The Fifth International Copper Meeting on Copper and Related Metals in Biology took place between 14 and 18 October 2006 in Alghero, Sardinia, and was organized by M. Solioz, D. Thiele and J. Mercer.
Introduction
Copper is an essential cofactor for several enzymes that catalyse many biochemical reactions crucial for normal cell physiology. Its ability to accept and donate single electrons as it changes oxidation state between Cu+ and Cu2+—that is, Cu(I) and Cu(II), respectively—makes copper an ideal biological cofactor; however, this ability also allows it to readily catalyse the formation of reactive oxygen species (ROS) through Fenton chemistry when unbound within the cell. Several specific trafficking pathways have evolved to ensure that most copper ions in the cell are either compartmentalized or protein bound, thereby maximizing the trade-off between their biological utility and toxicity. These pathways include Cu(I)-binding metallochaperones that shuttle copper to different subcellular sites for storage or the assembly of cupro-enzymes, and copper permeases and effluxers that act in a coordinated manner to modulate intracellular copper concentration within a relatively narrow range.
The Alghero conference highlighted progress in characterizing the function of copper permeases and the molecular genetic regulation of cellular copper levels. The importance of copper in neurobiology and the etiology of several neurological syndromes were also addressed, together with exciting new discoveries that link copper to both the immune response and programmed cell death.
Copper, immunity and pathogenicity
Although copper-catalysed Fenton chemistry has traditionally been viewed solely as a deleterious side-effect of the presence of copper ions, it now seems that organisms are exploiting its toxic potential to rid themselves of pathogens. Research presented at the meeting highlighted a new appreciation of the role of copper ions in the innate immune response. Activated macrophages and neutrophils are known to release oxygen radicals by a respiratory burst within phagosomes, which ultimately has a bactericidal function. The respiratory burst occurs through the activation of the NADPH oxidase complex, which produces superoxide anions that contribute to the accumulation of cytotoxic ROS within the phagosome. Changes in copper homeostasis are now recognized as a crucial component of this oxidative burst (Fig 1). M. Petris (Columbia, MO, USA) reported that cellular copper levels are elevated in macrophages exposed to cytokines and other mediators of inflammation. Similarly, copper deficiency is known to attenuate the antimicrobial activity of macrophages (Percival, 1998). D. Osman, from the group of J. Cavet (Manchester, UK), reported that Salmonella phagocytosed by macrophages shows enhanced copper-induced gene expression, which is consistent with an elevation in bacterial copper load. Petris reported that the enhancement of macrophage copper levels is associated with the coupled activity of the Ctr1 cuprous transporter and the copper-induced trafficking of the Menkes P-type ATPase (ATP7A) from the trans-Golgi network (TGN) to perinuclear vesicles and the phagosome. ATP7A was originally identified through its role in the serosal transfer of copper in mucosal cells. Patients with loss-of-function mutations in ATP7A present with profound copper deficiency during infancy, and dietary copper deficiency in animals is associated with higher rates of bacterial infection (Suttle & Jones, 1989).
Figure 1.
