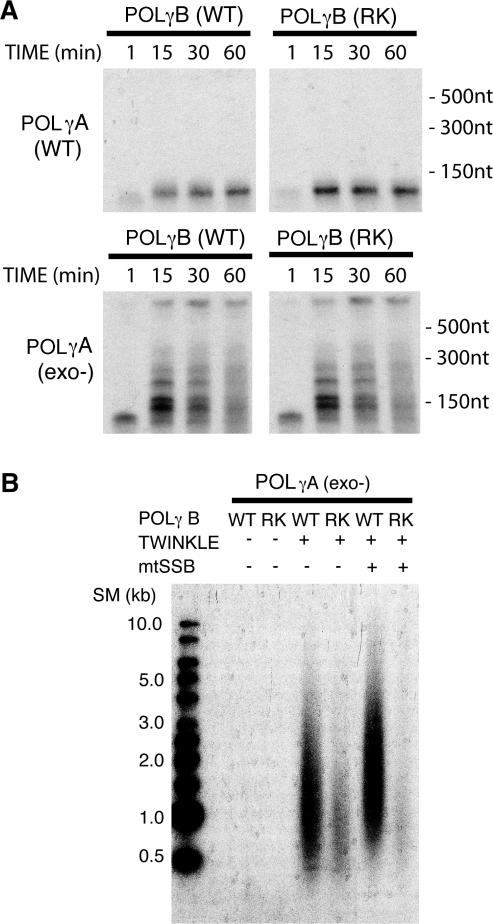
The functional role of the POLγB dsDNA-binding activity remained unclear. One explanation could be that this activity was required to stimulate the catalytic activity of POLγA on a duplex DNA template. Alternatively, the POLγB dsDNA-binding activity may be required for functional interactions with the TWINKLE helicase at the replication fork. To distinguish between these two possibilities, we utilized an exonuclease deficient version of POLγA in which a single amino acid substitution had abolished the 3′ to 5′ exonuclease activity. Similar mutations in other DNA polymerases confer a strand displacement activity that allows the polymerases to use duplex DNA as a template for DNA synthesis even in the absence of a DNA helicase (17,18). Wild type POLγ holoenzyme lacks strand displacement activity, but the impairment of the exonuclease activity allowed the polymerase to use dsDNA as template in the absence of TWINKLE (Figure 4A). Strand displacement DNA synthesis required the presence of POLγB (data not shown). In contrast, the dsDNA-binding activity was not required, since POLγB–RK (Figure 4A) and POLγB–RKK (data not shown) were both able to support DNA synthesis by POLγA-exo− on a duplex DNA template. We could therefore conclude that the POLγB dsDNA binding is not required to stimulate the catalytic activity of POLγA on a duplex DNA template.
Figure 4.
POLγB dsDNA-binding activity is required for TWINKLE stimulation of DNA synthesis. (A) Rolling-circle DNA synthesis without TWINKLE on the duplex DNA mini-circle template. The ability of wild-type and exonuclease deficient POLγA (150 fmol) to use this template for DNA synthesis were analyzed in the presence of either wild-type or RK mutant POLγB (450 fmol). The experiment was performed as described in materials and methods. Samples were taken at the times indicated and analyzed on a 10% denaturing polyacrylamide gel. (B) Constant amounts of TWINKLE (100 fmol), exonuclease-deficient POLγA (75 fmol), mtSSB (5 pmol), wild-type POLγB (150 fmol) and RK-mutant POLγB (150 fmol) were added as indicated. The proteins were incubated with the mini-circle template (10 fmol) at 37°C for 60 min as described in materials and methods. The replication products were analyzed on a 0.7% denaturing agarose gel.
POLγB dsDNA-binding activity is required for TWINKLE stimulation of DNA synthesis
We next monitored the ability of TWINKLE to stimulate the exonuclease deficient POLγ holoenzyme (Figure 4B). TWINKLE could stimulate DNA synthesis by the exo− POLγ holoenzyme and this stimulatory effect required the presence of POLγB. Notably, TWINKLE-dependent stimulation was impaired in the presence of either the POLγB–RK (Figure 4B) or POLγB–RKK mutant (data not shown). In the presence of these dsDNA-binding mutants, the amount of DNA synthesized was lower and the average length of the ssDNA molecules synthesized was shorter than with wt POLγB. Addition of mtSSB made the difference between wt and mutant POLγB even more striking (Figure 4B). We conclude that the dsDNA-binding activity of POLγB is required for TWINKLE-dependent stimulation of the POLγ holoenzyme.
