The Fifth International Conference on Na/Ca Exchange took place between 23 and 27 August 2006 in Brussels, Belgium, and was organized by A. Herchuelz. Image taken from the meeting poster showing hypothetical models for the Na/Ca exchanger (left) and the plasma membrane Ca2+-ATPase (right), courtesy of M. Hilge.
Introduction
Cytosolic Ca2+ regulates several cellular processes and its concentration is, in turn, finely regulated by various channels, pumps and exchangers. The Na/Ca exchanger (NCX) and the plasma membrane Ca2+-ATPase (PMCA) pump are two concurrent mechanisms for Ca2+ extrusion from the cell (Carafoli, 1994; Blaustein & Lederer, 1999; Philipson & Nicoll, 2000; Strehler & Treiman, 2004; Prasad et al, 2004). PMCA and NCX were cloned 17 and 19 years ago, respectively; four mammalian isoforms have been identified for the former (PMCA1–4) and three have been identified for the latter (NCX1–3; Carafoli, 1994; Philipson & Nicoll, 2000).
Na/Ca exchange (NCX) is a unique mechanism that allows Ca2+ extrusion from the cell against its gradient without energy consumption. Indeed, it is the entry of Na+ along its electrochemical gradient that provides the energy for Ca2+ extrusion. In addition, because NCX is electrogenic and voltage sensitive, it can reverse during cellular activation and contribute to Ca2+ entry into the cell (Blaustein & Lederer, 1999). Recently, the crucial role of NCX in cellular Ca2+ homeostasis has been definitively shown by the generation of mice with functional destruction of one Ncx gene. Homozygous Ncx1-deficient mice are not viable, and die between embryonic days 9 and 10, owing to the lack of development of the cardiovascular system (Wakimoto et al, 2000); therefore, NCX is essential for life.
PMCA belongs to the P-type family of transport ATPases, which form a phosphorylated intermediate during the reaction cycle. The carboxy (C)-terminal tail of PMCA contains an autoinhibitory domain that binds Ca2+-calmodulin. In the absence of Ca2+-calmodulin, this domain interacts with two regions of the protein located in the first and second intracellular loops; this interaction maintains the pump in an inactive state. Elevation of Ca2+-calmodulin relieves the inhibition and stimulates activity of the pump.
Traditionally, NCX is considered to have a low affinity but a high capacity for Ca2+, whereas PMCA has a high affinity but a low capacity for Ca2+ (Carafoli, 1994). Hence, NCX eliminates significant rises in intracellular Ca2+, whereas PMCA finely regulates the concentration of cytosolic free Ca2+ around its basal value (100 nM; Carafoli, 1994). In the β-cell and the heart, NCX seems to be the predominant mechanism for Ca2+ extrusion, accounting for approximately 70% and 90% of Ca2+ extrusion, respectively. In the heart, NCX can restore and control the level of basal Ca2+ on a beat-to-beat basis (Bers et al, 1996; Van Eylen et al, 1998).
Cloning the genes encoding NCX and PMCA has led to rapid advances in our knowledge of the role of these Ca2+-extrusion mechanisms in physiology and pathophysiology. This was clearly evident during the conference. Although the meeting was the fifth in a series, it was the first to bring together experts in both the NCX and the PMCA fields. It also celebrated the fortieth anniversary of the discovery of the two Ca2+-extrusion mechanisms. The speakers covered research on all aspects of these two proteins, ranging from molecular traits to new therapeutic opportunities.
Knockout models
As mentioned above, NCX is responsible for 90% of Ca2+ extrusion from the heart. So, if the gene encoding the exchanger were to be knocked out in the mouse, what would be the effect? Surprisingly, the answer is ‘almost nothing'. K. Philipson (Los Angeles, CA, USA) and his group once again took the conference by storm with a superb technical achievement using targeted knockout mice. Philipson showed that knockout cardiomyocytes adapt to a stimulus by decreasing Ca2+ influx into the cell rather than upregulating other Ca2+-efflux mechanisms (Fig 1). The adaptation might comprise important feedback mechanisms by which cardiomyocytes can limit Ca2+ influx in situations of compromised Ca2+-extrusion capacity (Henderson et al, 2004; Pott et al, 2005).
