Abstract
The interferon (IFN)-inducible, double-stranded RNA activated protein kinase (PKR) is a dual-specificity kinase, which has an essential role in the regulation of protein synthesis by phosphorylating the translation eukaryotic initiation factor 2 (eIF2). Here, we show the tyrosine (Tyr) phosphorylation of PKR in response to type I or type II IFNs. We show that PKR physically interacts with either Jak1 or Tyk2 in unstimulated cells and that these interactions are increased in IFN-treated cells. We also show that PKR acts as a substrate of activated Jaks, and is phosphorylated at Tyr 101 and Tyr 293 both in vitro and in vivo. Moreover, we provide strong evidence that both the induction of eIF2α phosphorylation and inhibition of protein synthesis by IFN are impaired in cells lacking Jak1 or Tyk2, which corresponds to a lack of induction of PKR tyrosine phosphorylation. We conclude that PKR tyrosine phosphorylation provides an important link between IFN signalling and translational control through the regulation of eIF2α phosphorylation.
Keywords: interferon, tyrosine phosphorylation, eIF2α kinase, mRNA translation, signal transduction
Introduction
Interferons (IFNs) are a family of related pleiotropic cytokines with potent antiviral, immunomodulatory and antiproliferative activities (O'Shea et al, 2004; Platanias, 2005). Stimulation of type I IFN (IFN-α/β) results in the rapid autophosphorylation and activation of the receptor-associated Janus kinase 1 (Jak1) and Tyrosine kinase 2 (Tyk2), which mediate the tyrosine phosphorylation of signal transducer and activator of transcription 1 (Stat1) and Stat2 (Platanias, 2005). Type II IFN (IFN-γ) transduces its signals through Jak1 and Jak2, which lead to the tyrosine phosphorylation of Signal transducer and activator of transcription 1 (Stat1) (Platanias, 2005). Activated and phosphorylated Stats form heterodimers and/or homodimers, and translocate to the nucleus to mediate the transcriptional induction of genes encoding proteins with antiviral, antiproliferative and/or immunoregulatory functions (Levy & Darnell Jr, 2002). Activation of the Jak–Stat pathway by IFNs is transient and downregulated at various levels, including dissociation of the receptor–ligand complex, inactivation of the positive regulators (Jaks, Stats) by dephosphorylation, inhibition of Stat-dependent transcription by protein inhibitors of activated Stats, regulation of nuclear localization and activation of specific suppressor proteins (SOCS; Kubo et al, 2003; Shuai & Liu, 2003).
Among the several type I IFN-inducible genes, the double-stranded RNA-activated protein kinase (PKR) functions as an important ‘checkpoint' against viral invasion through its ability to block protein synthesis and induce apoptosis (Gale Jr et al, 2000). PKR impairs translation initiation by phosphorylating the α-subunit of the eukaryotic initiation factor 2 (eIF2α) at Ser 51 (Dever, 2002). In addition to translation, PKR has been implicated in signal transduction pathways that lead to gene transcription in response to cytokines, growth factors and various forms of stress (Clemens, 2001). Thus, the eIF2α kinase acts as a link between transcriptional and translational pathways that regulate important biological processes such as virus infection, cell proliferation, transformation and apoptosis. Previous data showed the ability of PKR to autophosphorylate at tyrosine residues in bacteria and yeast (Icely et al, 1991; Lu et al, 1999), and phosphorylate an eIF2α mutant with the Ser51-Tyr substitution (Lu et al, 1999). Recently, we showed that PKR is a dual-specificity kinase in human and mouse cells, and that its autophosphorylation on specific tyrosine residues is required for its optimal activation (Su et al, 2006). Here, we extend these findings by identifying a novel mechanism of PKR activation by IFNs. Specifically, we show that both types of IFN induce the tyrosine phosphorylation of PKR, which is mediated by activated Jaks. Furthermore, we show that PKR tyrosine phosphorylation is an important link between the Jak–Stat pathway and the translational machinery in IFN-treated cells.