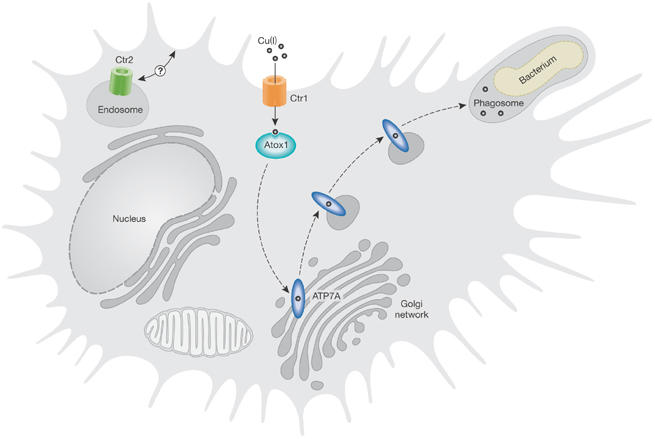
Potential role of copper in the respiratory burst reaction within phagosomes. The respiratory burst reaction in activated macrophages and neutrophils is a reactive oxygen species (ROS)-mediated antibacterial response. ROS production is metal-catalysed, with Fe(II) being considered the important metal ion. Compelling new evidence indicates that Cu(I) also has an important role in this reaction. Cu(I) is trafficked in the cytoplasm of eukaryotic cells by metallochaperone proteins to various intracellular sites of utilization. One such metallochaperone, Atox1, shuttles Cu(I) to a Cu(I) transporter, ATP7A, which resides within the trans-Golgi network, where it provides Cu(I) for the maturation of secreted cuproenzymes. An elevation in copper levels within macrophages is achieved by enhanced activity of the high-affinity plasma membrane Cu(I) permease Ctr1. This results in the relocation of ATP7A to both perinuclear vesicles and the phagosome. Delivery of Cu(I) to the phagosome might, in turn, enhance bactericidal activity during the phagocytosis of bacteria by catalysing the formation of ROS.
A role for copper in the inflammatory response is also consistent with observations reported by D. Geidroc (College Station, TX, USA) and D. Nies (Halle, Germany). Their data show that copper-efflux pumps analogous to the ATP7A transporter are important for bacterial copper resistance and are, in fact, virulence factors, as they are integral to the resistance of bacteria—such as Mycobacterium tuberculosis and Salmonella—to being killed by macrophages. Operons conferring copper resistance to bacteria are pathogenicity islands in Neisseria and Salmonella. Copper effluxers are therefore multifunctional, providing Cu(I) ions in TGN vesicles for secretory metalloproteins, moving Cu(I) into the circulation and across the placenta, and potentially delivering Cu(I) to phagosomes for antibacterial responses. The next step in this research is to use genetic approaches to confirm these exciting observations, thereby solidifying the importance of various mammalian copper transporters to the antimicrobial activity of macrophages.
A role for copper in neurodegenerative diseases
The Gitlin group recently discovered that ATP7A mediates N-methyl D-aspartate (NMDA) receptor-dependent copper release from hippocampal neurons, suggesting a role for copper in the modulation of synaptic activity of the NMDA receptor (Schlief et al, 2006). Released synaptic copper might also contribute to the deposition of amyloid β-peptide (Aβ) plaques in Alzheimer disease (AD). A. Bush (Melbourne, Vic, Australia) reported on copper coordination in Aβ fibrils and copper-induced crosslinking in Aβ aggregates. G. Multhaup (Berlin, Germany) showed that Aβ production is also influenced by the copper status of cells. The reduction of copper in AD tissue enhances Aβ production as copper modulates the activity of β-secretase, which is one of the enzymes that cleaves the amyloid precursor protein (APP) into Aβ. Bush reported that metal chelators related to 8-hydroxyquinoline are known to attenuate plaque formation in transgenic mouse models of AD. One variant, clioquinol, facilitates copper uptake into cells. Therefore, the question arises as to whether the efficacy of clioquinol in reducing Aβ plaque formation is correlated with the elevation in cellular copper and the subsequent inhibition of β-secretase activity or diminished crosslinking of Aβ fibrils. Other approaches to increase neuronal copper levels in an attempt to attenuate β-secretase activity were also discussed. Multhaup reported on the initial characterization of nanocarriers that might facilitate copper delivery to, and uptake across, the blood–brain barrier. However, whether target cells will ultimately have the capacity to effectively handle the large quantal delivery of unchaperoned copper atoms associated with each nanosphere remains unclear. C. Fernandez (Rosario, Argentina) reported that the in vitro aggregation of α-synuclein—a major component of Lewy bodies in Parkinson disease (PD)—is enhanced by copper ions in the micromolar concentration range, and that an amino-terminal domain of α-synuclein binds Cu(II). Elevated copper levels exist in the cerebrospinal fluid of PD patients, so copper binding might be a mediator of α-synuclein pathology. Copper therefore seems to have an expanding role in neurodegenerative disorders. If this is the case, the manipulation of copper levels in the brain might represent a viable and effective therapeutic strategy to treat neurodegenerative diseases such as AD and PD.
