Abstract
The ventral spinal cord contains a pool of motor neuron progenitors (pMNs), which sequentially generate motor neurons and oligodendrocytes in the embryo. The mechanisms responsible for the maintenance of pMNs are not clearly understood. We have identified a novel cyclin, cyclin Dx (ccndx), which is specifically expressed in pMNs in Xenopus. Here, we show that inhibition of ccndx causes paralysis in embryos. Furthermore, we show that maintenance of pMNs requires ccndx function. In addition, inhibition of ccndx results in the specific loss of differentiated motor neurons. However, the expression of interneuron or sensory neuron markers is unaffected in these embryos, suggesting that the role of ccndx is specifically to maintain pMNs. Thus, we have identified, for the first time, a tissue-specific cell-cycle regulator that is essential for the maintenance of a pool of neural progenitors in the vertebrate spinal cord.
Keywords: cell cycle, cyclin, Xenopus tropicalis, neurogenesis, motor neuron
Introduction
The G1 phase of the cell cycle is important during neurogenesis and histogenesis; therefore, factors controlling this phase of the cycle have received much attention. Interestingly, the D-type cyclins, which are G1-phase regulators, are expressed in dynamic and tissue-specific patterns during embryonic development, often in mutually exclusive cell types in the CNS (Wianny et al, 1998). Knockout studies of the three murine D-type cyclins have shown that they have distinct roles in regulating the differentiation of specific neuronal cell types. Cyclin D1−/− mice show a striking reduction in the thickness of all retinal layers owing to a decrease in proliferation and specific apoptosis of photoreceptor cells (Sicinski et al, 1995; Ma et al, 1998). The cerebella of cyclin D2−/− mice show a reduction in the number of granule cells and satellite interneurons, but not Golgi and basket interneurons. These neurons might arise from the same progenitors, suggesting that Cyclin D2 is required not only for proliferation but also for correct differentiation of different neuronal cell types in the cerebellum (Huard et al, 1999). Cyclin D3−/− mice are viable, but show lymphoid abnormalities (Sicinska et al, 2003). Mice lacking more than one of the D-type cyclins show phenotypes that represent the sum of the abnormalities observed in mice lacking individual cyclins (Ciemerych et al, 2002). Recent generation and characterization of mice lacking all the three D-cyclins unexpectedly exhibit relatively normal embryonic development until mid-gestation (Kozar et al, 2004). Interestingly, most cell types could proliferate in the absence of the D-type cyclins, with the exception of myocardial cells and haematopoietic stem cells, suggesting that only these cells critically require D-type cyclins for their proliferation (Kozar et al, 2004).
In Xenopus, a number of G1/S-phase cyclins have been identified including two D-type cyclins (Taieb & Jessus, 1996; Taieb et al, 1997), three E-type cyclins (Rempel et al, 1995; Chevalier et al, 1996) and two A-type cyclins (Minshull et al, 1990; Kobayashi et al, 1991). These G1 cyclins also show dynamic and tissue-restricted expression patterns during neurogenesis (Vernon & Philpott, 2003). However, a role for G1-phase cyclins during neurogenesis has not been addressed in Xenopus. We have isolated a novel D-type cyclin, which is specifically located within the ventral hindbrain and the ventral neural tube. This prompted us to investigate whether this novel cyclin, which we named Cyclin Dx (Ccndx), might regulate neurogenesis in the ventral neural tube.
Results and Discussion
Cyclin Dx is a novel D-type cyclin
We recently performed an enhanced large-scale functional screen using a full-length Xenopus tropicalis clone set with the aim of identifying novel genes involved in early embryogenesis (Chen et al, 2005). One of the genes isolated in the screen, TGas111k06, led to a consistent phenotype with regards to apoptosis and showed a highly restricted pattern of expression. Sequence analysis showed that clone TGas111k06 is a novel gene encoding a predicted protein of 290 amino acids with 55% sequence identity to Xenopus Cyclin D1 (Fig 1A). In addition, TGas111k06 contains a retinoblastoma (pRb)-binding domain (Dowdy et al, 1993; Ewen et al, 1993) and a cyclin box domain (Zwicker et al, 1999), which indicate that TGas111k06 might be a new member of D-type cyclins (Fig 1A).