Results And Discussion
Considering the autophosphorylation of PKR at specific tyrosine residues (Su et al, 2006) and the involvement of the eIF2α kinase in IFN signalling (Stark et al, 1998), we were interested in testing whether tyrosine phosphorylation of PKR is induced by IFNs. As shown in Fig 1A, treatment of the human monocytic U937 cells with IFN-γ resulted in a transient induction of PKR tyrosine phosphorylation (Fig 1A, panel a). In several experiments using human and mouse cell lines, we observed that the amount of tyrosine phosphorylated PKR differed between the cells (see below). We suggest that PKR is subject to dephosphorylation by specific tyrosine phosphatases, which could determine the levels of its tyrosine phosphorylation. Given the functional crosstalk between PKR and the T-cell protein tyrosine phosphatase (TC-PTP) in IFN-treated cells (Wang et al, 2006), we used EFM4 cells lacking TC-PTP and their TC-PTP reconstituted counterparts EFM4-R12 (Ibarra-Sanchez et al, 2001). We observed that a higher amount of PKR was tyrosine phosphorylated in cells lacking TC-PTP than in cells reconstituted with the tyrosine phosphatase in response to IFN-γ (Fig 1B). These data implied that TC-PTP directly mediates the dephosphorylation of PKR in IFN-treated cells. Another possible, but not mutually exclusive, interpretation might be that TC-PTP inactivates a kinase that is responsible for the tyrosine phosphorylation of PKR in IFN-treated cells. Interestingly, previous data have indicated a negative role of TC-PTP in Jak1 activation in response to IFNs (Simoncic et al, 2002), thus raising the possibility that PKR might be regulated by Jak1.
Figure 1.
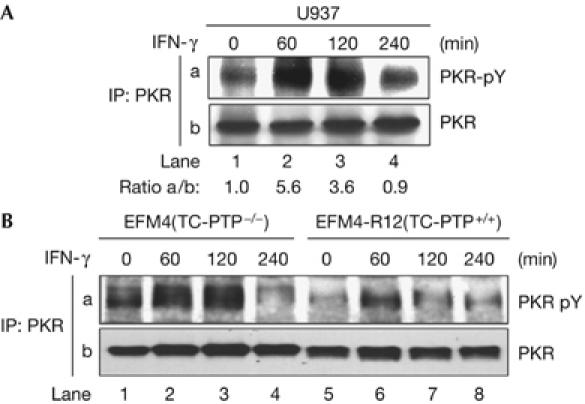
Induction of PKR tyrosine phosphorylation by interferons. The indicated cells were treated with IFN-γ for the indicated time periods. (A,B) Protein extracts were subjected to PKR immunoprecipitation (IP) followed by immunoblotting with the phosphotyrosine 4G10 monoclonal antibody (panel a). The total PKR protein levels are shown in panel b. The ratio of phosphorylated to total PKR for each lane is indicated. IFN, interferon; PKR, dsRNA-dependent protein kinase; TC-PTP, T-cell protein tyrosine phosphatase.
Jak kinases are upstream tyrosine kinases in the IFN signal cascade; therefore, we investigated whether PKR tyrosine phosphorylation is mediated by Jaks. We first tested for a possible substrate–kinase interaction between PKR and Jaks. Given the high protein sequence homology between Jaks (Yamaoka et al, 2004), we used Tyk2 as a Jak representative. We used recombinant glutathione-S-transferase (GST)-PKR (Wong et al, 2001) to pull down Tyk2 proteins with the vesicular stomatitis virus-G (VSV-G) epitope tag in the carboxyl terminus (Gauzzi et al, 1997; Li et al, 1999). We found that full-length (FL) PKR interacted with Tyk2 (supplementary Fig 1A online, lane 6) and that this interaction was primarily mediated by the C terminus of PKR (aa 263–551; lane 5), and to a lesser extent by the amino terminus of PKR (aa 1–262; lane 4). We then used various N-terminal truncated mutants of VSV-G-Tyk2 and GST-fusion proteins of the N terminus or the C terminus of PKR to map the interaction (supplementary Fig 1B online). We observed that the Janus homology (JH) 6–7 domains of Tyk2, both of which comprise the Band-4.1, ezrin, radixin, moesin (FERM) homology domain of the kinase (Yamaoka et al, 2004), bind to the N terminus of PKR (panel a). Further mapping of the interaction showed that the JH6 domain of Tyk2 is involved in the interaction with the N terminus of PKR (supplementary Fig 1C online, panel a, lane 6). Conversely, the JH5 of Tyk2, which lies between the SH2-like and FERM domains of the kinase (Yamaoka et al, 2004), is required for binding to the C terminus of PKR (supplementary Fig 1B online, panel b). The FERM domain of Jaks mediates its interactions with transmembrane proteins such as cytokine receptors and positively regulates their catalytic activity (Yamaoka et al, 2004). Thus, the FERM domain might recruit PKR to the receptor where Jak phosphorylation occurs, and this might account for the PKR tyrosine phosphorylation in response to IFNs.
