The establishment and spread of highly pathogenic avian influenza (HPAI) viruses of the H5N1 subtype in birds and coincident infections in humans since 2003 have raised concerns that we may be facing an influenza pandemic caused by an H5N1 influenza virus. In this brief Opinion piece, we consider the pandemic threat posed by H5N1 viruses and review the published data on the evaluation of H5N1 vaccines in preclinical and clinical studies.
HPAI H5N1 viruses have been isolated from avian species in more than 50 countries. As of 29 January 2007, 270 laboratory-confirmed cases of H5N1 infection in humans had been reported by the World Health Organization, 164 of which were fatal [1], resulting in a case fatality rate of approximately 60%.
In order to cause a pandemic, H5N1 viruses will have to acquire the ability to transmit efficiently from person to person. The H5 hemagglutinin (HA) is found in influenza viruses that typically infect avian species, so efficient person-to-person spread could happen if the H5N1 virus reassorts, or exchanges genes, with circulating human influenza viruses giving rise to a virus with the H5 HA (to which the population is not immune) in a gene constellation that confers the property of transmissibility. Alternatively, efficient person-to-person spread could occur if the H5N1 virus evolves and adapts to more efficient replication and transmissibility in the human population.
Two observations have led to questions about the likelihood of a reassortant H5N1 virus causing a pandemic. First, reassortant viruses have not been isolated despite ongoing H5N1 outbreaks in birds and infections in humans, even with concurrent circulation of human influenza viruses since 2003. Second, laboratory studies have found that reassortant viruses that derived the surface glycoprotein genes from an H5N1 virus and internal protein genes from an H3N2 influenza A virus were not efficiently transmitted and were somewhat less infectious to ferrets (an animal model for human influenza) than the wild-type H5N1 viruses [2]. The concern that an H5N1 virus could adapt to the human host and acquire mutations that confer transmissibility prompts very careful analysis of each cluster of human H5N1 infections that is reported ( [1,3–5]). At present, the data suggest that human-to-human transmission is inefficient and very limited. Nevertheless, from the standpoint of public health preparedness, it is important to move forward in developing approaches for dealing with H5N1 in humans.
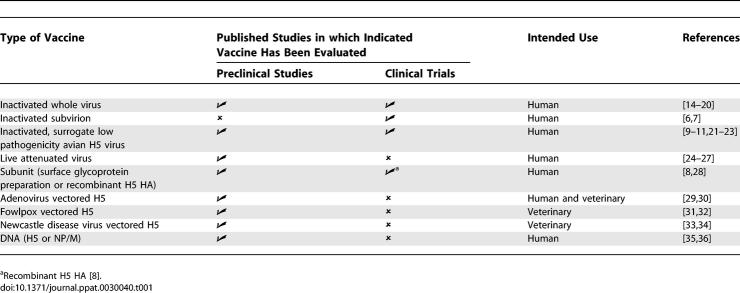
Vaccination is the preferred strategy for prevention and control of influenza. The most expeditious way to generate an H5N1 vaccine is to use licensed technology, such as inactivated or live attenuated vaccines. However, several practical and scientific challenges to the development of H5N1 vaccines exist. These include high pathogenicity of wild-type H5N1 influenza viruses, reduced yield of candidate vaccine viruses in embryonated hens' eggs compared to that of human influenza viruses, limited manufacturing capacity, and poor immunogenicity of the H5 HA. Despite these obstacles, several approaches have been used to generate candidate vaccines and a few have advanced to clinical trials (Table 1). Table 1 also includes data published on vaccines that are being developed for veterinary use.
Table 1.