Figure 1.
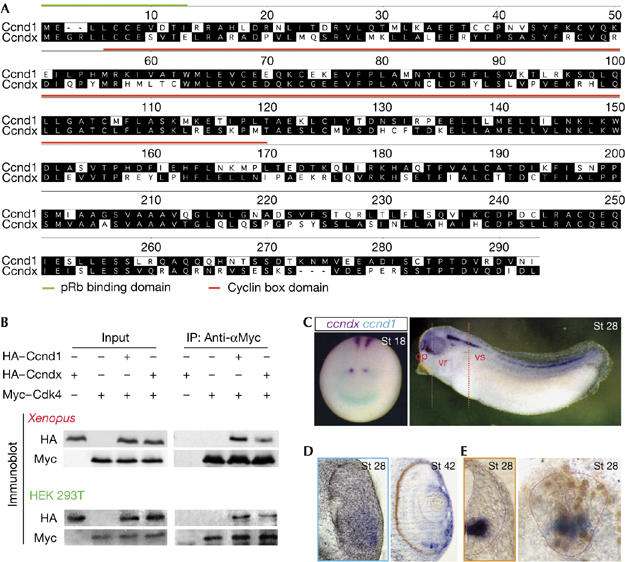
Cyclin Dx is a novel D-type cyclin in Xenopus. (A) Protein sequence alignment of X. tropicalis Ccnd1 and Ccndx. Predicted retinoblastoma (pRb)-binding domain (green line) and conserved cyclin-box domain (red line) are indicated. Residues that match the consensus exactly are shaded black. (B) Ccndx binds to Cdk4. Xenopus embryos were injected with the indicated messenger RNAs and HEK 293T cells were cotransfected with the indicated constructs and lysed for co-immunoprecipitation (IP) using the Myc antibody. The immunoblot was then probed using the antibodies, as indicated. (C–E) Comparison of the expression patterns of ccndx (purple) and ccnd1 (blue) shown by whole-mount double in situ hybridization at the neurula stage (St 18). The expression of ccndx is localized to the ventral spinal cord (vs), ventral retina (vr) and olfactory placode (op) at the tailbud stage (St 28). Transverse section in the eye (blue dash line) and spinal cord (orange dash line) shows that ccndx is localized to the ventral retina (D) and ventral spinal cord in a domain that includes the progenitors of motor neurons incorporating BrdU+ cells (E). The neural tube edge is demarcated with pink.
To confirm whether TGas111k06 encoded a novel D-type cyclin, we investigated whether it could bind to Cyclin-dependent kinase 4 (Cdk4) and phosphorylate pRb in vivo. Indeed, co-immunoprecipitation (co-IP) experiments showed that this novel cyclin protein could bind to Cdk4 in Xenopus embryos and HEK 293T cells (Fig 1B), resulting in hyperphosphorylation of pRb at serine 780, a Cdk4-specific site (Kitagawa et al, 1996; supplementary Fig S1 online). On the basis of these results, as well as the fact that this novel cyclin was more similar to mammalian Cyclin D1 than Cyclin D3, and the apparent lack of a clear cyclin D3 orthologue in the X. tropicalis genome, we have named this novel gene cyclin Dx (ccndx).
To test whether the expression pattern of ccndx was different from ccnd1, we performed whole-mount double in situ hybridization assays with both genes in X. tropicalis embryos (Fig 1C, left panel; supplementary Fig S2 online). At the late neural plate stage (St 18–20), ccndx expression was localized to the hindbrain and the ventral neural tube. By contrast, ccnd1 transcripts were highly enriched in the midbrain–hindbrain boundary (MHB) and anterior neural plate (Fig 1C, left panel). No obvious overlapping domain between ccnd1 and ccndx was shown by these assays, suggesting that each D-type cyclin might have specific functions in regulating local proliferation and/or differentiation, allowing them to function in a highly optimized manner (Ciemerych et al, 2002).
