Abstract
Background
β-catenin is a multifunctional protein involved in two apparently independent processes: cell-cell adhesion and signal transduction. β-catenin is involved in Wnt signaling pathway that regulates cellular differentiation and proliferation. In this study, we investigated the expression pattern of β-catenin and cyclin D1 using immunohistochemistry and searched for mutations in exon 3 of the β-catenin gene and Axin gene in esophageal squamous cell carcinoma.
Materials and methods
Samples were obtained from 50 esophageal cancer patients. Immunohistochemical staining for β-catenin and cyclin D1 was done. Mutational analyses of the exon3 of the β-catenin gene and Axin gene were performed on tumors with nuclear β-catenin expression.
Results
Four (8%) esophageal cancer tissues showed high nuclear β-catenin staining. Overexpression of cyclin D1 was observed in 27 out of 50 (54%) patients. All four cases that showed nuclear β-catenin staining overexpressed cyclin D1. No relationship was observed between the expression pattern of β-catenin and cyclin D1 and age, sex, tumor size, stage, differentiation grade, lymph node metastasis, response to chemotherapy, or survival. No mutational change was found in β-catenin exon 3 in the four cases with nuclear β-catenin staining. Sequencing analysis of the Axin cDNA revealed only a splicing variant (108 bp deletion, position 2302–2409) which was present in the paired normal mucosa.
Conclusion
A fraction of esophageal squamous cell carcinomas have abnormal nuclear accumulation of β-catenin accompanied with increased cyclin D1 expression. Mutations in β-catenin or axin genes are not responsible for this abnormal localization of β-catenin.
Background
β-catenin is a multifunctional protein involved in two apparently independent processes: cell-cell adhesion and signal transduction. β-catenin binds to both the cytoplasmic domain of cadherin and the amino-terminal domain of β-catenin and mediates cell adhesion. In addition to its function in cell-cell adhesion, β-catenin plays an important role in signal transduction; it is involved in the Wnt signaling pathway that regulates cellular differentiation and proliferation [1].
The level of free β-catenin is low in normal cells, since the protein is sequestered in a complex, which includes the adenomatous polyposis coli (APC) protein, a serine threonine glycogen kinase (GSK-3β) and conductin or Axin, leading to degradation of β-catenin by proteasome. The binding of β-catenin by APC requires phosphorylation of β-catenin by GSK-3β on 3 serine and 1 threonine residues, all of which are encoded by exon 3 of the β-catenin gene [2-4].
In colorectal cancers, mutations of APC or β-catenin result in stabilization of β-catenin and a significant accumulation of this protein within the cytoplasm [5]. Furthermore, increased β-catenin may translocate to the nuclei and could serve as a transcriptional factor by binding to the T-cell factor/lymphoid enhancing factor (Tcf-Lef) family [5], leading to transcription of specific genes stimulating tumor formation, such as cyclin-D1, c-myc, c-jun, fra-1, uPAR, ZO-1, MMP7, NBL4, DRCTNNB1A, MCP-3 [6-12]. However, the precise regulatory mechanisms remain to be resolved. Mutations, including large interstitial deletions involving exon 3 of the β-catenin gene, have also been found in several other tumors [5,13,14].
Recently, cyclin D1 has been identified as a target of the β-catenin/T-cell factor/lymphoid enhancer factor complex [2,15]. Cyclin D1 is expressed in the G1 phase of the cell cycle, and is thought to play a major role in the control of the cell cycle and cancer progression. Overexpression of cyclinD1 has been suggested to contribute to oncogenesis by disturbing the cell cycle and has been reported to be an important oncogenic factor in esophageal carcinoma [16].
Recent experiments demonstrated that Axin functions as a tumor suppressor in hepatocellular carcinoma [17]. The different domains of Axin possess binding capacity for APC, GSK-3β, β-catenin, PP2A, Dishevelled, and Axin itself [18,19]. As a scaffold protein of this multiprotein complex, Axin is able to bring β-catenin and GSK-3β into close proximity, thus facilitating β-catenin phosphorylation [20,21] and subsequent ubiquitin-mediated degradation by the proteasome system [22,23].