DISCUSSION
The mtDNA replication machinery is related to the machinery found in bacteriophage T7. The TWINKLE helicase displays high primary sequence similarity to the gene 4 helicase-primase. Furthermore the T7 DNA polymerase and POLγA are both classified as family A DNA polymerases (5,9). Studies of bacteriophage DNA replication may therefore provide essential insights into the mechanisms of mammalian mitochondrial DNA replication. The replisome of bacteriophage T7 consists of four proteins: the gene 2.5 ssDNA binding protein, the gene 4 helicase-primase, the T7 DNA polymerase and the processivity factor thioredoxin. Similar to the situation in the mitochondrial replisome, the T7 DNA polymerase/thioredoxin complex can catalyze processive DNA synthesis on ssDNA templates, but it cannot use duplex DNA as a template in the absence of the gene 4 helicase-primase (19,20). The T7 replication machinery can perform coordinated leading and lagging DNA synthesis. The gene 4 helicase-primase plays an essential role for the coordinated synthesis, since it provides binding sites for the two T7 DNA polymerases present at the fork, one on the leading strand and one on the lagging strand. A deletion of the 17 C-terminal residues of gene 4 helicase-primase results in an inability to physically interact with T7 DNA polymerase. This mutation does not affect DNA-dependent nucleotide hydrolysis, helicase and primase activities, but impairs the coupling of polymerase activity to the helicase activity. The C-terminally truncated helicase-primase is at least 10-fold less effective in stimulating DNA synthesis than the wild-type gene 4 protein (21,22).
The mitochondrial minimal replisome can perform leading strand DNA synthesis in vitro on a mini-circle template, but no lagging DNA synthesis is observed. Compared to the related T7 replisome, the mitochondrial replisome must have lost some key features, which are absolutely needed for coordinated leading and lagging strand DNA synthesis. In agreement with this notion, there is no primase activity present in the mitochondrial replisome and such an activity would be necessary for lagging strand DNA synthesis to occur (11). TWINKLE lacks an essential zinc-finger motif that is required for the primase activity of the related gene 4 protein (23,24). Furthermore, the physical interactions necessary for coordinated leading and lagging strand DNA synthesis in the phage T7 system are missing in the mitochondrial replisome. Even if we cannot formally exclude the possibility of transient interactions between TWINKLE and the POLγ holoenzyme during ongoing DNA synthesis, we have so far not observed direct protein–protein contacts between these two enzymes using an array of techniques, including surface plasmon resonance, gel filtration, and the yeast 2-hybrid system (data not shown). In spite of the absence of detectable protein–protein interactions with the TWINKLE helicase, the POLγ holoenzyme cannot be replaced by the T4 or T7 DNA polymerase at the reconstituted mtDNA replication fork (11). The absence of detectable interactions may suggest that the POLγ holoenzyme contributes to replisome function with specific molecular functions that are not present in the phage DNA polymerases. The ability of POLγB to bind dsDNA is such a unique feature that is not found in thioredoxin, the accessory subunit of the T7 DNA polymerase.
The accessory POLγB subunit increases both the processivity and polymerization rate of the POLγ holoenzyme on an ssDNA template. As demonstrated elsewhere and here, the dsDNA-binding activity of POLγB is not required for this stimulatory effect (6). A requirement for the dsDNA-binding activity is only observed on a duplex DNA template. The observed requirement is not due to effects on the catalytic activity of the POLγ holoenzyme. To demonstrate this point, we used an exonuclease deficient POLγ holoenzyme that is capable of strand displacement DNA synthesis in the absence of TWINKLE. This mutant requires POLγB for DNA synthesis, but is not affected by mutations that abolish the dsDNA-binding activity. Instead, the absolute requirement of the dsDNA-binding activity is only seen when the POLγ holoenzyme functions together with TWINKLE at the mtDNA replication fork.