We then tested the association of endogenous PKR and Jaks. We observed that PKR is coimmunoprecipitated with Jak1 from U937 (Fig 2A) and HT1080 cells (Fig 2B). These results also showed that PKR and Jak1 are held together in a preformed complex in untreated cells (Fig 2A,B, lane 1) and that treatment with IFN enhances the interaction of PKR with activated (tyrosine phosphorylated) Jak1 (Fig 2A, lanes 2–4; Fig 2B, lanes 2 and 3). To confirm this interaction further, we used 2fTGH cells and their isogenic derivative U1A cells lacking Tyk2, or U4A cells lacking Jak1 (McKendry et al, 1991), which are unresponsive to type I IFN or both types of IFN, respectively. We found that PKR was associated with either Jak1 or Tyk2 in a time-dependent manner in IFN-treated 2fTGH cells (Fig 2C,D). The specificity of these interactions was confirmed by using immunoprecipitation with an irrelevant mouse IgG antibody (Fig 2A, lane 5; supplementary Fig 2 online) and by the use of extracts from U4A or U1A cells (Fig 2C, lane 5; Fig 2D, lane 6). Interestingly, the interaction of endogenous PKR and Tyk2 proteins was diminished in cells lacking Jak1 (Fig 2D, lanes 4 and 5). Our data indicated that the association of endogenous PKR and Tyk2 proteins was enhanced in the presence of Jak1. That is, Jak1 might assume a structural role that facilitates PKR binding to Tyk2 in vivo. Alternatively, PKR interaction with Tyk2 might be facilitated by phosphorylation of each protein or both proteins by Jak1.
Figure 2.
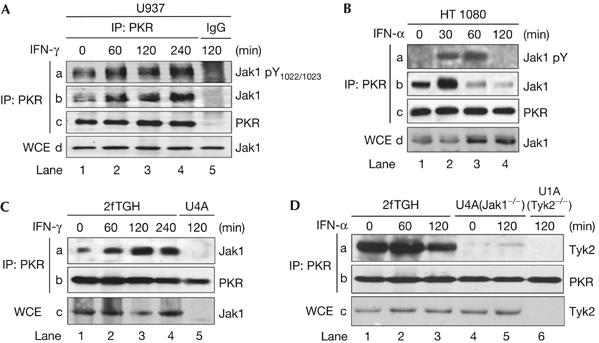
Physical interactions between Jaks and PKR. (A–D) The indicated cells were treated with either IFN-α2b or IFN-γ for the indicated time periods. Protein extracts were subjected to PKR immunoprecipitation (IP) followed by immunoblotting with the indicated antibodies. For the detection of tyrosine-phosphorylated Jak1, the phosphospecific pTyr1022/1023 antibody (A, panel a) or the phosphotyrosine 4G10 antibody (B, panel a) was used. The total levels of Jak1 or Tyk2 were detected by immunoblotting of whole-cell extracts (WCE; A,B, panel d; C,D, panel c). IFN, interferon; PKR, dsRNA-dependent protein kinase.
The essential role of activated Jaks in PKR tyrosine phosphorylation was determined in U1A and U4A cells. In these cells, we detected the tyrosine phosphorylation of PKR in the parental 2fTGH cells (Fig 3A, panel a, lanes 2 and 3) but not in U4A (Jak1−/−) cells (lanes 5 and 6) on treatment with IFN-α. We also observed that a higher amount of eIF2α was bound to PKR in 2fTGH cells than in U4A cells (Fig 3A, panel c, compare lanes 1 and 4) and that this interaction was induced in 2fTGH cells after IFN-α treatment (Fig 3A, panel c, lanes 2 and 3). These data indicated that the presence of Jak1 favours the interaction between PKR and eIF2α and that PKR tyrosine phosphorylation contributes positively to this association. The ability of Jak1 to mediate the tyrosine phosphorylation of PKR was further verified in transient expression of both proteins in Jak1-deficient U4A cells. We used the catalytically inactive Flag-tagged PKRLys296Arg (PKRK296R) together with either wild-type (WT) or the kinase-dead (KD) K896R mutant of Jak1 (Muller et al, 1993; Fig 3B). Immunoblot analysis with an phosphotyrosine antibody showed tyrosine phosphorylation of Flag–PKRK296R by WT Jak1 but not by the KD mutant of Jak1. The fact that recombinant active Jak2 was able to phosphorylate GST-PKRK296R in vitro (Fig 3C, panel a, lane 2) supported the conclusion that PKR is a substrate of Jaks.
