Polarity, which is defined as a structural and/or functional asymmetry, is a fundamental property of eukaryotic cells. Some types of cell are intrinsically polarized in situ, for example, epithelial cells and neurons, whereas others become polarized transiently in response to external cues, for example, migrating cells, lymphocytes and phagocytes. During metazoan development, cell polarity is necessary for the establishment of the body axis, morphogenetic movements and cell-fate determination in a range of contexts. Cell fate is often specified by asymmetric cell division, in which fate determinants become differentially distributed between daughter cells (reviewed by Suzuki & Ohno, 2006; Betschinger & Knoblich, 2004).
During the past decade, it has become apparent that polarity is largely regulated by a conserved set of proteins referred to as the partition-defective (PAR) proteins (Macara, 2004). In Caenorhabditis elegans zygotes and Drosophila oocytes, a complex consisting of Par3, Par6 and atypical protein kinase C (aPKC) localizes selectively to the anterior pole of the egg cortex (Etemad-Moghadam et al, 1995; Benton & St Johnston, 2003), whereas in both invertebrate and vertebrate epithelia this complex concentrates at apical junctional complexes and/or the apical plasma membrane (Wodarz et al, 2000; Izumi et al, 1998). A second kinase, Par1, localizes to the posterior pole of C. elegans zygotes (Guo & Kemphues, 1995) and Drosophila oocytes (Benton & St Johnston, 2003), and to the basolateral membranes of epithelia (Bohm et al, 1997). Maintenance of these non-overlapping distributions is thought to be the result of mutually antagonistic phosphoregulation: encroachment of the Par3–Par6–aPKC complex into the posterior pole of the egg is limited by the Par1-mediated phosphorylation of Par3, which disrupts its interaction with aPKC (Benton & St Johnston, 2003). Conversely, phosphorylation of Par1 by aPKC inhibits both its kinase activity and its ability to localize to the anterior pole of the cell (Suzuki et al, 2004; Hurov et al, 2004). These mutually antagonistic phosphorylation events therefore function to maintain cellular asymmetry in the presence of continuing cytoplasmic diffusion.
The polarized distribution of aPKC is also crucial for asymmetric cell division. In several model systems—including neuroblasts and sensory organ precursor (SOP) cells in Drosophila, and mammalian epidermal basal cells—a polarized distribution of the Par3–Par6–aPKC complex is necessary for the proper segregation of fate determinants between daughter cells (Betschinger & Knoblich, 2004; Lechler & Fuchs, 2005).
One such cell-fate determinant, Numb, was originally identified as a regulator of Drosophila SOP differentiation (Uemura et al, 1989); however, it has subsequently been found to function in a range of other binary cell-fate decisions in both neuronal and non-neuronal tissues (Betschinger & Knoblich, 2004; Roegiers & Jan, 2004). In Drosophila, Numb localizes opposite the Par3–Par6–aPKC complex in both mitotic SOP cells undergoing the pI division (Rhyu et al, 1994) and neuroblasts (Knoblich et al, 1995), and becomes segregated into one of the two daughter cells. The adoption of distinct cell fates by the daughter cell that contains Numb is thought to result largely from the ability of Numb to functionally antagonize the Notch signalling pathway through a direct interaction with the cytoplasmic domain of Notch (Betschinger & Knoblich, 2004). In mammals, Numb is widely expressed and is essential for normal development. Mice lacking Numb exhibit profound defects in angiogenic remodelling and neural-tube closure, and lack several neuronal cell lineages (Zhong et al, 2000; Zilian et al, 2001). An asymmetric distribution of Numb has been reported in mitotic neural progenitors in the mouse forebrain (Zhong et al, 1996), retinal precursors (Cayouette et al, 2001), isolated cortical progenitors (Shen et al, 2002) and muscle satellite cells (Conboy & Rando, 2002). In mammalian epithelial cells, endogenous Numb is localized primarily to the basolateral cortex (Dho et al, 2006), indicating that Numb also maintains a polarized distribution in non-mitotic cells.
Although it is clear that the polarized distribution of Numb is essential for its function, the mechanisms by which this pattern is achieved are poorly understood. In the 24 January issue of The EMBO Journal, Jane McGlade and colleagues reported that Numb is a substrate for aPKC, and that this phosphorylation is necessary to maintain its asymmetric localization in both mammalian epithelial cells and mitotic Drosophila SOP cells (Smith et al, 2007).