Figure 1.
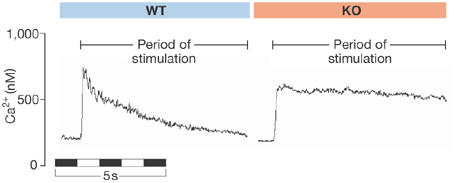
Ca2+ response of isolated myocytes to the application of caffeine. Caffeine was added just before the rapid rises in Ca2+. Caffeine induces the release of Ca2+ stores from the sarcoplasmic reticulum. In the myocyte from a wild-type (WT) mouse, Ca2+ rises and then declines as it is removed from the cell by the Na/Ca exchanger (NCX). In a myocyte in which the NCX has been knocked out (KO) , Ca2+ rises and remains elevated.
In addition to autoregulatory mechanisms, the myocardium might undergo long-term adaptations to the absence of NCX. Through a proteomics analysis, Philipson observed that one protein of unknown function changes its mobility on two-dimensional gels in response to the ablation of NCX. He speculated that this protein has an important role in the regulation of excitation-contraction coupling and could therefore be another example of the robustness of nature.
Studies of isolated bladder smooth muscle by G. Shull (Cincinnati, OH, USA) using mice with null mutations in Pmca1 and Pmca4 indicate that both isoforms affect contractility (Liu et al, 2006). By contrast, studies of cardiac function in Pmca4-null and Pmca1-heterozygous mutant mice reveal no alterations in contractility or relaxation. These observations indicate that PMCAs do not have a crucial role in cardiac muscle contractile performance, but might be involved in signalling functions that regulate other aspects of cardiac biology (see below).
Structure–function relationships of the exchanger
NCX1 is regulated by the binding of intracellular Ca2+ to a site on the large cytoplasmic loop known as the calcium-binding domain 1 (CBD1). It is anticipated that Ca2+ binding to CBD1 results in a conformational change that, in turn, triggers the transformation of NCX1 into an activated state. The crystal (Nicoll et al, 2006) and nuclear-magnetic resonance (NMR; Hilge et al, 2006) structures of CBD1 have recently been solved. CBD1 contains an immunoglobulin-like fold with four Ca2+ ions bound at one end. M. Hilge (Nijmegen, The Netherlands) identified a second binding domain, called CBD2, and found that in the absence of Ca2+ the Ca2+-binding half of CBD1 becomes unstructured, whereas CBD2 maintains its integrity (Hilge et al, 2006). The NMR structures also made it possible for Hilge to construct a model for the entire regulatory exchanger loop, with CBD1 and CBD2 organized in an anti-parallel fashion (Fig 2). D. Nicoll (Los Angeles, CA, USA) introduced the fluorescent amino acid tryptophan at various locations in CBD1 to examine the effects of Ca2+ binding on its fluorescence. Tryptophans introduced into the Ca2+-binding half of CBD1 showed significant Ca2+-induced changes in fluorescence, whereas those introduced into the distal half of CBD1 were unaffected. In addition, Cys 387 is located near the Ca2+-binding region of CBD1, and the sulphydryl modification of this residue is Ca2+-dependent. The chemical data therefore support the NMR observation of unfolding in the Ca2+-binding region of CBD1 when Ca2+ is removed. Whether this unfolding relates to the movement of the exchanger into an inactive state remains to be determined.
Figure 2.