Figure 3.
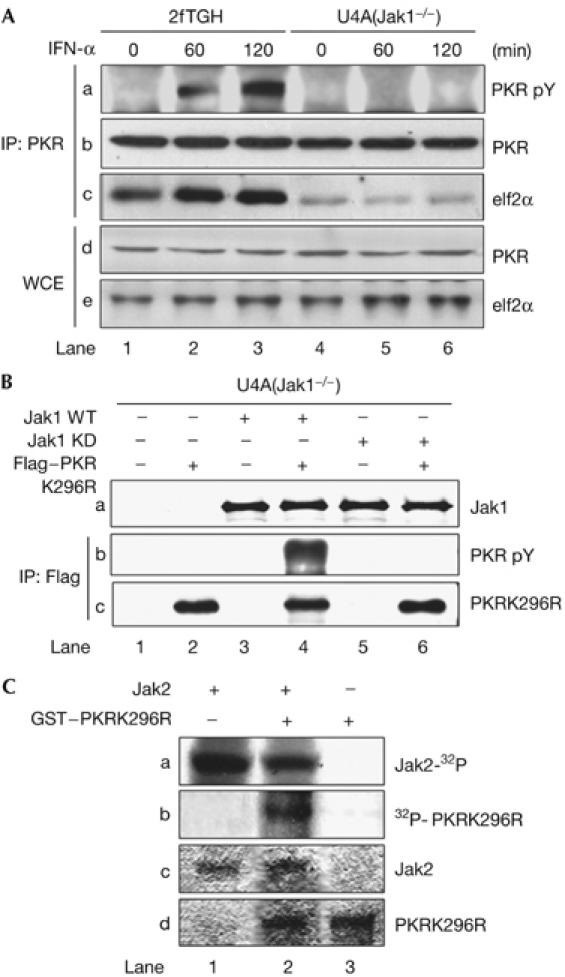
Tyrosine phosphorylation of PKR by Jaks. (A) 2fTGH and U4A cells were stimulated with IFN-α2b for the indicated time periods. Protein extracts were immunoprecipitated (IP) for PKR followed by immunoblotting with phosphotyrosine 4G10 antibody (panel a), PKR monoclonal antibody (panel b) and eIF2α antibody (panel c). The levels of PKR (panel d) and eIF2α (panel e) in WCE were detected by immunoblotting. (B) U4A cells were transfected with the catalytically inactive Flag–PKRK296R complementary DNA in the absence or presence of either wild-type (WT) or a kinase-dead (KD) K296R mutant of Jak1 cDNA. Protein extracts were immunoprecipitated for Flag followed by immunoblotting with phosphotyrosine 4G10 (panel b) or PKR antibody (panel c). Jak1 levels (panel a) were detected by immunoblotting of WCE. (C) Recombinant murine Jak2 and GST–PKRK296R were subjected to in vitro phosphorylation with [γ-32P]ATP and autoradiography (panels a and b). The phosphorylated proteins were detected after an equal time of exposure. Total Jak2 (panel c) and GST–PKRK296R (panel d) levels were detected by Coomassie blue staining. IFN, interferon; PKR, dsRNA-dependent protein kinase; PKRK296R, PKRLys296Arg; WCE, whole-cell extracts.
Considering the capacity of PKR to autophosphorylate at specific tyrosine residues (Su et al, 2006), we examined next whether site-specific tyrosine phosphorylation of PKR is also mediated by Jaks. Immunoblot analyses with phosphospecific antibodies (Su et al, 2006) showed that recombinant Jak2 was able to phosphorylate the catalytically inactive GST-PKRK296R at Tyr 101, which is within the dsRNA-binding motif II of PKR, and Tyr 293, which is within the catalytic subdomain II of the eIF2α kinase (Fig 4A, panels a and b). Furthermore, we observed that phosphorylation of PKR at either Tyr 101 (Fig 4B) or Tyr 293 (Fig 4C) was induced in 2fTGH cells on stimulation with either IFN-α (Fig 4B,C) or IFN-γ (Fig 4D). However, induction of site-specific phosphorylation of PKR was undetected in cells lacking either Tyk2 (Fig 4B,C) or Jak1 (Fig 4D). In fact, basal levels of phosphorylated Tyr 101 and Tyr 293 were undetectable in Tyk2-deficient U1A cells (Fig 4B,C, panel a), indicating that Tyk2 might contribute to the basal tyrosine phosphorylation of PKR in these cells. Contrary to this, phosphorylated Tyr 293 was detected in Jak1-deficient U4A cells; however, the intensity of the signal did not increase after IFN-γ treatment (Fig 4D, panel a). Collectively, these data suggest that IFN treatment induces PKR phosphorylation at Tyr 101 and Tyr 293 through the activation of Jak1 and Tyk2.