Copper trafficking and regulation of gene expression
Mutations in the copper-efflux pump ATP7B predispose individuals to Wilson's disease, which is characterized by the accumulation of toxic levels of copper in the liver and brain. A related syndrome in dogs was mapped to the copper metabolism domain-containing 1 (COMMD1 or Murr1) locus (van De Sluis et al, 2002), and a physical interaction between ATP7B and COMMD1 has since been shown (Tao et al, 2003). Although there is speculation about the functional significance of this interaction with regard to ATP7B-dependent copper export from the cell, the focus of the researchers at the meeting was on other physiological functions of COMMD1. Studies presented by B. van de Sluis (Utrecht, The Netherlands) indicate that COMMD1 might physically interact with the catalytic α-subunit of hypoxia-inducible factor 1 (HIF-1α), and that early embryonic lethality in COMMD1-knockout mice is associated with increased expression of HIF-1 target genes; however, additional studies are required to ensure that the loss of COMMD1 function is directly responsible for the observed HIF-1 activation. C. Duckett (Ann Arbor, MI, USA) further showed that COMMD1 is present in both the nucleus and the cytoplasm, and that the nuclear pool regulates gene expression by modulating nuclear factor-κB (NF-κB) activity. In the cytoplasm, COMMD1 binds the X-linked inhibitor of apoptosis (XIAP); in turn, this E3 ubiquitin ligase binds caspases, and suppresses both caspase 3-dependent and caspase 9-dependent apoptotic signalling. Duckett showed that copper has an unexpected role in this pathway, through its ability to induce the degradation of XIAP (Mufti et al, 2006). These findings suggest that the link between copper and programmed cell death might contribute to the pathology of copper-overload disorders.
K. Wood from the group of D. Thiele (Durham, NC, USA) presented intriguing data on the ability of superoxide dismutase 1 (Sod1) to regulate the activity of the copper-dependent nuclear transcription factor Mac1 in Saccharomyces cerevisiae. The transcriptional activation function of Mac1 is dependent on the ability of Sod1 to disproportionate superoxide anions. Although the link between Sod1 and Mac1 is not clear, it is possible that Mac1 activity is inhibited in ROS-stressed cells as a mechanism to attenuate copper-catalysed Fenton chemistry. These findings emphasize the crosstalk between individual copper-trafficking and sensory pathways in the cell. We look forward to future studies elucidating the mechanisms that allow for such crosstalk, which will be invaluable in understanding the regulatory hierarchy among individual copper-trafficking pathways. This, in turn, will enhance our appreciation of how the cell ultimately makes decisions about its copper status and the allocation of its total copper quota.
Regulation of cellular copper levels
Cellular copper levels are controlled by the interplay between the ATPase exporters and the Ctr family of copper permeases. J. Mercer (Melbourne, Vic, Australia) reported on the interplay between the two P-type ATPase effluxers in the mammary gland. Copper is taken up preferentially in the mammary gland of lactating animals and in the liver of non-lactating animals. In the mammary gland both ATP7A and ATP7B are functional, with the latter exporting copper to milk and the former apparently having a back-up protective role to remove excess copper from epithelial cells. An important aspect of copper homeostasis in many cell types is therefore the regulated trafficking of both ATP7A and ATP7B for specialized functions. For example, the trafficking of ATP7B from the TGN to vesicles juxtaposed to the bile caniliculus is important for the excretion of copper in bile. Although both ATPases exhibit copper-mediated trafficking in certain cell types, S. Lutsenko (Portland, OR, USA) reported that ATP7B alone fails to be transported in a copper-dependent manner in primary kidney cells. The mechanistic basis for these important tissue-specific differences in ATP7B trafficking remains unknown.