Esophageal squamous cell carcinoma is an aggressive disease with a poor prognosis, and the genetic mechanism of its carcinogenesis remains to be solved. The progression of this tumor is associated with multiple genetic alterations, including loss of heterozygosity in chromosomes 3p, 5q, 9p, 9q, 13q, 17p, 17q and 18q, and amplification of epidermal growth factor receptor (EGFR), HER-2, c-myc, and cyclin D1 [24]. The most frequent genetic alteration in esophageal squamous cell carcinoma is a point mutation in the p53 gene (40–60%) that occurs at a relatively early stage of tumor development [13,25]. However, Wnt signal pathway in esophageal cancer has not been extensively studied.
In this paper, we investigated the expression pattern of β-catenin and cyclin D1 in esophageal squamous cell carcinoma. We found aberrant localization of β-catenin in a minor proportion of the tumor and mutation in exon 3 of the β-catenin gene (CTNNB1) and Axin gene was studied in these samples.
Materials and methods
Tissue samples
Samples were obtained from 50 esophageal cancer patients who had undergone operations at the Department Surgery II, Nagoya City University Medical School between 1996 and 2000. They were classified according to the guideline for the clinical and pathologic studies on carcinoma of the esophagus [26]. All samples for RT-PCR were frozen immediately in liquid nitrogen and stored -80°C until analysis. All the tissues for immunohistochemistry was fixed in formalin and embedded in paraffin.
Immunohistochemical staining for β-catenin and cyclin D1
Immunohistochemical staining was done with anti-β-catenin monoclonal antibody (Transduction Lab.) and anti-cyclin D1 monoclonal antibody (Oncogene Research Product). A formalin-fixed paraffin-embedded tissue block was cut and de-waxed. Each section was treated for 15 min in citric-acid buffer (pH 6.0) with autoclave at 120°C for antigen retrieval, and was immunostained with the standard indirect ABC technique. Counterstaining was done with hematoxylin. When more than 20% of the cells were stained, the staining was scored as positive.
RNA extraction and reverse transcriptional reaction
Total RNA was extracted from the esophageal cancer tissue, and normal esophageal mucosa taken from the mucosa as far apart from the tumor as possible, using Isogen kit (Nippon gene, Tokyo, Japan). The concentration of the RNA was adjusted to 200 ng/ml, using spectrophotometer. Reverse transcriptional reaction was carried out at 42°C for 90 min and 95°C for 5 min followed by incubation at 72°C for 15 min, using 1 μg RNA, 0.5 mg oligo(dTi) primer, Superscript II enzyme (Gibco BRL, Gaithersburg, MD).
Mutational analyses of the exon3 of the β-catenin and axin genes
Polymerase chain reaction (PCR) and direct sequencing analysis were performed on the four tumors with nuclear β-catenin expression. Four microliter of cDNA mixture was subjected to amplification in 80 μl containing 0.8 U of Taq polymerase, 10×PCR buffer, 25 μM each of dATP, dCTP, dGTP, dTTP, 25 μM of MgCl2 and 80 pmol each of 5' and 3' primers. PCR conditions for β-catenin were initial denaturation for 5 min at 95°C, followed by 38 cycles of denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec, extension at 72°C for 30 sec and a final extension at 72°C for 5 min. PCR conditions for axin were initial denaturation for 5 min at 95°C, followed by 38 cycles of denaturation at 94°C for 60 sec, annealing at 54°C for 60 sec, extension at 72°C for 60 sec and a final extension at 72°C for 5 min. The primer pair used for amplification of the entire exon 3 of the β-catenin gene and four sets of primers for axin gene are shown in the Table 1. The identities of PCR products were analyzed by a 3% agarose gel electrophoresis. After electrophoresis, the PCR products were purified from agarose gels using a QIAquick gel extraction kit (Qiagen, Hilden, Germany) and amplified with the primer set shown in Table 1. Sequencing was performed with the ABI 3100 DNA Sequencer (Applied Biosystems, Foster City, Calif.).
Table 1.