Figure 4.
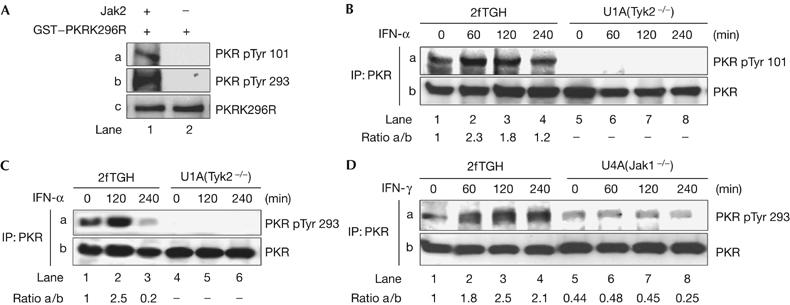
Tyrosine phosphorylation of PKR by interferons. (A) Recombinant Jak2 and GST–PKRK296R were subjected to phosphorylation using non-radioactive ATP. The reactions were then subjected to immunoblotting with the indicated phosphospecific (panels a and b) and pan-specific (panel c) antibodies against PKR. (B–D) 2fTGH, U1A or U4A cells were stimulated with IFN-α2b (B,C) or IFN-γ (D) for the indicated time periods. Protein extracts were immunoprecipitated (IP) for PKR followed by immunoblotting with the indicated PKR phosphospecific antibodies (panel a). Total PKR in the immunoprecipitates was detected by immunoblotting (panel b). The ratio of phosphorylated to total PKR for each lane is indicated. ATP, adenosine triphosphate; GST, glutathione S-transferase; IFN, interferon; PKR, dsRNA-dependent protein kinase; PKRK296R, PKRLys296Arg.
Given that tyrosine autophosphorylation of PKR is required for its correct functioning (Su et al, 2006), we next examined the potential impact of tyrosine phosphorylation of PKR by Jaks in vivo. Autophosphorylation of PKR at threonine (Thr) 446 within its kinase loop is required for its optimal activation and phosphorylation of eIF2α (Romano et al, 1998; Dey et al, 2005). We observed that IFN-α treatment induces Thr 446 phosphorylation of PKR in 2fTGH cells but not in Jak1-deficient U4A cells (Fig 5A, panel a). Furthermore, we observed a correlation between tyrosine (Fig 3A, panel a) and Thr 446 phosphorylation of PKR (Fig 5A), which is consistent with a positive role of both types of phosphorylation in PKR activation. In keeping with the increased interaction between endogenous PKR and eIF2α in response to IFN-α (Fig 3A, panel c), we observed an induction of eIF2α phosphorylation in IFN-treated 2fTGH cells but not in cells deficient in either Jak1 or Tyk2 (Fig 5B,C). This suggested that PKR phosphorylation by Jaks is necessary for eIF2α phosphorylation in IFN-treated cells. To assess the biological relevance of eIF2α phosphorylation, we measured the overall protein synthesis in IFN-α-treated 2fTGH and Tyk2-deficient U1A cells. We observed that treatment with IFN-α for 2 h resulted in an approximate 40% reduction of global protein synthesis in 2fTGH cells but not in U1A cells (Fig 5D); this is consistent with the defect of eIF2α phosphorylation in the Tyk2-deficient cells (Fig 5C). We also observed an approximate 40% inhibition of global protein synthesis in IFN-α-treated 2fTGH cells in the presence of actinomycin D (Fig 5D), which was used to block the transcription of IFN-inducible genes. The role of PKR in IFN-induced inhibition of protein synthesis was further determined in PKR+/+ and PKR−/− mouse embryonic fibroblasts (MEFs; Abraham et al, 1999; Durbin et al, 2002). We observed that IFN-γ stimulation resulted in an approximate 30% decrease of protein synthesis in the IFN-treated PKR+/+ MEFs but not in PKR−/− MEFs (Fig 5E). These data further indicated the inhibitory role of PKR in protein synthesis in IFN-treated cells.
Figure 5.