In the cell, copper-ion movement to the efflux pumps is mediated by the antioxidant protein 1 (Atx1 or Atox1) metallochaperone. The structural basis for Cu(I) transfer from Atx1 to the yeast P-type ATPase was recently described (Banci et al, 2006). L. Banci (Florence, Italy) confirmed that Cu(I) movement from Atx1 to the ATPase occurs through ligand–exchange reactions, a possibility that was first proposed by T. O'Halloran and colleagues (Pufahl et al, 1997).
Thiele, who presented the inaugural Arturo Leone lecture, discussed the importance of the Ctr family of copper transporters in eukaryotes, with an emphasis on the function of Ctr1 and Ctr2. An enterocyte-specific CTR1-knockout caused a profound copper deficiency in many tissues and a resultant reduction in the activity of several copper-dependent enzymes (Nose et al, 2006); however, surprisingly, mucosal copper levels were elevated. This observation is counterintuitive, as the ablation of Ctr1 in mucosal cells would be expected to attenuate mucosal copper levels. In addition, copper levels in some tissues, such as the kidney, remain relatively unaffected in the enterocyte-specific CTR1-null mouse. Kidney copper levels are similarly unaffected in CTR1-heterozygous mice (Lee et al, 2001). Therefore, what mechanisms allow for the relative insensitivity of the kidney, but not, for example, the brain to loss of Ctr1 function? Although the answer is not immediately apparent, data presented on Ctr2 by several investigators offer at least two intriguing possibilities. J. Bertinato (Ottawa, Canada) and P. van den Berghe (Utrecht, The Netherlands) both presented data in support of Ctr2-mediated copper import into the cell, although there was some discussion as to whether this occurs by virtue of its localization to the plasma membrane. One possibility is that under normal conditions Ctr2 is intracellularly localized, whereas in some tissues, such as the kidney, it is able to relocalize to the plasma membrane and functionally compensate for the loss of Ctr1. A second possibility is that differences exist in the relative abundance of Ctr1 and Ctr2 within tissues, such that the latter is the major copper permease present in some tissues but not others. Furthering our understanding of the interplay between Ctr1 and Ctr2 in the regulation of copper uptake into mammalian cells will be crucial for understanding the differential sensitivity of some tissues to the manipulation of Ctr1 copy number.
The cardiac hypertrophy observed in enterocyte-specific CTR1-null animals is reversed by a single injection of copper salt (Nose et al, 2006), suggesting that copper can be effectively re-utilized in animals. W. Schaffner (Zurich, Switzerland) reported that copper re-utilization is also observed in copper-deficient Drosophila. This situation is analogous to the well-established iron re-utilization cycle in animals, although the mechanisms by which copper is recycled for its subsequent re-utilization have yet to be described.
The mechanism of copper uptake by the Ctr1 permease is being resolved. V. Unger (New Haven, CT, USA) reported preliminary cryo-electron microscopy reconstruction data on human Ctr1, which indicate that structural dynamics in the trimeric structure are important in moving Cu(I) across the lipid bilayer. The current working model of Ctr1-mediated copper transport indicates that a Cu(I)-bound closed state in the cytoplasmic facing domain facilitates Cu(I) transfer to cytosolic metallochaperones, such as Atx1, through transient interactions and ligand–exchange reactions analogous to those observed between Atx1 and the yeast P-type ATPase (Banci et al, 2006). We eagerly await further developments on this front, as new information on the relationship between Ctr1 structure and function will be crucial for our mechanistic understanding of how copper is taken up by the cell and transferred to a number of competing soluble metallochaperones for its delivery to various subcellular locations.
The status of cellular copper in mammalian cells is also modulated through a signalling pathway that originates from the mitochondria. E. Shoubridge (Montreal, Canada) reported that mutations in Sco1, Sco2 and other human cytochrome oxidase assembly factors result in a profound copper-deficiency phenotype that is both tissue-specific and allele-specific, and results from an enhanced rate of copper efflux from the cell. Although the molecular mechanisms and signalling pathways have yet to be resolved, the associated copper-deficiency phenotype highlights yet another important interplay between cellular metabolism and the regulation of copper homeostasis. Elucidation of these interplays might provide the mechanistic basis of the tissue specificity of clinical phenotypes associated with mutations in these genes.