The McGlade laboratory has previously shown that Numb can be displaced from cortical binding sites in non-polarized cells by treatment with phorbol esters or by the activation of certain G-protein-coupled receptors that signal through PKC (Dho et al, 2006). In their more recent study, the authors show that Numb is phosphorylated directly by PKC in vitro and in vivo, and they use mass spectroscopy to identify two evolutionarily conserved PKC sites—Ser 7 and Ser 295—that seem to be preferentially phosphorylated by aPKC isoforms. A mutant construct lacking both of the phosphorylation sites (Numb2A) localized normally to the cortex of non-polarized (HeLa) cells; however, in contrast to the wild-type protein, the mutant was not displaced by either phorbol ester treatment or expression of a constitutively active mutant of aPKCζ. These observations indicate that phosphorylation of Numb by aPKC is sufficient to destabilize its interaction with cortical binding partners.
In mammalian epithelial cells, the Par3–Par6–aPKC complex localizes predominantly to apically-located tight junctions, where it is necessary for the establishment of epithelial polarity (Suzuki & Ohno, 2006; Macara 2004). As noted above, the McGlade laboratory has previously shown that endogenous Numb localizes primarily to the basolateral cortex of polarized Madin–Darby canine kidney (MDCK) cells (Dho et al, 2006), thereby raising the possibility that aPKC might negatively regulate the association of Numb with apical membranes. Now the authors show directly that the distributions of aPKC and Numb in MDCK cells do not significantly overlap and are, in fact, complementary. Furthermore, in contrast to the wild-type protein, the phosphorylation-deficient Numb2A mutant fails to segregate to the basolateral pole in MDCK monolayers, and instead is uniformly distributed on both apical and basolateral membranes. In addition, the depletion of endogenous aPKCλ—but not PKCα—by RNA interference also resulted in the association of endogenous Numb with the apical membrane, indicating that the PKC-mediated phosphorylation of Numb at these regulatory sites is indeed isoform-specific. Together, these findings clearly show that phosphorylation of Numb by aPKCλ is necessary for its exclusion from the apical pole of MDCK cells.
As mentioned above, both Ser 7 and Ser 295 are evolutionarily conserved. To confirm that aPKC-mediated phosphorylation is a conserved mechanism for regulating Numb localization, the authors next examined the behaviour of a panel of phosphorylation-site mutants in Drosophila SOP cells. Mass-spectroscopy analysis of dNumb indicated that Ser 52 and possibly Ser 304, which correspond to Ser 7 and Ser 295, respectively, in mouse Numb, were indeed phosphorylated by aPKC in vitro, as were several other conserved serine residues. In dividing SOP (pI) cells, Numb typically concentrates at the anterior cell cortex, opposite aPKC (Fig 1). Wild-type dNumb, which was ectopically expressed in pI cells by using a tissue-specific promoter, localized similarly to the endogenous protein. As in mammalian cells, mutation of a single serine, Ser52Ala, was insufficient to randomize the distribution of the mutant protein. However, a mutant lacking all five conserved PKC sites (Numb5A) failed to concentrate at the anterior cell border and clearly extended to the posterior cortex. Interestingly, a mutant in which Ser 52 was retained but the other four PKC sites were mutated (Numb4A) localized normally, implying that phosphorylation of Ser 52 is necessary, but not sufficient, for displacement of Numb from the posterior pole of the cell. Together, these observations indicate that phosphorylation of Numb by aPKC is an evolutionarily conserved mechanism for achieving its asymmetric distribution in polarized cells. It should be noted that localization of Numb is at least in part dependent on its interaction with Lethal giant larvae (Lgl) through the adaptor protein Partner of numb (Pon). Importantly, association of Lgl with the cell cortex is also inhibited by aPKC-mediated phosphorylation (Betschinger et al, 2003). However, because non-phosphorylatable Numb mutants can associate with the cortex even in the presence of endogenous aPKC, alternative Lgl-independent modes of interaction must exist.
Figure 1.
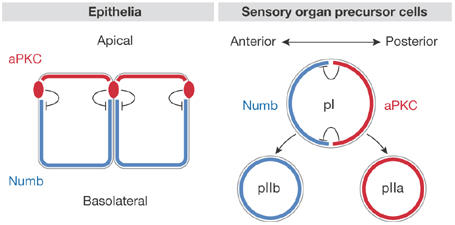
A conserved mechanism for the maintenance of Numb asymmetry in polarized cells. In epithelial cells, atypical protein kinase C (aPKC) is concentrated in apical junctional complexes and/or the apical cell cortex at steady state, whereas Numb is restricted to basolateral membranes. Encroachment of Numb into the apical pole of the cell results in its phosphorylation by aPKC, preventing its association with apical membranes. In mitotic Drosophila sensory organ precursor cells (pI), aPKC is concentrated in the posterior cortex. Phosphorylation-dependent displacement of Numb from the posterior pole causes Numb to concentrate anteriorly. Upon cell division, Numb is concentrated in the anterior daughter cell (pIIb), which adopts a developmental fate different than that of the posterior daughter (pIIa).