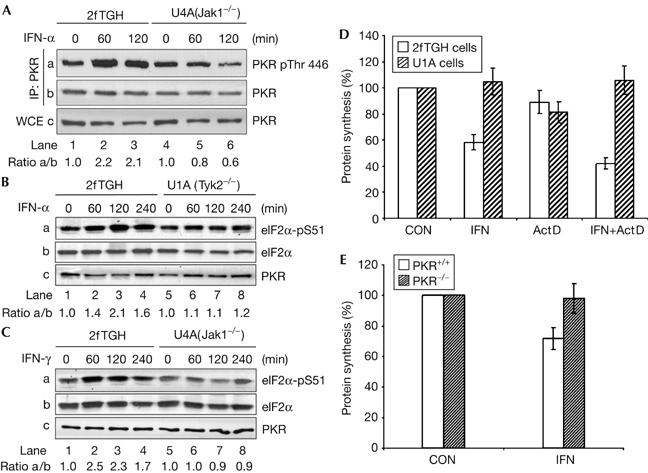
Regulation of eIF2α phosphorylation and protein synthesis by interferons. 2fTGH, U4A (A, C) or U1A (B) cells were stimulated with either IFN-α2b or IFN-γ for the indicated time periods. Total protein extracts were subjected to immunoblotting with the indicated antibodies. (D) 2fTGH and U1A cells were left untreated or treated with IFN-α2b in the absence or presence of 0.5 μg ml−1 actinomycin D (Act D) for 2 h. Cells were subjected to [35S]-methionine labelling for 1 h. (E) PKR+/+ and PKR−/− MEFs were left untreated or treated with IFN-γ for 2 h followed by [35S]-methionine labelling for an additional hour. (D,E) The graphs show the levels of incorporation of radioactivity into protein as percentages of the corresponding control (CON) values. The data are from three independent experiments performed in duplicate. eIF, eukaryotic initiation factor 2; IFN, interferon; MEF, mouse embryonic fibroblasts 2; PKR, dsRNA-dependent protein kinase; WCE, whole-cell extracts.
Despite the significant advances in the Jak–Stat signalling pathway, Stats have remained the only known substrates of activated Jaks (O'Shea et al, 2002). Our work identifies PKR as a novel substrate of Jaks; however, the molecular mechanisms leading to PKR phosphorylation by Jaks in vivo are not fully understood. One possibility is a direct interaction of PKR with Jak1 and/or Tyk2 in the vicinity of the IFN receptor, where the activation of Jaks occurs. Given that both the N- and C-terminal domains of PKR are required for the interaction with Tyk2 in vitro (supplementary Fig 1 online), it is possible that direct binding to Jaks maintains PKR in a conformation that makes the specified tyrosine residues of the eIF2α kinase accessible to phosphorylation. Tyrosine phosphorylation of PKR might also be required to enhance its binding to activated Jaks, given the induction of the interactions between PKR and either Jak1 or Tyk2 in response to IFNs. Alternatively, PKR might be bound to Jaks through an intermediate protein, the function of which as an adaptor protein is also modulated by phosphorylation. We also show that Jak1 is not required for the tyrosine phosphorylation of PKR in response to dsRNA (supplementary Fig 3 online), which is consistent with previous data showing that dsRNA signalling proceeds independently of Jak activation (Bandyopadhyay et al, 1995). The physiological relevance of our findings might be underscored by the distinct biological effects mediated by dsRNA and IFNs during virus infection. That is, at an early phase of infection, PKR activation by dsRNA is linked not only to the inhibition of viral protein synthesis (Samuel, 2001) but also to the induction of IFN-β gene expression through the activation of nuclear factor-κB (Williams, 2001). At a later phase of infection, the produced IFN acts in an autocrine and a paracrine manner (Honda et al, 2006) to activate Jaks and fortify cells through pathways that include the activation of PKR and expression of IFN-inducible genes. In addition to translation (Fig 5), PKR assumes a transcriptional role in IFN-treated cells through the modulation of Stat1 function (Wong et al, 1997, 2001; Wang et al, 2006). Therefore, functional interplay between these transcriptional and translational pathways might be required to control the efficient expression of IFN-inducible genes and also the duration and strength of IFN action. Moreover, regulation of messenger RNA translation by IFNs uses various pathways, such as the induction of P56 protein (Guo et al, 2000; Hui et al, 2003) and phosphorylation of eIF4E-binding protein 4E-BP1 (Platanias, 2005). The coordinated action of these pathways with the eIF2α phosphorylation pathways might also be an important determinant of the biological actions of IFNs.