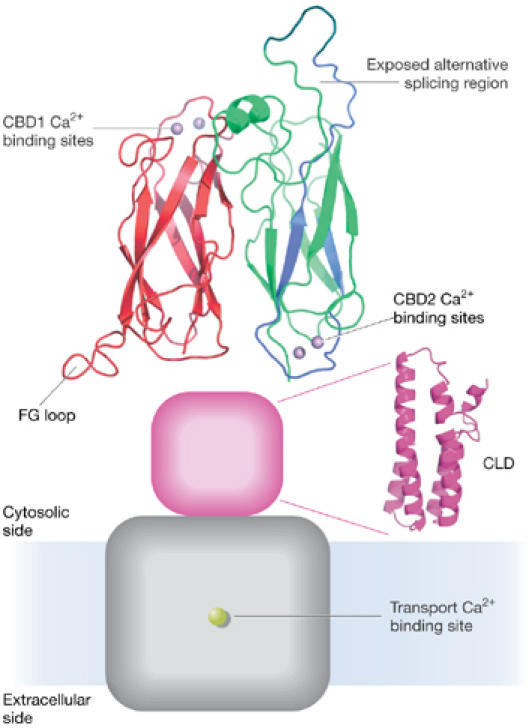
Model of the intact Na/Ca exchanger. The Na/Ca exchanger is composed of two anti-parallel oriented Ca2+-binding domains CBD1 (red) and CBD2 (green), as well as a third cytosolic domain shown in blue and a transmembrane domain shown in grey. CLD, catenin-like domain; FG loop, loop between the F and G β-strands.
Hilge also presented a representative structure for each of the two main splice-variant classes of NCX, which contain the mutually exclusive CBD2-encoding exons A and B. In contrast to CBD2-A, CBD2-B displayed unstructured Ca2+-binding sites under physiological conditions. This lack of ability of exon B-containing isoforms could lead to reduced Ca2+ fluxes in non-excitable cells that contain exon B compared with excitable cells that contain exon A.
Regulation of the exchanger
J. Reeves (Newark, NY, USA) questioned the physiological relevance of current concepts of NCX regulation by cytosolic Ca2+ and Na+. He pointed out that cytosolic Ca2+ concentrations during normal cardiac function exceed the apparent dissociation constant for allosteric Ca2+ activation (0.3 μM). Reeves provided a possible solution to this dilemma by showing that interactions between NCX and F-actin interfere with allosteric Ca2+ activation in transfected cells (Condrescu & Reeves, 2006). Reeves also found that elevated concentrations of cytosolic Na+ induce a mode of activity that no longer requires allosteric Ca2+ activation (Urbanczyk et al, 2006). This mode of activity seems to be dependent on phosphatidyl-4,5-bisphosphate (PIP2), indicating that PIP2 levels might determine whether elevated Na+ produces inactivation or constitutive behaviour. The effects of PIP2 were explored further in Chinese hamster ovary (CHO) cells expressing the metabotropic (M1) acetylcholine receptor, in which carbachol reduces PIP2 levels by 90%. The results showed that carbachol addition did not inhibit NCX activity as expected, suggesting that the role of PIP2 in regulating NCX activity is more complex than previously thought.
M. Ottolia (Los Angeles, CA, USA) used fluorescence resonance-energy transfer (FRET) to measure conformational changes that occur in the exchanger. She engineered the CBD1 of the exchanger with the cyan (CFP) and yellow (YFP) variants of the green fluorescent protein fused to its amino (N) terminus and C terminus, respectively (YFP-CBD-CFP). Her data indicate that the Ca2+-binding domain of NCX detects fast changes in Ca2+ that occur during excitation-contraction coupling, suggesting that NCX is regulated by Ca2+ on a beat-to-beat basis (Ottolia et al, 2004). As YFP-CBD-CFP exists as a soluble protein, it might be exposed to a Ca2+ environment that is different from that at the sarcolemma. To overcome this problem, she engineered a full-length exchanger with YFP and CFP inserted at positions 371 and 508. Finally, Ottolia created exchangers with YFP, or CFP, inserted at different locations within the large intracellular loop. She obtained data indicating that the distance between the large intracellular loops of two or more adjacent exchangers decreases in the presence of Ca2+, which suggests NCX oligomerization.