Primer sequences and annealing temperatures for PCR
| Primer set | Position | Annealing temperature |
| β-catenin exon3 | ||
| F:GTC TGA GGA GCA GCT TCA GT | 77–96 | 56°C |
| R:CAT TAG TGG GAT GAG CAG CA | 559–578 | |
| Axin | ||
| F1:GCG GGA CAG ATT GAT TCA CT | 28–47 | 54°C |
| R1:TCG GCA GGT ATC CAG ATA TG | 811–830 | |
| F2:TCT GTA GTG ACC AGA GCT CT | 771–790 | 54°C |
| R2:GAC GAT GGA TCG CCG TCC T | 1380–1398 | |
| F3:AGT TCG CGG AGG AGC TCA T | 1272–1290 | 54°C |
| R3:CCT CAA TGA TCC ACT GCA TG | 2011–2030 | |
| F4:AGG ATG CGG AGA AGA ACC AG | 1986–2005 | 54°C |
| R4:TCC TGA GTA CGA GGT CAT CT | 2770–2789 |
Statistical method
χ-square test was used to analyze the relationship between the two variables. A p value of less than 0.05 was considered significant.
Results
Immunohistochemical study for β-catenin
Fifty esophageal cancer tissues and paired normal esophageal mucosa were stained for β-catenin using immunohistochemistry. In all the normal esophageal mucosa, β-catenin staining was restricted to the plasma membrane. The intensity of the staining did not vary very much among individuals. In most of the 50 esophageal cancer tissues, the β-catenin staining was at the plasma membrane. However, four cases (8%) of esophageal cancer tissue were judged as showing widespread nuclear staining. And in these cases, β-catenin was not expressed on the plasma membrane (Fig. 1a). Normal esophageal mucosa of these cases showed membrane staining of β-catenin, and did not show nuclear staining (Fig. 1b). 46 other esophageal cancer samples showed either plasma membrane or cytoplasmic staining or both. No relationship was observed between β-catenin expression pattern and age, sex, tumor size, stage, differentiation grade, lymph node metastasis, response to chemotherapy, or survival (χ-square test, Table 2).
Figure 1.
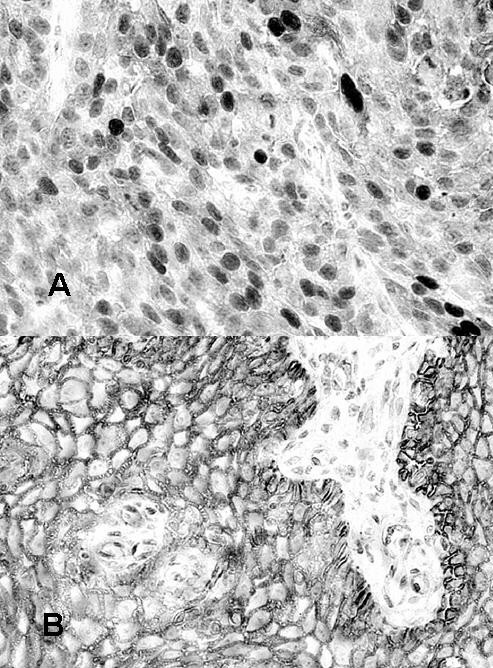
Immunohistochemical staining of β-catenin in normal and cancer tissues of the esophagus. In this cancer tissues, β-catenin was expressed in the nuclei, and was hardly detectable at the plasma membrane (a). In normal esophageal epithelium, β-catenin was expressed at the cell membrane (b).
Table 2.