Methods
Cell culture and treatments. All cells were maintained in DMEM (Invitrogen, Burlington, ON, Canada) with exception of U937 cells, which were cultured in RPMI 1640 (Invitrogen). The media were supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen) and 100 U/ml penicillin/streptomycin. For IFN treatment, cells were incubated with 1,000 IU/ml of human IFN-α2b (Intron A; Schering-Plough, Kenilworth, NJ, USA), 100 IU/ml of human IFN-γ (BD Biosciences, Franklin Lakes, NJ, USA) or 100 IU/ml of mouse IFN-γ (Invitrogen).
In vitro Jak2 kinase assay. Twenty microlitres of agarose-bound purified mouse Jak2 (Millipore, Charlottesville, VA, USA) were washed twice with 1 ml Jak2 kinase buffer consisting of 50 mM NaCl, 10 mM HEPES (pH 7.4), 5 mM MgCl2, 5 mM MnCl2 and 0.1 mM sodium orthovandadate. The agarose-bound Jak2 was resuspended in 15 μl of kinase buffer containing 10 μCi [γ-32P]ATP (ICN Biomedicals Inc., Irvine, CA, USA) and 10 ng of purified GST-PKRK296R, and the kinase reaction was carried out at 30°C for 30 min. Proteins were subjected to SDS–polyacrylamide gel electrophoresis and autoradiography. Some kinase reactions were carried out as above in the presence of cold 1 μM ATP followed by immunoblotting with phosphospecific antibodies.
In vivo [35S]-methionine labelling. [35S]-methionine labelling was carried out as described previously (Kazemi et al, 2004).
Further experimental details are listed in the Supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
Acknowledgments
We thank G. Stark for 2fTGH, U1A and U4A cells, S. Pellegrini for Tyk2 constructs and M.L. Tremblay for EFM4 and EFM4-R12 cells. This work has been supported by a grant from the Cancer Research Society of Canada to A.E.K. D.B. is a research student of the Terry Fox Foundation through an award from the National Cancer Institute of Canada. J.F.R. is the recipient of a Pre-doctoral Traineeship Award from the US Army.
References
- Abraham N et al. (1999) Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem 274: 5953–5962 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay SK, Leonard GT Jr, Bandyopadhyay T, Stark GR, Sen GC (1995) Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J Biol Chem 270: 19624–19629 [DOI] [PubMed] [Google Scholar]
- Clemens MJ (2001) Initiation factor eIF2α phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol 27: 57–89 [DOI] [PubMed] [Google Scholar]
- Dever TE (2002) Gene-specific regulation by general translation factors. Cell 108: 545–556 [DOI] [PubMed] [Google Scholar]
- Dey M, Cao C, Dar AC, Tamura T, Ozato K, Sicheri F, Dever TE (2005) Mechanistic link between PKR dimerization, autophosphorylation and eIF2αa substrate recognition. Cell 122: 901–913 [DOI] [PubMed] [Google Scholar]
- Durbin RK, Mertz SE, Koromilas AE, Durbin JE (2002) PKR protection against intranasal vesicular stomatitis virus infection is mouse strain dependent. Viral Immunol 15: 41–51 [DOI] [PubMed] [Google Scholar]
- Gale M Jr, Tan SL, Katze MG (2000) Translational control of viral gene expression in eukaryotes. Microbiol Mol Biol Rev 64: 239–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauzzi MC, Barbieri G, Richter MF, Uze G, Ling L, Fellous M, Pellegrini S (1997) The amino-terminal region of Tyk2 sustains the level of interferon α receptor 1, a component of the interferon alpha/β receptor. Proc Natl Acad Sci USA 94: 11839–11844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Hui DJ, Merrick WC, Sen GC (2000) A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J 19: 6891–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Takaoka A, Taniguchi T (2006) Type I interferon [correction of inteferon] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25: 349–360 [DOI] [PubMed] [Google Scholar]
- Hui DJ, Bhasker CR, Merrick WC, Sen GC (2003) Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. J Biol Chem 278: 39477–39482 [DOI] [PubMed] [Google Scholar]
- Ibarra-Sanchez MJ, Wagner J, Ong MT, Lampron C, Tremblay ML (2001) Murine embryonic fibroblasts lacking TC-PTP display delayed G1 phase through defective NF-κB activation. Oncogene 20: 4728–4739 [DOI] [PubMed] [Google Scholar]
- Icely PL, Gros P, Bergeron JJ, Devault A, Afar DE, Bell JC (1991) TIK, a novel serine/threonine kinase, is recognized by antibodies directed against phosphotyrosine. J Biol Chem 266: 16073–16077 [PubMed] [Google Scholar]