Interaction with other proteins
PMCAs are essential for the long-term maintenance of intracellular Ca2+ homeostasis; however, they are also intimately involved in the spatial and temporal control of Ca2+ signalling. E. Strehler (Rochester, MN, USA) highlighted several of the recently discovered protein partners that can interact with PMCA isoforms in regulatory and signalling cross-talk. In fact, the role of most of the new PMCA-interacting proteins is now beginning to be unravelled. Work by several groups has shown that PDZ proteins bind to the C-terminal tail of specific PMCA isoforms, and recruit them to macromolecular complexes involved in nitric oxide signalling, pre-synaptic and post-synaptic Ca2+ signalling, and actin-cytoskeleton remodelling.
E. Cartwright and L. Neyses (Manchester, UK) have shown that PMCA interacts with several proteins, including Ras-associated factor 1, calcineurin and syntrophin. This led them to propose that PMCA and its interacting proteins have an important and previously unknown function in cardiac signalling, with physiological as well as pathological relevance (Fig 3; Cartwright et al, 2005; Oceandy et al, 2006). To determine the functional role of the ubiquitous isoform PMCA4, and the effect of its deletion on the mouse, they used a systems biology approach. By analysing more than 100 parameters, they carried out the most complete phenotyping of any calcium-transporter gene knockout mouse. Their analysis showed that although PMCA4 is ubiquitously expressed, its function is highly tissue-specific, with physiological/pathophysiological relevance in sperm and the cardiovascular system.
Figure 3.
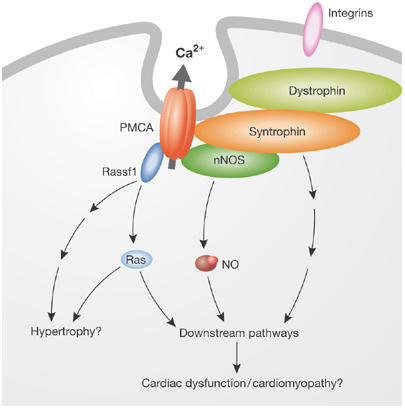
Representation of the plasma membrane Ca2+-ATPase signalling complex in the heart. Plasma membrane Ca2+-ATPase (PMCA) is located in invaginations of the plasma membrane (caveolae) and is tethered to the dystrophin complex through syntrophin; downstream effectors are regulated by the pump, notably neuronal nitric oxide synthase (nNOS), nitric oxide (NO) and the Ras pathway. Rassf1, Ras-associated factor 1.
Na/Ca exchange and cell death
Recently, D. Bano (Leicester, UK) made the interesting observation that NCX3 is cleaved by the Ca2+-dependent proteases calpains during brain ischaemia and in neurons undergoing excitotoxity, with resulting Ca2+ deregulation and neuronal death (Bano et al, 2005). At the meeting, he reported on a role for calpains in the regulation of nucleo-cytoplasmic transport, resulting in chromatin condensation and nuclear disassembly. He is currently investigating how Ca2+ and calpains regulate various distinct cellular pathways, and whether spatial and/or temporal constraints are in place.
L. Annunziato (Naples, Italy) previously showed that several strategies for inhibiting NCX caused a significant worsening of the brain damage induced by the occlusion of the middle cerebral artery (Annunziato et al, 2004). He also observed a selective upregulation of NCX1 and NCX3 messenger RNA levels in regions of the brain that survive an ischaemic insult (Pignataro et al, 2004; Boscia et al, 2006). He therefore directed his attention to understanding the transductional and transcriptional pathways responsible for NCX upregulation. Annunziato showed that the brain isoform of Akt, which is a downstream effector of neurotrophic factors such as nerve growth factor, can exert a neuroprotective effect by modulating the expression and activity of NCX1-3. These observations might contribute to the development of targeted compounds that could reduce brain damage induced by ischaemia.
Na/Ca exchange and skin colour
In 2005, an important genetic component of human skin colour was identified. The allelic frequency of a single nucleotide polymorphism (SNP) in the coding region of the putative NCX solute carrier 24 member 5 (SLC24A5) was found to vary notably between Caucasian populations and those of African ancestry (The International HapMap Consortium, 2005). Concurrently, a group of zebrafish researchers discovered that another mutation in the zebrafish orthologue of SLC24A5 was responsible for the hypo-pigmented phenotype of the golden mutant (Lamason et al, 2005), and showed that the human SNP was associated with skin colour variation in admixed groups of African-Americans and African-Caribbeans.