In tailbud-stage embryos (St 28), ccndx expression remained localized to the ventral hindbrain and spinal cord, with further domains apparent in the ventral retina and olfactory region (right panel in Fig 1C and St 28, Fig 1D). Interestingly, ccndx is expressed in the apical ciliary margin zone (CMZ) of ventral retina at the tadpole stage (St 42, Fig 1D). Further investigation of the expression of ccndx in the ventral spinal cord showed that it is highly localized to the domain containing the progenitors of motor neurons (pMNs; Fig 1E, left panel) and in proliferating cells as shown by bromodeoxyuridine (BrdU) incorporation (Fig 1E, right panel). The highly localized expression pattern of ccndx prompted us to examine whether it is crucial for the maintenance of pMNs.
Loss of ccndx function leads to paralysis
We designed morpholino antisense oligonucleotides (MOs) to block either ccndx protein translation or mRNA splicing (Fig 2A). Each MO was designated by its target location: for example, ATG for the initiation codon; e1i1 for the exon 1–intron 1 boundary. To assess whether MOs targeting the splice junctions could interfere with the endogenous ccndx transcripts in vivo, we injected experimental and control MOs into one-cell stage embryos, allowed them to develop until the late neurula stage (St 20), and performed reverse transcription–PCR (RT–PCR) using primers located in exon1 and exon3 of the ccndx gene. As a control, a MO containing five mismatches to the e1i1 sequence control MO (Ct MO) was used. Control MO-injected embryos contained correctly spliced ccndx mRNA, whereas embryos injected with MOs targeting the splice junctions resulted in incorrectly spliced products or complete loss of amplified products (Fig 2B). Ccndx morphants appeared to be morphologically normal at the tailbud stage (St 25), apart from a small delay in development and a slight anterior/posterior truncation (supplementary movie online). This result was consistent with a reduction in body size observed in D-type cyclin-deficient mice (Ciemerych et al, 2002; Kozar et al, 2004). Further inspection showed that the main defect in ccndx morphants was a lack of hatching response or to touch stimulation even after St 35 (early tadpole stage), when primary neurons readily mediate an escape response in Ct MO-injected embryos or uninjected control embryos (see supplementary movie online). At the tadpole stage (St 40), ccndx MO-injected embryos showed a defect in the formation of the ventral retina (Fig 2D), which was consistent with ccndx expression in this region (Fig 1C). All MOs designed to inhibit ccndx function gave rise to the same paralysed phenotype, suggesting that the phenotype was specific (supplementary Fig S3 online). As ccndx is maternally expressed (supplementary Fig S2 online), and we were particularly interested in the function of the zygotic transcripts in the CNS, we performed most of our subsequent knockdown experiments using combined splice MOs (e1i1+e2i2; Dx MO).
Figure 2.
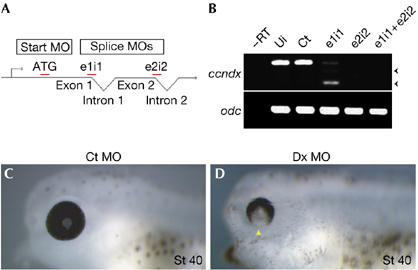
Phenotype of ccndx-deficient embryos. (A) Diagram depicting the target location of the different MOs. (B) Reverse transcription (RT)–PCRs of MO-injected embryos. MOs targeting splice junctions result in the shift of bands (truncated, arrowheads) or absence of transcripts relative to the controls. Ui, uninjected embryos; Ct, 5-mismatch e1i1 control MO. (C,D) Embryos injected with ccndx (Dx) MOs have defects in the formation of the ventral retina (D, arrowhead), relative to control MO-injected embryos (C). Ct MO, 5-mismatched e1i1 control MO; Dx MO, e1i1+e2i2 combined MOs; MO, morpholino antisense oligonucleotides; St 40, stage 40.