In addition to Ser 7 and Ser 295, McGlade and colleagues identified seven other phosphorylation sites on mouse Numb, implying that other aspects of Numb function are also regulated by phosphorylation events. Numb is a member of a family of cytoplasmic adaptor proteins, referred to as clathrin-associated sorting proteins (CLASPS), which link transmembrane cargo proteins, such as Notch, to the clathrin endocytic machinery (Traub, 2005). Numb interacts directly with α-adaptin, which is a component of the adaptor protein 2 (AP-2) clathrin adaptor complex (Santolini et al, 2000); this interaction is necessary for the ability of Numb to antagonize Notch signalling (Berdnik et al, 2002), presumably by mediating its endocytosis. The Drosophila Numb-associated kinase (NAK) is closely related to the mammalian adaptin-associated kinase (AAK), which regulates the association of cargo with the AP-2 complex (Conner & Schmid, 2002). It will be interesting to determine whether mammalian Numb is phosphorylated by AAK, and whether this affects its interaction with the endocytic machinery. Numb has also been localized to endosomal compartments in non-polarized cells, where it might function in post-endocytic recycling (Smith et al, 2004). As polarized recycling of membrane constituents is an important mechanism for maintaining cell polarity, it will also be fascinating to determine whether Numb selectively associates with basolaterally-directed recycling pathways, and whether phosphorylation regulates this function as well.

References
- Benton R, St Johnston D (2003) Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115: 691–704 [DOI] [PubMed] [Google Scholar]
- Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA (2002) The endocytic protein α-adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell 3: 221–231 [DOI] [PubMed] [Google Scholar]
- Betschinger J, Knoblich JA (2004) Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol 14: R674–R685 [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA (2003) The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422: 326–330 [DOI] [PubMed] [Google Scholar]
- Bohm H, Brinkmann V, Drab M, Henske A, Kurzchalia TV (1997) Mammalian homologues of C. elegans PAR-1 are asymmetrically localized in epithelial cells and may influence their polarity. Curr Biol 7: 603–606 [DOI] [PubMed] [Google Scholar]
- Cayouette M, Whitmore AV, Jeffery G, Raff M (2001) Asymmetric segregation of Numb in retinal development and the influence of the pigmented epithelium. J Neurosci 21: 5643–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Rando TA (2002) The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3: 397–409 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL (2002) Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol 156: 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dho SE, Trejo J, Siderovski DP, McGlade CJ (2006) Dynamic regulation of mammalian numb by G protein-coupled receptors and protein kinase C activation: structural determinants of numb association with the cortical membrane. Mol Biol Cell 17: 4142–4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etemad-Moghadam B, Guo S, Kemphues KJ (1995) Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83: 743–752 [DOI] [PubMed] [Google Scholar]
- Guo S, Kemphues KJ (1995) par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81: 611–620 [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H (2004) Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 14: 736–741 [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S (1998) An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol 143: 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN (1995) Asymmetric segregation of Numb and Prospero during cell division. Nature 377: 624–627 [DOI] [PubMed] [Google Scholar]
- Lechler T, Fuchs E (2005) Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437: 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara IG (2004) Par proteins: partners in polarization. Curr Biol 14: R160–R162 [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN (1994) Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76: 477–491 [DOI] [PubMed] [Google Scholar]
- Roegiers F, Jan YN (2004) Asymmetric cell division. Curr Opin Cell Biol 16: 195–205 [DOI] [PubMed] [Google Scholar]
- Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP (2000) Numb is an endocytic protein. J Cell Biol 151: 1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S (2002) Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development 129: 4843–4853 [DOI] [PubMed] [Google Scholar]
- Smith CA, Dho SE, Donaldson J, Tepass U, McGlade CJ (2004) The cell fate determinant numb interacts with EHD/Rme-1 family proteins and has a role in endocytic recycling. Mol Biol Cell 15: 3698–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA et al. (2007) aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J 26: 468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ohno S (2006) The PAR-aPKC system: lessons in polarity. J Cell Sci 119: 979–987 [DOI] [PubMed] [Google Scholar]
- Suzuki A et al. (2004) aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol 14: 1425–1435 [DOI] [PubMed] [Google Scholar]
- Traub LM (2005) Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta 1744: 415–437 [DOI] [PubMed] [Google Scholar]
- Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN (1989) numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell 58: 349–360 [DOI] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E (2000) Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol 150: 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN (1996) Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron 17: 43–53 [DOI] [PubMed] [Google Scholar]
- Zhong W, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, Jan YN (2000) Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci USA 97: 6844–6849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilian O, Saner C, Hagedorn L, Lee HY, Sauberli E, Suter U, Sommer L, Aguet M (2001) Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol 11: 494–501 [DOI] [PubMed] [Google Scholar]