R. Ginger (Sharnbrook, UK) described the results of another independent study conducted in a population of South Asian ancestry. By using a whole-genome scanning approach she found that the non-synonymous SNP in SLC24A5 is strongly associated with natural variation of skin colour in this population, with an allelic frequency difference between the lightest and darkest subjects of 39%. Amino-acid sequence alignments revealed that SLC24A5 encodes a protein similar to those members of the NCX family that are potassium-dependent.
NCX inhibitors and new therapeutic opportunities
NCX inhibitors have been proposed to be cardioprotective. However, their protective effects depend on whether predominant Ca2+ entry during ischaemia/reperfusion is mediated through reverse-mode NCX or reduced Ca2+ extrusion through forward-mode NCX. To address whether inhibition of NCX is beneficial or detrimental, E. Murphy (Research Triangle Park, NC, USA) used a model developed by Philipson, involving cardiac-specific ablation of Ncx. Ncx-knockout hearts exhibited delayed ischaemic contracture, reduced maximum contracture and improved post-ischaemic function. They also had smaller infarcts following ischaemia and reperfusion, and a lower rate of ATP reduction with better preservation of Na+ homeostasis. The smaller increase in Na+ resulted in less reverse-mode Ncx and therefore a smaller increase in Ca2+ during ischaemia. The rise in Ca2+ levels during ischaemia and early reperfusion seems to result in cell death largely owing to activation of the mitochondrial permeability transition pore.
T. Iwamoto (Fukuoka, Japan) reported that the specific NCX inhibitor SEA0400 lowers arterial blood pressure in salt-dependent or ouabain-dependent hypertensive rat models, but not in normotensive rats or other types of hypertensive rat. In addition, heterozygous Ncx1-knockout mice are resistant to developing salt-dependent hypertension (Iwamoto et al, 2004). He also reported that adrenocorticotropic hormone (ACTH)-induced hypertension, which is related to Cushing's syndrome, was attenuated in a heterozygous Ncx1-knockout compared with wild-type mice. These findings suggest that salt-dependent or ACTH-dependent hypertension is triggered by Ca2+ entry through NCX1 in arterial smooth muscle. Endogenous cardiac glycosides, which might contribute to salt-dependent or ACTH-dependent hypertension, seem to be necessary for NCX1-mediated hypertension. Therefore, vascular NCX1 seems to be a new therapeutic or diagnostic target for salt-dependent hypertension.
Conclusions
By the end of the meeting, the general feeling was that considerable progress had been made since the last conference on NCX held in Banff, Canada, five years ago. In particular, progress has been made in understanding the structure, regulation and functioning of NCX, and its role in cell function, dysfunction, death and skin pigmentation. In addition to a fundamental understanding of the genetics and molecular processes, we have promising leads for new therapeutic approaches. The meeting was the first to bring together experts in both the NCX and the PMCA fields to broaden the perspective and expand the discussions on, for example, the respective role of the two carriers in intracellular Ca2+ homeostasis. The experience was rewarding for researchers from both fields, who suggested that this format should be repeated in future meetings.

Denis Noble

Andre Herchuelz
Acknowledgments
We thank all participants of the meeting for their contributions to this report. Space limitations prevented the citation of many worthy contributions.