ccndx is required to maintain pMNs in the spinal cord
To investigate whether ccndx affects dorso–ventral (D–V) patterning of the neural tube, we cloned the X. tropicalis orthologues of irx3 and nkx6 and used them as markers for the patterning of neuronal progenitors in the spinal cord (Briscoe et al, 2000). Transverse sections of the spinal cord showed that these genes exhibited a similar pattern of expression along the D–V axis in the neural tube in X. tropicalis, as compared with amniotic embryos (see Ct MO in Fig 3B). Compared with Ct MO-injected embryos, neither the distribution of the domain of dorsal neuronal progenitors (irx3; 77%, n=52) nor the domain of ventral neuronal progenitors (nkx6; 79%, n=82) were affected in the ccndx morphants (Fig 3B). However, the expression of olig2, a marker of pMNs (Novitch et al, 2001), was absent in ccndx morphant embryos. These results indicate that the function of ccndx was to maintain pMNs, and not the patterning of neuronal progenitors in the spinal cord (Fig 3C; whole-mount in situs are shown in supplementary Fig S4 online).
Figure 3.
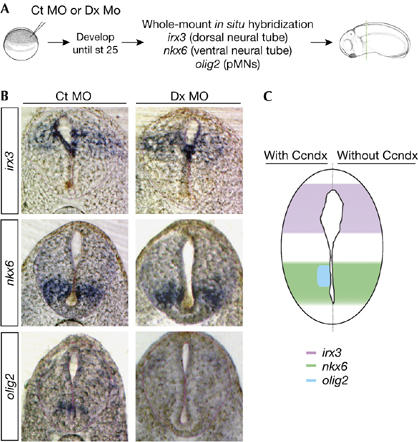
Ccndx function is required to maintain motor neuron progenitors. (A) Experimental design. Embryos were injected at the one-cell stage, fixed at the early tailbud stage and analysed with the markers indicated. (B,C) The maintenance of pMNs is lost when ccndx function is inhibited, as assayed by olig2 expression. However, neither the patterning of dorsal progenitors (irx3) nor ventral progenitors (nkx6) is affected by the loss of ccndx function. Ct MO, 5-mismatched e1i1 control MO; Dx MO, e1i1+e2i2 combined MOs; MO, morpholino antisense oligonucleotides; pMNs, progenitors of motor neurons.
The expression domain of ccndx is broader than pMN marker olig2 (Figs 1C, 3B); therefore, we investigated whether ccndx is required for ventral interneuron differentiation, in addition to motor neuron differentiation. We cloned the differentiated motor neuron marker isl1 (Pfaff et al, 1996) and the interneuron marker evx1 (Moran-Rivard et al, 2001) from X. tropicalis. Isl1 was expressed in the domain of differentiated motor neurons in the ventral neural tube and the domain of the Rohon-Beard (RB) sensory neurons in the dorsal spinal cord, whereas the expression of evx1 was highly restricted in the ventral interneuron domain (see Ct MO in Fig 4B).
Figure 4.