- Kazemi S, Papadopoulou S, Li S, Su Q, Wang S, Yoshimura A, Matlashewski G, Dever TE, Koromilas AE (2004) Control of α subunit of eukaryotic translation initiation factor 2 (eIF2α) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2α-dependent gene expression and cell death. Mol Cell Biol 24: 3415–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A (2003) Suppressors of cytokine signaling and immunity. Nat Immunol 4: 1169–1176 [DOI] [PubMed] [Google Scholar]
- Levy DE, Darnell JE Jr (2002) Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3: 651–662 [DOI] [PubMed] [Google Scholar]
- Li S, Labrecque S, Gauzzi MC, Cuddihy AR, Wong AH, Pellegrini S, Matlashewski GJ, Koromilas AE (1999) The human papilloma virus (HPV)-18 E6 oncoprotein physically associates with Tyk2 and impairs Jak–STAT activation by interferon-α. Oncogene 18: 5727–5737 [DOI] [PubMed] [Google Scholar]
- Lu J, O'Hara EB, Trieselmann BA, Romano PR, Dever TE (1999) The interferon-induced double-stranded RNA-activated protein kinase PKR will phosphorylate serine, threonine or tyrosine at residue 51 in eukaryotic initiation factor 2α. J Biol Chem 274: 32198–32203 [DOI] [PubMed] [Google Scholar]
- McKendry R, John J, Flavell D, Muller M, Kerr IM, Stark GR (1991) High-frequency mutagenesis of human cells and characterization of a mutant unresponsive to both α and γ interferons. Proc Natl Acad Sci USA 88: 11455–11459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M et al. (1993) The protein tyrosine kinase JAK1 complements defects in interferon-α/β and -γ signal transduction. Nature 366: 129–135 [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Gadina M, Schreiber RD (2002) Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell 109(Suppl): S121–S131 [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Pesu M, Borie DC, Changelian PS (2004) A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov 3: 555–564 [DOI] [PubMed] [Google Scholar]
- Platanias LC (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 5: 375–386 [DOI] [PubMed] [Google Scholar]
- Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, Herring C, Mathews MB, Qin J, Hinnebusch AG (1998) Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2α kinases PKR and GCN2. Mol Cell Biol 18: 2282–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE (2001) Antiviral actions of interferons. Clin Microbiol Rev 14: 778–809, table [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K, Liu B (2003) Regulation of JAK–STAT signalling in the immune system. Nat Rev Immunol 3: 900–911 [DOI] [PubMed] [Google Scholar]
- Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ (2002) The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol 12: 446–453 [DOI] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD (1998) How cells respond to interferons. Annual Review of Biochemistry 67: 227–264 [DOI] [PubMed] [Google Scholar]
- Su Q, Wang S, Baltzis D, Qu LK, Wong AH, Koromilas AE (2006) Tyrosine phosphorylation acts as a molecular switch to full-scale activation of the eIF2α RNA-dependent protein kinase. Proc Natl Acad Sci USA 103: 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Raven JF, Baltzis D, Kazemi S, Brunet DV, Hatzoglou M, Tremblay ML, Koromilas AE (2006) The catalytic activity of the eukaryotic initiation factor-2α kinase PKR is required to negatively regulate Stat1 and Stat3 via activation of the T-cell protein-tyrosine phosphatase. J Biol Chem 281: 9439–9449 [DOI] [PubMed] [Google Scholar]
- Williams BR (2001) Signal integration via PKR. Sci STKE 2001: RE2. [DOI] [PubMed] [Google Scholar]
- Wong AH, Tam NW, Yang YL, Cuddihy AR, Li S, Kirchhoff S, Hauser H, Decker T, Koromilas AE (1997) Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways. EMBO J 16: 1291–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AH, Durbin JE, Li S, Dever TE, Decker T, Koromilas AE (2001) Enhanced antiviral and antiproliferative properties of a STAT1 mutant unable to interact with the protein kinase PKR. J Biol Chem 276: 13727–13737 [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Saharinen P, Pesu M, Holt VE III, Silvennoinen O, O'Shea JJ (2004) The Janus kinases (Jaks). Genome Biol 5: 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information