Concluding remarks
The Alghero conference was held in memory of Arturo Leone, who passed away unexpectedly at the end of 2005. Leone was one of the founding organizers of the international copper meetings, and was a wonderful colleague for those of us in the biometal community. He made several key contributions to the field, the most important of which was his prescient use of bio-reporters to identify a defect in intracellular copper availability in Menkes fibroblasts (Leone et al, 1985). Another invaluable contribution was galvanizing investigators around the world to meet regularly to discuss their work on copper in an open, friendly and collaborative environment. Leone's charisma and plain old charm—necessary to pull the meeting off not once, but on multiple occasions—was unique. It is our hope that future meetings will continue to uphold his original vision of the conference and provide further insights into several important questions regarding cellular copper trafficking. These include the emerging role for mitochondria in the regulation of cellular copper homeostasis and crosstalk between individual copper-trafficking pathways. Progress on these, and other fronts, will ultimately enhance our understanding of how the cellular copper quota is prioritized not only under basal conditions but also in response to physiological and pathophysiological stimuli that either notably reduce its bioavailability or allow its excessive accumulation. This might, in turn, lead to the development of therapeutic strategies to treat relevant human disorders, and assist with the implementation of practical guidelines and legislation concerned with human consumption of, and exposure to, copper within our environment.

Scot C. Leary

Dennis R. Winge
Acknowledgments
We thank all of our colleagues for sharing their research findings with us, particularly those who described unpublished work. We apologize to all those participants whose research findings we were unable to discuss owing to space limitations.
References
- Banci L, Bertini I, Cantini F, Felli IC, Gonnelli L, Hadjiliadis N, Pierattelli R, Rosato A, Voulgaris P (2006) The Atx1–Ccc2 complex is a metal-mediated protein–protein interaction. Nat Chem Biol 2: 367–368 [DOI] [PubMed] [Google Scholar]
- Lee J, Prohaska JR, Thiele DJ (2001) Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA 98: 6842–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone A, Pavlakis GN, Hamer DH (1985) Menkes' disease: abnormal metallothionein gene regulation in response to copper. Cell 40: 301–309 [DOI] [PubMed] [Google Scholar]
- Mufti AR et al. (2006) XIAP Is a copper binding protein deregulated in Wilson's disease and other copper toxicosis disorders. Mol Cell 21: 775–785 [DOI] [PubMed] [Google Scholar]
- Nose Y, Kim BE, Thiele DJ (2006) Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab 4: 235–244 [DOI] [PubMed] [Google Scholar]
- Percival SS (1998) Copper and immunity. Am J Clin Nutr 67: S1064–S1068 [DOI] [PubMed] [Google Scholar]
- Pufahl RA, Singer CP, Peariso KL, Lin SJ, Schmidt PJ, Fahrni CJ, Culotta VC, Penner-Hahn JE, O'Halloran TV (1997) Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science 278: 853–856 [DOI] [PubMed] [Google Scholar]
- Schlief ML, West T, Craig AM, Holtzman DM, Gitlin JD (2006) Role of the Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc Natl Acad Sci USA 103: 14919–14924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle NF, Jones DG (1989) Recent developments in trace element metabolism and function: trace elements, disease resistance and immune responsiveness in ruminants. J Nutr 119: 1055–1061 [DOI] [PubMed] [Google Scholar]
- Tao TY, Liu F, Klomp L, Wijmenga C, Gitlin JD (2003) The copper toxicosis gene product Murr1 directly interacts with the Wilson disease protein. J Biol Chem 278: 41593–41596 [DOI] [PubMed] [Google Scholar]
- van De Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C (2002) Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 11: 165–173 [DOI] [PubMed] [Google Scholar]