On an ssDNA template the DNA synthesis rate of the POLγ holoenzyme is about 360 nt/s, whereas the mitochondrial replisome synthesizes DNA at a rate of about 180 nt/s on a duplex template. The slower rate observed for the replisome, may suggest that the TWINKLE helicase dictates the speed of the mtDNA replication fork and that the trailing POLγ holoenzyme is slowed down by the TWINKLE helicase ahead. A DNA polymerase trailing behind a slower DNA helicase will ensure that the two factors stay together at the replication fork. The POLγB subunit may have two crucial roles in this process. First, as mentioned above there is a delicate balance between the polymerase and exonuclease mode in the POLγ holoenzyme. If the polymerase is tailgating a slow moving DNA helicase, it could be stimulated to enter the exonuclease mode. The POLγB subunit effectively prevents this by inhibiting the POLγA exonuclease activity. Second, the dsDNA-binding capacity of POLγB may be required to ensure that the POLγ holoenzyme stays bound to the template behind the slow-moving TWINKLE helicase. This idea is seemingly in contrast to findings by others and us, showing that the dsDNA-binding activity of POLγB is not required to stabilize interactions with a primed ssDNA template. However, one must remember that these investigations of primed ssDNA template interactions were done in the presence of ddNTP in order to trap the polymerase gamma in a ternary complex. These experiments are based on the knowledge that DNA polymerases form stable complex with a primed DNA template, provided that the primer template is terminated by a ddNTP and that the next dNTP specified by the template is simultaneously present. At the actual replication fork, the polymerase may instead be idling between the polymerase and exonuclease mode behind the slow-moving TWINKLE helicase. Under these conditions, the polymerase may be much more prone to leave the template and dsDNA-binding activity could be of crucial importance. In future work, we will address this and related possibilities to further clarify the molecular function of the dsDNA-binding activity of POLγB.
Acknowledgements
This work was supported by grants to M.F. from the Swedish Research Council, Åke Wiberg foundation, The Swedish Society of Medicine, the Emil and Wera Cornell's foundation and European Commission (fp6 EUMITOCOMBAT). Funding to pay Open Access publication charge was provided by the Swedish Research Council.
References
- 1.Shadel GS, Clayton DA. Mitochondrial DNA maintenance in vertebrates. Annu. Rev. Biochem. 1997;66:409–435. doi: 10.1146/annurev.biochem.66.1.409. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Barcelo JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, Pommier Y. Human mitochondrial topoisomerase I. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10608–10613. doi: 10.1073/pnas.191321998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Farr CL, Kaguni LS. Accessory subunit of mitochondrial DNA polymerase from Drosophila embryos. Cloning, molecular analysis, and association in the native enzyme. J. Biol. Chem. 1997;272:13640–13646. doi: 10.1074/jbc.272.21.13640. [DOI] [PubMed] [Google Scholar]
- 4.Carrodeguas JA, Kobayashi R, Lim SE, Copeland WC, Bogenhagen DF. The accessory subunit of Xenopus laevis mitochondrial DNA polymerase gamma increases processivity of the catalytic subunit of human DNA polymerase gamma and is related to class II aminoacyl-tRNA synthetases. Mol. Cell. Biol. 1999;19:4039–4046. doi: 10.1128/mcb.19.6.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaguni LS. DNA polymerase gamma, the mitochondrial replicase. Annu. Rev. Biochem. 2004;73:293–320. doi: 10.1146/annurev.biochem.72.121801.161455. [DOI] [PubMed] [Google Scholar]
- 6.Carrodeguas JA, Pinz KG, Bogenhagen DF. DNA binding properties of human pol gammaB. J. Biol. Chem. 2002;277:50008–50014. doi: 10.1074/jbc.M207030200. [DOI] [PubMed] [Google Scholar]
- 7.Carrodeguas JA, Theis K, Bogenhagen DF, Kisker C. Crystal structure and deletion analysis show that the accessory subunit of mammalian DNA polymerase gamma, Pol gamma B, functions as a homodimer. Mol. Cell. 2001;7:43–54. doi: 10.1016/s1097-2765(01)00153-8. [DOI] [PubMed] [Google Scholar]