Clinico-pathological characteristics and β-catenin and cyclin-D1 protein expression in 50 patients with esophageal cancer
| age/sex | follow-up(M) | out comea) | T | N | stage | lyb) | vc) | diffd) | β-catenine) | cyclin D1 | |
| 1 | 67M | 31 | D.O | 1 | 0 | I | 0 | 0 | M | M | + |
| 2 | 46M | 24 | A | 1 | 0 | I | 0 | 0 | M | C | - |
| 3 | 68M | 26 | A | 1 | 1 | I | 1 | 1 | W | N+C+M | + |
| 4 | 56M | 11 | A | 1 | 0 | I | 0 | 0 | M | M | - |
| 5 | 68M | 18 | A | 1 | 0 | I | 0 | 0 | M | M | + |
| 6 | 66M | 36 | D.E | 3 | 0 | II | 2 | 1 | M | C+M | - |
| 7 | 73F | 38 | A | 2 | 1 | II | 1 | 0 | W | M | - |
| 8 | 69M | 2 | D.O | 1 | 2 | II | 0 | 0 | W | M | - |
| 9 | 60M | 32 | A | 1 | 2 | II | 0 | 0 | W | M | + |
| 10 | 58M | 16 | D.E | 1 | 1 | II | 1 | 1 | P | N+C | + |
| 11 | 79F | 30 | D.E | 3 | 0 | III | 1 | 1 | W | N+C | + |
| 12 | 69M | 8 | D.E | 3 | 2 | III | 1 | 0 | M | C+M | - |
| 13 | 58M | 12 | D.E | 3 | 1 | III | 1 | 1 | P | M | + |
| 14 | 66M | 11 | D.E | 3 | 1 | III | 1 | 1 | M | C | - |
| 15 | 66F | 39 | D.E | 3 | 2 | III | 1 | 1 | P | C | - |
| 16 | 54F | 16 | D.E | 3 | 3 | III | 1 | 0 | W | C+M | - |
| 17 | 52F | 15 | D.E | 3 | 2 | III | 2 | 1 | W | M | + |
| 18 | 68M | 12 | D.E | 3 | 1 | III | U | U | n.a. | M | + |
| 19 | 68M | 26 | D.E | 3 | 2 | III | 1 | 2 | W | M | - |
| 20 | 68M | 12 | D.E | 3 | 1 | III | 2 | 1 | M | M | + |
| 21 | 57F | 14 | D.E | 3 | 2 | III | 2 | 2 | W | M | + |
| 22 | 61F | 6 | D.E | 3 | 2 | III | 1 | 0 | W | M | - |
| 23 | 63M | 28 | D.O | 3 | 3 | III | 3 | 1 | M | C | + |
| 24 | 47F | 22 | A | 3 | 2 | III | 1 | 0 | W | M | + |
| 25 | 50M | 29 | A | 3 | 2 | III | 1 | 1 | P | C+M | + |
| 26 | 68M | 9 | D.E | 3 | 2 | III | 1 | 1 | M | M | - |
| 27 | 70F | 4 | D.E | 3 | 2 | III | 1 | 0 | W | M | - |
| 28 | 56M | 18 | A | 3 | 2 | III | 1 | 0 | W | M | - |
| 29 | 68M | 9 | D.E | 4 | 0 | III | U | U | U | M | + |
| 30 | 68M | 15 | D.E | 3 | 3 | III | 2 | 2 | P | M | - |
| 31 | 75M | 6 | D.E | 4 | 3 | IV | U | U | W | M | - |
| 32 | 51F | 9 | D.E | 4 | 2 | IV | 2 | 2 | M | M | + |
| 33 | 67M | 2 | D.E | 4 | 2 | IV | U | U | n.a. | M | - |
| 34 | 55M | 6 | D.E | 4 | 4 | IV | U | U | M | M | - |
| 35 | 51M | 2 | D.E | 2 | 4 | IV | 0 | 0 | M | M | + |
| 36 | 69M | 8 | D.E | 4 | 3 | IV | U | U | W | C | + |
| 37 | 62M | 7 | D.E | 4 | 2 | IV | 1 | 1 | W | M | - |
| 38 | 45M | 8 | D.E | 4 | 3 | IV | 1 | 0 | W | M | + |
| 39 | 46M | 3 | D.E | 4 | 4 | IV | 2 | 1 | M | M | + |
| 40 | 52M | 14 | D.E | 3 | 4 | IV | 1 | 1 | M | C | + |
| 41 | 72M | 5 | D.E | 3 | 4 | IV | 2 | 2 | M | M | - |
| 42 | 53M | 13 | D.E | 4 | 2 | IV | 1 | 0 | M | M | + |
| 43 | 76M | 11 | D.E | 4 | 3 | IV | 1 | 1 | M | M | + |
| 44 | 64M | 6 | D.O | 4 | 2 | IV | 1 | 1 | M | M | - |
| 45 | 51M | 6 | D.E | 4 | 4 | IV | U | U | W | M | - |
| 46 | 58M | 22 | A | 4 | 3 | IV | U | U | M | C+M | - |
| 47 | 69M | 2 | D.E | 3 | 4 | IV | 2 | 1 | W | C | + |
| 48 | 57M | 13 | D.E | 4 | 2 | IV | 2 | 2 | M | M | - |
| 49 | 72M | 14 | D.E | 4 | 1 | IV | 1 | 1 | W | C | - |
| 50 | 60M | 18 | A | 4 | 4 | IV | 2 | 1 | M | N+C | + |
All the samples except cases 18 (unknown) and 33 (adenosquamous carcinoma) were squamous cell carcinomas.