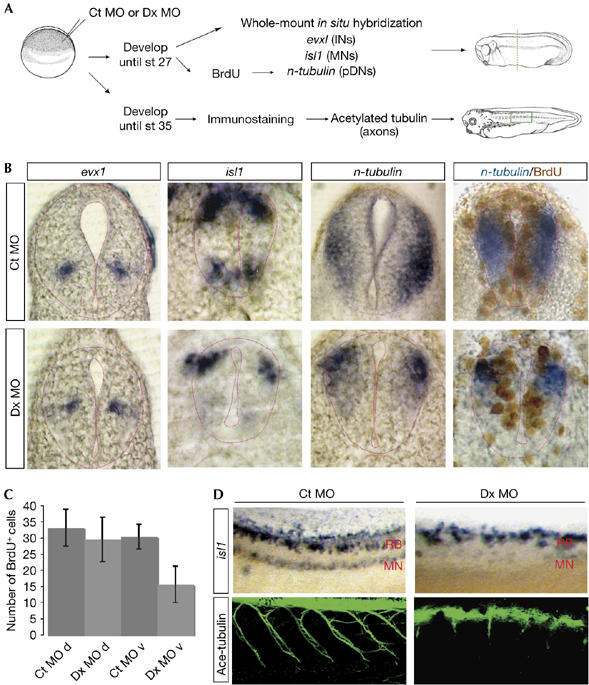
Ccndx function is required for motor neuron differentiation. (A) Experimental design. Embryos were injected at the one-cell stage, fixed at the late tailbud or tadpole stages and analysed with the markers or antibody indicated. INs, interneurons; MNs, motor neurons; pDNs: pan-differentiated neurons. (B) Inhibition of ccndx results in the specific loss of differentiated motor neurons (isl1 in MN domain and n-tubulin at the ventral spinal cord), but interneurons are not affected (evx1). (C) Cell proliferation is reduced in the ventral spinal cord of ccndx morphants, as assayed by BrdU incorporation. d, dorsal spinal cord; v, ventral spinal cord. (D) Inhibition of ccndx causes the loss of differentiated motor neurons, assayed by isl1, and failure of the embryos to extend motor neuron axons, as assayed by immunofluorescence of acetylated tubulin. Ct MO, 5-mismatched e1i1 control MO; Dx MO, e1i1+e2i2 combined MOs; MO, morpholino antisense oligonucleotides; RB, Rohon-Beard sensory neurons.
Embryos lacking ccndx function were analysed for the expression of isl1, evx1 and n-tubulin, a general marker for differentiated neurons (Fig 4A; whole-mount in situ results are shown in the supplementary Fig S4 online). The expression of isl1 was largely reduced in the domain of differentiated motor neurons, whereas isl1 expression in the Rohon-Beard sensory neuron domain was unaffected (64%, n=55; Fig 4B,D). This finding provided a good internal control for the specificity of the effect of knocking down ccndx function in the ventral spinal cord. Furthermore, all MOs targeting ccndx gave rise to this phenotype, confirming the specificity of the result (data not shown). In addition, n-tubulin was specifically eliminated in the ventral, but not the dorsal, neural tube in the ccndx-deficient embryos (65%, n=48; Fig 4B). Finally, the expression of the interneuron marker, evx1, was not affected by inhibiting ccndx (Fig 4B), thus providing strong evidence that the function of ccndx is very specific for motor neuron differentiation in the spinal cord.
To determine whether the loss of pMNs was caused by a failure of these cells to proliferate, we counted BrdU+ nuclei in consecutive sections along the spinal cord, covering a distance of 100 μm (average of five sections per embryo). We found that although the number of BrdU+ nuclei in the dorsal spinal cord was unaffected (Ct: 32±5.5 nuclei, n=5 embryos; Dx: 29±6.6 nuclei, n=9), there was a significant reduction in BrdU+ nuclei in the ventral domain (Fig 4C; Ct: 30±3.8 nuclei, n=5; Dx: 15±5.4 nuclei, n=9; representative sections are shown in Fig 4B). The decrease of about 15 BrdU+ nuclei in 100 μm corresponds to the number of BrdU+ cells that normally express ccndx in the spinal cord over 100 μm (15±5 cells, n=3; representative section shown in Fig 1E). Thus, we conclude that ccndx is required for the continued proliferation of the pMNs in the ventral spinal cord.
To examine whether motor axon formation was affected by inhibiting ccndx, we stained tadpoles with an antibody for acetylated tubulin. In control tadpoles, the tracts of motor neuron axons travelled from the spinal cord down the intermyotomal cleft. By contrast, axon projections were severely disrupted in the ccndx morphant tadpoles (Fig 4D). This result confirmed that the phenotype of paralysis in ccndx morphants was due to the defect in motor neuron formation.