References
- Annunziato L, Pignataro G, Di Renzo G (2004) Pharmacology of brain Na/Ca exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev 56: 633–654 [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P (2005) Cleavage of the plasma membrane Na/Ca exchanger in excitotoxicity. Cell 120: 275–285 [DOI] [PubMed] [Google Scholar]
- Bers DM, Bassani JW, Bassani RA (1996) Na-Ca exchange and Ca fluxes during contraction and relaxation in mammalian ventricular muscle. Ann NY Acad Sci 779: 430–442 [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79: 763–864 [DOI] [PubMed] [Google Scholar]
- Boscia F, Gala R, Pignataro G, de Bartolomeis A, Cicale M, Ambesi-Impiombato A, Di Renzo G, Annunziato L (2006) Permanent focal brain ischemia induces isoform-dependent changes in the pattern of Na+/Ca2+ exchanger gene expression in the ischemic core, periinfarct area, and intact brain regions. J Cereb Blood Flow Metab 26: 502–517 [DOI] [PubMed] [Google Scholar]
- Carafoli E (1994) Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J 8: 993–1002 [PubMed] [Google Scholar]
- Cartwright EJ, Schuh K, Neyses L (2005) Calcium transport in cardiovascular health and disease: the sarcolemmal calcium pump enters the stage. J Mol Cell Cardiol 39: 403–406 [DOI] [PubMed] [Google Scholar]
- Condrescu M, Reeves J (2006) Actin-dependent regulation of the cardiac Na/Ca exchanger. Am J Physiol 290: 691–701 [DOI] [PubMed] [Google Scholar]
- Henderson SA et al. (2004) Functional adult myocardium in the absence of Na/Ca exchange: cardiac specific knockout of NCX1. Cir Res 95: 604–611 [DOI] [PubMed] [Google Scholar]
- Hilge M, Aelen J, Vuister GW (2006) Ca2+ regulation in the Na/Ca exchanger involves two markedly different Ca2+ sensors. Mol Cell 22: 15–25 [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T (2004) Salt-sensitive hypertension is triggered by Ca2+ entry via Na/Ca exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199 [DOI] [PubMed] [Google Scholar]
- Lamason RL et al. (2005) SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310: 1782–1786 [DOI] [PubMed] [Google Scholar]
- Liu L, Ishida Y, Okunade G, Shull GE, Paul RJ (2006) Role of plasma membrane Ca2+-ATPase in contraction-relaxation processes of the bladder: evidence from PMCA gene ablated mice. Am J Physiol Cell Physiol 290: 1239–1247 [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Sawaya MR, Kwon S, Cascio D, Philipson KD, Abramson J (2006) The crystal structure of the primary Ca2+ sensor of the Na+/Ca2+ exchanger reveals a novel Ca2+ binding motif. J Biol Chem 281: 21577–21581 [DOI] [PubMed] [Google Scholar]
- Oceandy D, Buch MH, Cartwright EJ, Neyses L (2006) The emergence of plasma membrane calcium pump as a novel therapeutic target for heart disease. Mini Rev Med Chem 6: 583–588 [DOI] [PubMed] [Google Scholar]
- Ottolia M, Philipson KD, John S (2004) Conformational changes of the Ca2+ regulatory site of the Na/Ca exchanger detected by FRET. Biophys J 87: 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson KD, Nicoll D (2000) Sodium-calcium exchange: a molecular perspective. Ann Rev Physiol 62: 111–133 [DOI] [PubMed] [Google Scholar]
- Pignataro G et al. (2004) Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke 35: 2566–2570 [DOI] [PubMed] [Google Scholar]
- Pott CH, Philipson KD, Goldhaber JI (2005) Excitation-contraction coupling in Na/Ca exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ Res 97: 1288–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad V, Okunade GW, Miller ML, Shull GE (2004) Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun 322: 1192–1203 [DOI] [PubMed] [Google Scholar]
- Strehler EE, Treiman M (2004) Calcium pumps of plasma membrane and cell interior. Curr Mol Med 4: 323–335 [DOI] [PubMed] [Google Scholar]
- The International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437: 1299–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanczyk J, Chernysh O, Condrescu M, Reeves JP (2006) Sodium-calcium exchange does not require allosteric Ca activation at high cytosolic sodium concentrations. J Physiol 575: 693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eylen F, Albuquerque J, Herchuelz A (1998) Contribution of Na/Ca exchange to Ca2+ outflow and entry in the rat pancreatic β-cell. Diabetes 47: 1873–1880 [DOI] [PubMed] [Google Scholar]
- Wakimoto K et al. (2000) Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem 275: 36991–36998 [DOI] [PubMed] [Google Scholar]