a) D.O; dead with other disease, A; alive D.E; dead with esophageal cancer
b) ly; grade of lymphatic invasion, U; unknown c) v; grade of venous invasion, U; unknown
d) differentiation, W; well differentiated, M; moderately differentiated, P; poorly differentiated, U; unknown, n.a.; not applicable
e) localization by immunostaining, M; membrane, C; cytoplasmic N; nuclear staining
Immunohistochemical staining for cyclin D1
We then analyzed the expression of cyclin D1, one of the possible downstream targets of the Wnt signal pathway, using immunohistochemistry. In all the normal esophageal mucosa, cyclin D1 staining was negative (Fig. 2a). Overexpression of cyclin D1 was observed in the tumor nuclei in 27 out of 50 (54%) patients (Fig 2b). All of the four cases that showed widespread nuclear β-catenin staining overexpressed cyclin D1. When these 4 cases were excluded, there was no cooperation between the staining pattern of β-catenin and cyclin D1 expression. No relationship was observed between the overexpression of cyclin D1 and age, sex, tumor size, stage, differentiation grade, lymph node metastasis, response to chemotherapy, or survival (Table 2).
Figure 2.
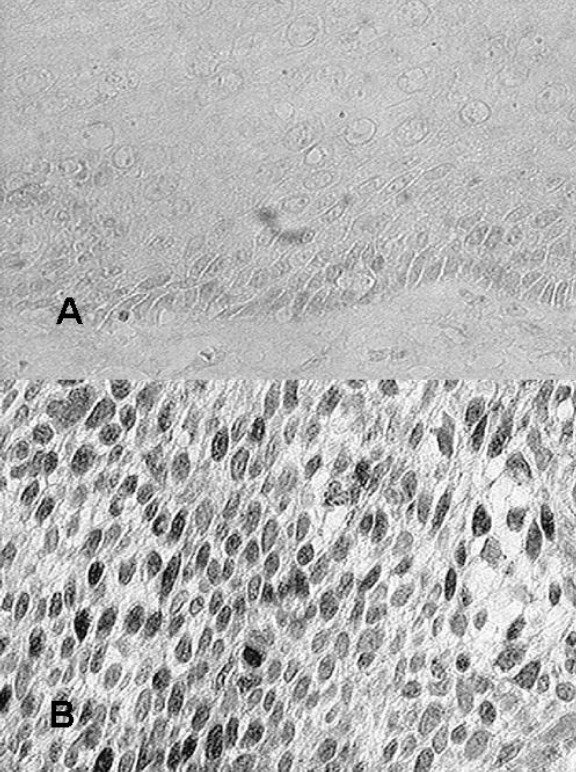
Immunohistochemical staining of cyclin D1 expression in normal and cancer tissues of the esophagus. In all normal esophageal mucosa, cyclin D1 staining was negative (a). In some cancer tissues, cyclin D1 was expressed at the nuclei (b).
PCR and direct sequencing analysis of the β-catenin gene
To investigate the mechanism of β-catenin nuclear localization, we amplified the β-catenin gene by PCR using the primer pair flanking the entire exon 3. All four cases showed the single band of 502 bp, and none showed an aberrant PCR product within the amplified region. Then we determined the sequence of each band by direct sequencing. No mutational change was found in any of these cases (data not shown).
PCR and direct sequencing analysis of the Axin gene
We then searched for mutational changes in the Axin gene. Axin gene mutation may have caused the abnormal distribution of β-catenin. We examined the Axin cDNA in four cases showing nuclear localization of β-catenin protein. Sequencing analysis of the Axin cDNA revealed a splicing variant (108 bp deletion, position 2302–2409) and a normal cDNA in two of the cases tested. We sequenced the cDNA from the normal mucosa in these cases, and the same variant was found. This deletion affected the whole exon 9 (Fig. 3). Thus neither β-catenin exon 3 nor Axin gene mutation was responsible for the aberrant localization of β-catenin in the 4 cases.