Summary
Here, we describe a novel D-type cyclin, ccndx, which is specifically expressed in pMNs in Xenopus and that is essential for the maintenance of these cells (Fig 5). Maintenance of a pool of pMNs is likely to be essential for the generation of motor neuron subtypes and oligodendrocytes as the tadpole grows. The identification of a pMN-specific cyclin supports a model in which specific cyclins have essential roles in the maintenance of the specific progenitor pools of neural cells in the CNS (Kowalczyk et al, 2004; Berger et al, 2005). It will be interesting to determine whether similar mechanisms are involved in regulating the maintenance of different pools of stem cells and/or progenitor cells in the spinal cord in other vertebrates.
Figure 5.
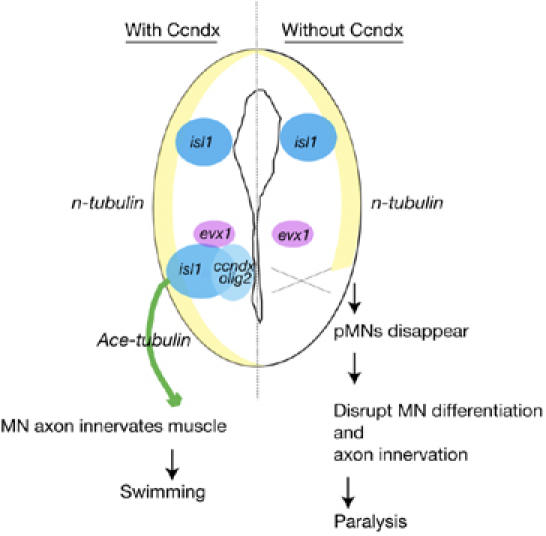
Summary of Cyclin Dx function. In the spinal cord, ccndx is specifically expressed in pMNs and ensures that adequate numbers of differentiated motor neurons are generated, which are needed for the establishment of the constitution of functional motor circuits, and proper swimming and escape responses. In the absence of ccndx, tadpoles are paralysed owing to the loss of pMNs. However, the expression of interneuron (evx1) or sensory neurons (isl1 and the n-tubulin in dorsal sensory neuron domain) is not affected when ccndx is inhibited. Thus, ccndx is a novel localized neuronal regulator that is essential for the maintenance of a pool of neural progenitor cells in the vertebrate spinal cord. MN, motor neurons; pMNs, progenitors of motor neurons.
Methods
Isolation of ccndx and plasmid constructs. Ccndx (TGas111k06 or GenBank accession code DQ508541) was identified in a gain-of-function screen (Chen et al, 2005). Other X. tropicalis clones used here were identified by searching the X. tropicalis Full-Length Project database (Gilchrist et al, 2004). These include ccnd1 (TGas026a07), evx1 (TTbA025j08), irx3 (TNeu131p22), isl1 (TTpA069e10) and nkx6 (TNeu010j02). The olig2 was cloned from embryos by RT–PCR and subcloned into pCR-BluntII-TOPO. Tagged constructs used here include X. laevis Myc-tagged cdk4 in FTX5 (a gift from Angel R. Nebreda; Dinarina et al, 2005), X. tropicalis HA-tagged ccnd1 and ccndx in pcHA (BamHI–XhoI).
Whole-mount in situ hybridization, BrdU detection, immunofluorescence and histology. Whole-mount in situ hybridization and double in situ hybridization of X. tropicalis embryos were performed as described (Chen et al, 2005). BrdU incorporation was then detected by using the substrate diaminobenzidine (Sigma-Aldrich, Dorset, UK) as previously described (Carruthers et al, 2003). Some specimens were sectioned after staining and these were post-fixed overnight in MEMFA (0.1 M MOPS pH 7.5, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde) and then embedded in gelatin/albumin mixture and solidified with glutaraldehyde. Sections (20–30 μm) were cut on a Leica VT1000M vibrotome, mounted in 90% glycerol and photographed with Nomarski optic. Embryos for immmunofluorescence staining were fixed in Dent's solution and incubated with the primary antibody (acetylated tubulin, 1/300; Sigma), followed by the secondary antibody, goat anti-mouse Alexa488 (1/200; Invitrogen, Paisley, UK) staining.