- 8.Fan L, Kim S, Farr CL, Schaefer KT, Randolph KM, Tainer JA, Kaguni LS. A novel processive mechanism for DNA synthesis revealed by structure, modeling and mutagenesis of the accessory subunit of human mitochondrial DNA polymerase. J. Mol. Biol. 2006;358:1229–1243. doi: 10.1016/j.jmb.2006.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, Wanrooij S, Garrido N, Comi G, Morandi L, et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 2001;28:223–231. doi: 10.1038/90058. [DOI] [PubMed] [Google Scholar]
- 10.Korhonen JA, Gaspari M, Falkenberg M. TWINKLE has 5′ ->3′ DNA helicase activity and is specifically stimulated by mitochondrial single-stranded DNA-binding protein. J. Biol. Chem. 2003;278:48627–48632. doi: 10.1074/jbc.M306981200. [DOI] [PubMed] [Google Scholar]
- 11.Korhonen JA, Pham XH, Pellegrini M, Falkenberg M. Reconstitution of a minimal mtDNA replisome in vitro. EMBO J. 2004;23:2423–2429. doi: 10.1038/sj.emboj.7600257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longley MJ, Clark S, Yu Wai Man C, Hudson G, Durham SE, Taylor RW, Nightingale S, Turnbull DM, Copeland WC, Chinnery PF. Mutant POLG2 disrupts DNA polymerase gamma subunits and causes progressive external ophthalmoplegia. Am. J. Hum. Genet. 2006;78:1026–1034. doi: 10.1086/504303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaspari M, Falkenberg M, Larsson NG, Gustafsson CM. The mitochondrial RNA polymerase contributes critically to promoter specificity in mammalian cells. EMBO J. 2004;23:4606–4614. doi: 10.1038/sj.emboj.7600465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falkenberg M, Lehman IR, Elias P. Leading and lagging strand DNA synthesis in vitro by a reconstituted herpes simplex virus type 1 replisome. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3896–3900. doi: 10.1073/pnas.97.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakubovskaya E, Chen Z, Carrodeguas JA, Kisker C, Bogenhagen DF. Functional human mitochondrial DNA polymerase gamma forms a heterotrimer. J. Biol. Chem. 2006;281:374–382. doi: 10.1074/jbc.M509730200. [DOI] [PubMed] [Google Scholar]
- 16.Johnson AA, Johnson KA. Exonuclease proofreading by human mitochondrial DNA polymerase. J. Biol. Chem. 2001;276:38097–38107. doi: 10.1074/jbc.M106046200. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Trego KS, Song L, Parris DS. 3′ to 5′ exonuclease activity of herpes simplex virus type 1 DNA polymerase modulates its strand displacement activity. J. Virol. 2003;77:10147–10153. doi: 10.1128/JVI.77.18.10147-10153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabor S, Richardson CC. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 1987;84:4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber HE, Russel M, Model P, Richardson CC. Interaction of mutant thioredoxins of Escherichia coli with the gene 5 protein of phage T7. The redox capacity of thioredoxin is not required for stimulation of DNA polymerase activity. J. Biol. Chem. 1986;261:15006–15012. [PubMed] [Google Scholar]
- 20.Nakai H, Richardson CC. The effect of the T7 and Escherichia coli DNA-binding proteins at the replication fork of bacteriophage T7. J. Biol. Chem. 1988;263:9831–9839. [PubMed] [Google Scholar]
- 21.Hamdan SM, Marintcheva B, Cook T, Lee SJ, Tabor S, Richardson CC. A unique loop in T7 DNA polymerase mediates the binding of helicase-primase, DNA binding protein, and processivity factor. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5096–5101. doi: 10.1073/pnas.0501637102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notarnicola SM, Mulcahy HL, Lee J, Richardson CC. The acidic carboxyl terminus of the bacteriophage T7 gene 4 helicase/primase interacts with T7 DNA polymerase. J. Biol. Chem. 1997;272:18425–18433. doi: 10.1074/jbc.272.29.18425. [DOI] [PubMed] [Google Scholar]
- 23.Shutt TE, Gray MW. Bacteriophage origins of mitochondrial replication and transcription proteins. Trends Genet. 2006;22:90–95. doi: 10.1016/j.tig.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Shutt TE, Gray MW. Twinkle, the mitochondrial replicative DNA helicase, is widespread in the eukaryotic radiation and may also be the mitochondrial DNA primase in most eukaryotes. J. Mol. Evol. 2006;62:588–599. doi: 10.1007/s00239-005-0162-8. [DOI] [PubMed] [Google Scholar]