Figure 3.
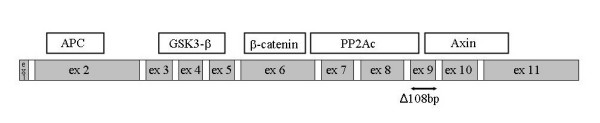
Genetic alterations of the Axin gene in esophageal squamous cell carcinoma. Splicing variant is indicated by two-headed arrows.
Discussion
In this paper, we searched for changes in some of the proteins and genes involved in the Wnt signaling pathway in esophageal cancer. We identified abnormal nuclear accumulation of β-catenin protein in 4 (8%) out of the 50 esophageal cancer samples. The aberrant location of this protein was probably function suggested by the increased expression of cyclin D1 in all 4 of these cases. However, we were unable to find any genetic alterations responsible for this aberrant β-catenin distribution.
Ninomiya et al. have reported that overexpression of β-catenin was observed in 3 of 22 cases studied (13.6%). Although no mutation of the β-catenin gene was observed, the common silent mutation of the APC gene was found in all the cases [27]. De Castro et al. have reported that 18% cases of the esophageal squamous cell carcinoma showed overexpression of β-catenin in the cytoplasm and nuclei of tumor cells. They found no genetic alteration of the β-catenin gene [13]. We observed nuclear expression of β-catenin in 4 cases (8%). This is a frequency similar to those reported by other researchers. No mutational change in the β-catenin gene was found in any of the cases we studied, as reported by others [13].
In esophageal adenocarcinomas, Krishnadath et al. observed a correlation between reduced expression of β-catenin and poor prognosis [28]. Furthermore, in esophageal adenocarcinomas, the nucleus was stained in some tumors by β-catenin immunostaining. Lin et al. have also reported immunohistochemical data on β-catenin in esophageal cancer but did not find correlation with malignant behavior of the tumor [29]. In another study β-catenin and cyclin D1 expression was correlated with survival of esophageal cancer patients [30]. In other series of esophageal cancers, no mutation in the mutation cluster region of APC and exon3 of β-catenin genes was detected [7].
It has been reported that immunohistochemical examination of cyclin D1 expression may provide important prognostic information for esophageal cancer [31]. Takeuchi et al. have reported that the overexpression of cyclin D1 may be a useful prognostic indicator in patients with squamous cell carcinomas of the esophagus [32]. And Zhai et al. have reported that overexpression of cyclin D1 was highly associated with nuclear accumulation of β-catenin in ovarian endometrioid adenocarcinomas [33]. In our studies, no relationship was observed between cyclin D1 expression pattern and clinicopathological features. In accordance with the report by Zhai et al. all four cases in our study showing nuclear localization of β-catenin protein overexpressed cyclin D1.
A number of studies have shown that Axin is critical for mediating the down regulation of β-catenin [34,35]. One recent publication reported reduced axin repression in ESCC [36]. We looked for Axin gene mutations in esophageal squamous cell carcinoma, but found only a splicing variant.
In conclusion, the nuclear localization of β-catenin occurs in a fraction of esophageal carcinomas. All the cases showing nuclear localization of β-catenin protein overexpressed cyclin D1 which may have contributed to the oncogenesis of the esophageal carcinoma. The abnormal localization of β-catenin apparently did not result from the genetic alterations of either the β-catenin or Axin gene.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
YF and JK planned the experiment. JK performed most of the experiments with the help and supervision of NH and HI. TN, YS, YT HS, NS, MK, and YK provided the surgical materials. JK and YF wrote the paper.
All authors read and approved final version of manuscript.
Acknowledgments
Acknowledgements
The authors thank Mrs. Shinobu Makino for immunohistochemical staining.
Contributor Information
Junzo Kudo, Email: dr_kudo@yahoo.co.jp.
Tadashi Nishiwaki, Email: dr_kudo@yahoo.co.jp.
Nobuhiro Haruki, Email: dr_kudo@yahoo.co.jp.
Hideyuki Ishiguro, Email: dr_kudo@yahoo.co.jp.
Yasuyuki Shibata, Email: dr_kudo@yahoo.co.jp.