Immunoblotting, co-immunoprecipitations and pRb phosphorylation analysis. Co-IP in Xenopus was performed as described previously (Dinarina et al, 2005). Xenopus embryos were injected at the two-cell stage and were analysed at stage 10. For immunoblotting, Myc–HRP and HA–HRP-conjugated antibodies (1/2000; Roche, Lewes, UK) were used. HEK 293T cells were cultured, co-transfected and lysed as described previously (Voigt & Papalopulu, 2006). Western analysis using phosphoRb S780 (Abcam; 1/1000) and Rb (Sigma; 1/2500) antibodies was performed on H293T-transfected cell lysates.
Morpholino design and reverse transcription–PCR. Splice junctions were identified by aligning complementary DNA sequences with genomic sequences. X. tropicalis embryos were injected with 10–20 ng total MOs before first cleavage. MO sequences are listed as follows. Control MO: 5-mis ccndx e1i1, 5′-ACcTACgTCCAcCATCCAcCAGcTG-3′ (mismatch bases are lowercases), ATG MO: Ccndx ATG, 5′-CACAGCATAACAGCCTTCCCTCCAT-3′, splice MOs (intron sequences are lowercase): e1i1, 5′-acgtacCTCCAGCATCCAGCAGGTG-3′; and e2i2, 5′-ggacacttaccAGCAGTTCTTTGTC-3′.
Total RNA was isolated from MO-injected embryos by using the TRIzol reagent according to the manufacturer's instructions (Invitrogen). cDNA was synthesized using Avian Myeloblastosis Virus (AMV) reverse transcriptase (Roche) and PCR was performed using Taq polymerase (Roche) according to established protocols. Primer sequences are written 5′–3′ as follows: Dx forward, 5′-TGGAGGGAAGGCTGTTATGCTGTG; reverse, 5′-GCCCGCTGCACACTGGAT.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Fig S1
Supplementary Fig S2
Supplementary Fig S3
Supplementary Fig S4
Supplementary Movie
Acknowledgments
We are grateful to N. Papalopulu, J. Pines, S. Ohnuma and A. Roberts for invaluable discussions, and A. Nebrada for providing the Myc-tagged Cdk4 construct. This work was funded by a Wellcome Trust Senior Research Fellowship (E.A.), a National Institutes of Health grant RR13221 (E.A.) and a Taiwanese Government Studentship (90104) (J.A.C.).
References
- Berger C, Pallavi SK, Prasad M, Shashidhara LS, Technau GM (2005) A critical role for cyclin E in cell fate determination in the central nervous system of Drosophila melanogaster. Nat Cell Biol 7: 56–62 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J (2000) A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101: 435–445 [DOI] [PubMed] [Google Scholar]
- Carruthers S, Mason J, Papalopulu N (2003) Depletion of the cell-cycle inhibitor p27(Xic1) impairs neuronal differentiation and increases the number of ElrC(+) progenitor cells in Xenopus tropicalis. Mech Dev 120: 607–616 [DOI] [PubMed] [Google Scholar]
- Chen JA, Voigt J, Gilchrist M, Papalopulu N, Amaya E (2005) Identification of novel genes affecting mesoderm formation and morphogenesis through an enhanced large scale functional screen in Xenopus. Mech Dev 122: 307–331 [DOI] [PubMed] [Google Scholar]
- Chevalier S, Couturier A, Chartrain I, Le Guellec R, Beckhelling C, Le Guellec K, Philippe M, Ford CC (1996) Xenopus cyclin E, a nuclear phosphoprotein, accumulates when oocytes gain the ability to initiate DNA replication. J Cell Sci 109: 1173–1184 [DOI] [PubMed] [Google Scholar]
- Ciemerych MA, Kenney AM, Sicinska E, Kalaszczynska I, Bronson RT, Rowitch DH, Gardner H, Sicinski P (2002) Development of mice expressing a single D-type cyclin. Genes Dev 16: 3277–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarina A, Perez LH, Davila A, Schwab M, Hunt T, Nebreda AR (2005) Characterization of a new family of cyclin-dependent kinase activators. Biochem J 386: 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, Weinberg RA (1993) Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73: 499–511 [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Sherr CJ, Matsushime H, Kato J, Livingston DM (1993) Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73: 487–497 [DOI] [PubMed] [Google Scholar]
- Gilchrist MJ, Zorn AM, Voigt J, Smith JC, Papalopulu N, Amaya E (2004) Defining a large set of full-length clones from a Xenopus tropicalis EST project. Dev Biol 271: 498–516 [DOI] [PubMed] [Google Scholar]
- Huard JM, Forster CC, Carter ML, Sicinski P, Ross ME (1999) Cerebellar histogenesis is disturbed in mice lacking cyclin D2. Development 126: 1927–1935 [DOI] [PubMed] [Google Scholar]
- Kitagawa M et al. (1996) The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J 15: 7060–7069 [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Minshull J, Ford C, Golsteyn R, Poon R, Hunt T (1991) On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol 114: 755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, Ciemerych MA, Sicinski P, Kaczmarek L (2004) The critical role of cyclin D2 in adult neurogenesis. J Cell Biol 167: 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K et al. (2004) Mouse development and cell proliferation in the absence of D-cyclins. Cell 118: 477–491 [DOI] [PubMed] [Google Scholar]
- Ma C, Papermaster D, Cepko CL (1998) A unique pattern of photoreceptor degeneration in cyclin D1 mutant mice. Proc Natl Acad Sci USA 95: 9938–9943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J, Golsteyn R, Hill CS, Hunt T (1990) The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J 9: 2865–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Rivard L, Kagawa T, Saueressig H, Gross MK, Burrill J, Goulding M (2001) Evx1 is a postmitotic determinant of v0 interneuron identity in the spinal cord. Neuron 29: 385–399 [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM (2001) Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 31: 773–789 [DOI] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM (1996) Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell 84: 309–320 [DOI] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL (1995) Maternal Xenopus Cdk2–cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem 270: 6843–6855 [DOI] [PubMed] [Google Scholar]
- Sicinska E et al. (2003) Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 4: 451–461 [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazeli A, Gardner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA (1995) Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82: 621–630 [DOI] [PubMed] [Google Scholar]
- Taieb F, Jessus C (1996) Xenopus cyclin D2: cloning and expression in oocytes and during early development. Biol Cell 88: 99–111 [PubMed] [Google Scholar]
- Taieb F, Chartrain I, Chevalier S, Haccard O, Jessus C (1997) Cyclin D2 arrests Xenopus early embryonic cell cycles. Exp Cell Res 237: 338–346 [DOI] [PubMed] [Google Scholar]
- Vernon AE, Philpott A (2003) The developmental expression of cell cycle regulators in Xenopus laevis. Gene Expr Patterns 3: 179–192 [DOI] [PubMed] [Google Scholar]
- Voigt J, Papalopulu N (2006) A dominant-negative form of the E3 ubiquitin ligase Cullin-1 disrupts the correct allocation of cell fate in the neural crest lineage. Development 133: 559–568 [DOI] [PubMed] [Google Scholar]
- Wianny F, Real FX, Mummery CL, Van Rooijen M, Lahti J, Samarut J, Savatier P (1998) G1-phase regulators, cyclin D1, cyclin D2, and cyclin D3: up-regulation at gastrulation and dynamic expression during neurulation. Dev Dyn 212: 49–62 [DOI] [PubMed] [Google Scholar]
- Zwicker J, Brusselbach S, Jooss KU, Sewing A, Behn M, Lucibello FC, Muller R (1999) Functional domains in cyclin D1: pRb-kinase activity is not essential for transformation. Oncogene 18: 19–25 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig S1
Supplementary Fig S2
Supplementary Fig S3
Supplementary Fig S4
Supplementary Movie