Yukio Terashita, Email: dr_kudo@yahoo.co.jp.
Hironori Sugiura, Email: dr_kudo@yahoo.co.jp.
Noriyuki Shinoda, Email: dr_kudo@yahoo.co.jp.
Masahiro Kimura, Email: dr_kudo@yahoo.co.jp.
Yoshiyuki Kuwabara, Email: dr_kudo@yahoo.co.jp.
Yoshitaka Fujii, Email: yosfujii@med.nagoya-cu.ac.jp.
References
- Korinek V, Barker N, Willert K, Molenaar M, Roose J, Wagenaar G, Markman M, Lamers W, Destree O, Clevers H. Two members of the Tcf family implicated in Wnt/beta-catenin signaling during embryogenesis in the mouse. Mol Cell Biol. 1998;18:1248–1256. doi: 10.1128/mcb.18.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Shiozaki H, Doki Y, Yamamoto M, Utsunomiya T, Kawanishi K, Fukuchi N, Inoue M, Tsujinaka T, Monden M. Cytoplasmic beta-catenin in esophageal cancers. Int J Cancer. 1999;84:174–178. doi: 10.1002/(SICI)1097-0215(19990420)84:2<174::AID-IJC14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci USA. 1995;92:3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Wijnhoven BP, Nollet F, De Both NJ, Tilanus HW, Dinjens WN. Genetic alterations involving exon 3 of the beta-catenin gene do not play a role in adenocarcinomas of the esophagus. Int J Cancer. 2000;86:533–537. doi: 10.1002/(SICI)1097-0215(20000515)86:4<533::AID-IJC15>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Fujita M, Furukawa Y, Nagasawa Y, Ogawa M, Nakamura Y. Down-regulation of monocyte chemotactic protein-3 by activated beta-catenin. Cancer Res. 2000;60:6683–6687. [PubMed] [Google Scholar]
- Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J, Holcombe RF, Waterman ML. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet. 2001;28:53–57. doi: 10.1038/88264. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Furukawa Y, Daigo Y, Miyoshi Y, Nagasawa Y, Nishiwaki T, Kawasoe T, Fujita M, Satoh S, Miwa N, Fujii Y, Nakamura Y. Isolation and characterization of human NBL4, a gene involved in the beta-catenin/tcf signaling pathway. Jpn J Cancer Res. 2000;91:597–603. doi: 10.1111/j.1349-7006.2000.tb00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasoe T, Furukawa Y, Daigo Y, Nishiwaki T, Ishiguro H, Fujita M, Satoh S, Miwa N, Nagasawa Y, Miyoshi Y, Ogawa M, Nakamura Y. Isolation and characterization of a novel human gene, DRCTNNB1A, the expression of which is down-regulated by beta-catenin. Cancer Res. 2000;60:3354–3358. [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Hanski C. Target genes of beta-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA. 1999;96:1603–1608. doi: 10.1073/pnas.96.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro J, Gamallo C, Palacios J, Moreno-Bueno G, Rodriguez N, Feliu J, Gonzalez-Baron M. beta-catenin expression pattern in primary oesophageal squamous cell carcinoma. Relationship with clinicopathologic features and clinical outcome. Virchows Arch. 2000;437:599–604. doi: 10.1007/s004280000266. [DOI] [PubMed] [Google Scholar]
- Saito T, Oda Y, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, Tsuneyoshi M. beta-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumours. J Pathol. 2001;195:222–228. doi: 10.1002/path.942. [DOI] [PubMed] [Google Scholar]
- Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450–456. [PubMed] [Google Scholar]
- Research Committee on Malignancy of Esophageal Cancer J.S.f.E.D. Prognostic significance of Cyclin D1 and E-Cadherin in patients with esophageal squamous cell carcinoma: multiinstitutional retrospective analysis. Research Committee on Malignancy of Esophageal Cancer, Japanese Society for Esophageal Diseases. J Am Coll Surg. 2001;192:708–718. doi: 10.1016/S1072-7515(01)00840-7. [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Hsu W, Zeng L, Costantini F. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem. 1999;274:3439–3445. doi: 10.1074/jbc.274.6.3439. [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/S0960-9822(98)70226-X. [DOI] [PubMed] [Google Scholar]
- Hinoi T, Yamamoto H, Kishida M, Takada S, Kishida S, Kikuchi A. Complex formation of adenomatous polyposis coli gene product and axin facilitates glycogen synthase kinase-3 beta-dependent phosphorylation of beta-catenin and down-regulates beta-catenin. J Biol Chem. 2000;275:34399–34406. doi: 10.1074/jbc.M003997200. [DOI] [PubMed] [Google Scholar]
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sorensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T. Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 2001;61:7039–7043. [PubMed] [Google Scholar]
- Montesano R, Hollstein M, Hainaut P. Genetic alterations in esophageal cancer and their relevance to etiology and pathogenesis: a review. Int J Cancer. 1996;69:225–235. doi: 10.1002/(SICI)1097-0215(19960621)69:3<225::AID-IJC13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Bennett WP, Hollstein MC, Metcalf RA, Welsh JA, He A, Zhu S, Kusters I, Resau JH, Trump BF, Lane DP, Harris CC. p53 mutation and protein accumulation during multistage human esophageal carcinogenesis. Cancer Res. 1992;52:6092–6097. [PubMed] [Google Scholar]
- Japanese Society for esophageal disease . Guidelines for the clinical and pathologic studies on carcinoma of the esophagus, 9th edition. Tokyo: Kanehara Publish Co; 1999. [Google Scholar]
- Ninomiya I, Endo Y, Fushida S, Sasagawa T, Miyashita T, Fujimura T, Nishimura G, Tani T, Hashimoto T, Yagi M, Shimizu K, Ohta T, Yonemura Y, Inoue M, Sasaki T, Miwa K. Alteration of beta-catenin expression in esophageal squamous-cell carcinoma. Int J Cancer. 2000;85:757–761. doi: 10.1002/(SICI)1097-0215(20000315)85:6<757::AID-IJC3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Krishnadath KK, Tilanus HW, van Blankenstein M, Hop WC, Kremers ED, Dinjens WN, Bosman FT. Reduced expression of the cadherin-catenin complex in oesophageal adenocarcinoma correlates with poor prognosis. J Pathol. 1997;182:331–338. doi: 10.1002/(SICI)1096-9896(199707)182:3<331::AID-PATH860>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lin Y, Wu MY, Li DR, Wu XY, Zheng RM. Prognostic and clinocopathological features of E-cadherin, alpha-catenin, beta-catenin, gamma-catenin and cyclin d1 expression in human esophageal squamous cell carcinoma. World J Gastroenterol. 2004;10:3235–3239. doi: 10.3748/wjg.v10.i22.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki H, doki Y, Kawanishi K, Shamma A, Yano M, Inoue M, Monden M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery. 2000;127:552–561. doi: 10.1067/msy.2000.105028. [DOI] [PubMed] [Google Scholar]
- Nagasawa S, Onda M, Sasajima K, Makino H, Yamashita K, Takubo K, Miyashita M. Cyclin D1 overexpression as a prognostic factor in patients with esophageal carcinoma. J Surg Oncol. 2001;78:208–214. doi: 10.1002/jso.1152. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Ozawa S, Ando N, Shih CH, Koyanagi K, Ueda M, Kitajima M. Altered p16/MTS1/CDKN2 and cyclin D1/PRAD-1 gene expression is associated with the prognosis of squamous cell carcinoma of the esophagus. Clin Cancer Res. 1997;3:2229–2236. [PubMed] [Google Scholar]
- Zhai Y, Wu R, Schwartz DR, Darrah D, Reed H, Kolligs FT, Nieman MT, Fearon ER, Cho KR. Role of beta-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas. Am J Pathol. 2002;160:1229–1238. doi: 10.1016/s0002-9440(10)62550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Wu R, Zhai Y, Fearon ER, Cho KR. Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001;61:8247–8255. [PubMed] [Google Scholar]
- Nakajima M, Fukuchi M, Miyazaki T, Masuda N, Kato H, Kuwano H. Reduced expression of Axin correlates with tumour progression of oesophageal squamous cell carcinoma. Br J Cancer. 2003;88:1734–1739. doi: 10.1038/sj.bjc.6600941. [DOI] [PMC free article] [PubMed] [Google Scholar]