Vaccine Strategies against H5N1 Influenza that Have Been Evaluated in Preclinical and Clinical Studies
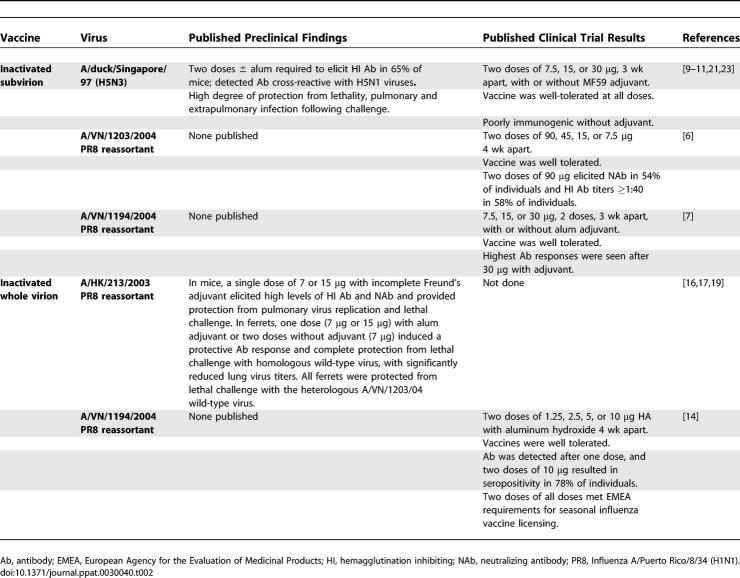
Perhaps the most significant scientific challenge for the development and licensure of pandemic vaccines for humans is that assessment of vaccine efficacy for humans will have to be inferred from preclinical studies in experimental animals and immunogenicity studies in humans, as it will not be possible to assess the efficacy of a pandemic vaccine in a clinical trial before a pandemic begins. Table 2 summarizes the preclinical and clinical findings from inactivated H5N1 vaccines evaluated in humans to date. Preclinical studies of influenza vaccines are generally conducted in mice or ferrets. In most cases, the 1997 and 2003 H5N1 vaccine candidates were promising in terms of immunogenicity and efficacy, with complete protection of animals from lethal H5N1 infection, and significant, if not complete, reduction of pulmonary viral replication following challenge. Preclinical data in ferrets have not been published on the 2004 H5N1 vaccines that were evaluated in clinical trials, so data are not available to directly assess how accurately preclinical studies would have predicted the outcome of evaluation of these vaccines in humans.
Table 2.
Summary of Preclinical and Clinical Findings for Inactivated H5N1 Virus Vaccines Evaluated in Humans
In clinical trials, inactivated virus vaccines based on H5N1 viruses isolated in 2004 [6,7], a recombinant H5 HA subunit vaccine based on an H5N1 virus isolated in 1997 expressed in a baculovirus vector [8], and an inactivated virus vaccine based on a surrogate low pathogenicity avian H5N3 virus [9–11], were poorly immunogenic when administered to volunteers without adjuvant. Clinical trials of H1N1 influenza vaccines in 1977 established that whole virion vaccines are more immunogenic than split-virion vaccines (in which the virus particles are disrupted by detergent treatment to obtain a preparation enriched for the surface antigens) [12,13]; however, the former are also more reactogenic than the latter. Consistent with this observation, in recent trials in humans of an alum-adjuvanted inactivated H5N1 virus vaccine, much lower doses of a whole virion vaccine elicited higher levels of antibody compared to a split-virion vaccine [7,14]. Despite the fact that the difference in immunogenicity of whole virion and split-virion vaccines was well established, preclinical studies of inactivated H5 virus vaccines in mice and ferrets have generally been performed using whole virion preparations with adjuvant, while the vaccine preparation evaluated in clinical trials is a purified split-virion vaccine. It is important to note that preclinical data will not be predictive of clinical trial results if the vaccine formulations that are tested in preclinical studies are different from those evaluated in clinical trials.
Clinical trials have demonstrated that the immunogenicity of H5 vaccines can be enhanced by an increased dose of the HA, the use of adjuvants, use of multiple doses, or use of a whole virion vaccine. More studies are needed to directly compare findings from preclinical and clinical evaluation of pandemic influenza vaccines to establish whether animal models can be used to guide decisions on which vaccine candidates to take forward for evaluation in humans. Although there is no evidence that H5N1 viruses have yet acquired pandemic potential, the consequences of such an event are serious enough that preparation for a possible pandemic is essential.
Abbreviation
- HA
hemagglutinin
Footnotes
Kanta Subbarao and Catherine Luke are with the Laboratory of Infectious Diseases, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, United States of America.
Competing interests. Our laboratory has a cooperative research and development agreement with MedImmune Vaccines to develop vaccines against potential pandemic strains of influenza.
Author contributions. KS and CL wrote the paper.
Funding. This research was supported in part by the Intramural Research Program of the US National Institutes of Health, National Institute of Allergy and Infectious Diseases.
References
- World Health Organization. Disease outbreak news. 2007. Available: http://www.who.int/csr/don/en. Accessed 2 February 2007.
- Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A. 2006;103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- Oner AF, Bay A, Arslan S, Akdeniz H, Sahin HA, et al. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N Engl J Med. 2006;355:2179–2185. doi: 10.1056/NEJMoa060601. [DOI] [PubMed] [Google Scholar]
- Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–2194. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: Phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Wilkinson BE, Masseoud F, Hu-Primmer J, Battaglia R, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001;19:1732–1737. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: A randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–1693. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) Vaccine: A potential priming strategy. J Infect Dis. 2005;191:1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- Wright PF, Dolin R, La Montagne JR. From the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Center for Disease Control, and the Bureau of Biologics of the Food and Drug Administration. Summary of clinical trials of influenza vaccines–II. J Infect Dis. 1976;134:633–638. doi: 10.1093/infdis/134.6.633. [DOI] [PubMed] [Google Scholar]
- Wright PF, Thompson J, Vaughn WK, Folland DS, Sell SH, et al. Trials of influenza A/New Jersey/76 virus vaccine in normal children: An overview of age-related antigenicity and reactogenicity. J Infect Dis. 1977;136(Suppl):S731–S741. doi: 10.1093/infdis/136.supplement_3.s731. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang J, Dong X, Fang H, Chen J, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: A phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- Subbarao K, Chen H, Swayne D, Mingay L, Fodor E, et al. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology. 2003;305:192–200. doi: 10.1006/viro.2002.1742. [DOI] [PubMed] [Google Scholar]
- Webby RJ, Perez DR, Coleman JS, Guan Y, Knight JH, et al. Responsiveness to a pandemic alert: Use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov AS, Webby RJ, Govorkova EA, Krauss S, Webster RG. Efficacy of H5 influenza vaccines produced by reverse genetics in a lethal mouse model. J Infect Dis. 2005;191:1216–1220. doi: 10.1086/428951. [DOI] [PubMed] [Google Scholar]
- Nicolson C, Major D, Wood JM, Robertson JS. Generation of influenza vaccine viruses on Vero cells by reverse genetics: An H5N1 candidate vaccine strain produced under a quality system. Vaccine. 2005;23:2943–2952. doi: 10.1016/j.vaccine.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis. 2006;194:159–167. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- Horimoto T, Takada A, Fujii K, Goto H, Hatta M, et al. The development and characterization of H5 influenza virus vaccines derived from a 2003 human isolate. Vaccine. 2006;24:3669–3676. doi: 10.1016/j.vaccine.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, et al. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Kuboki N, Okazaki K, Ninomiya A, Tanaka H, et al. Avirulent avian influenza virus as a vaccine strain against a potential human pandemic. J Virol. 1999;73:8303–8307. doi: 10.1128/jvi.73.10.8303-8307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-protectiveness and immunogenicity of influenza A/Duck/Singapore/3/97(H5) vaccines against infection with A/Vietnam/1203/04(H5N1) virus in ferrets. J Infect Dis. 2006;194:1040–1043. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- Li S, Liu C, Klimov A, Subbarao K, Perdue ML, et al. Recombinant influenza A virus vaccines for the pathogenic human A/Hong Kong/97 (H5N1) viruses. J Infect Dis. 1999;179:1132–1138. doi: 10.1086/314713. [DOI] [PubMed] [Google Scholar]
- Lu X, Edwards LE, Desheva JA, Nguyen DC, Rekstin A, et al. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine. 2006;24:6588–6593. doi: 10.1016/j.vaccine.2006.05.039. [DOI] [PubMed] [Google Scholar]
- Desheva JA, Lu XH, Rekstin AR, Rudenko LG, Swayne DE, et al. Characterization of an influenza A H5N2 reassortant as a candidate for live-attenuated and inactivated vaccines against highly pathogenic H5N1 viruses with pandemic potential. Vaccine. 2006;24:6859–6866. doi: 10.1016/j.vaccine.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Claas EC, van Amerongen G, de Jong JC, Osterhaus AD. ISCOM vaccine induced protection against a lethal challenge with a human H5N1 influenza virus. Vaccine. 1999;17:1355–1358. doi: 10.1016/s0264-410x(98)00390-9. [DOI] [PubMed] [Google Scholar]
- Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol. 2006;80:1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao CL, Yu KZ, Jiang YP, Jia YQ, Tian GB, et al. Protection of chickens against highly lethal H5N1 and H7N1 avian influenza viruses with a recombinant fowlpox virus co-expressing H5 haemagglutinin and N1 neuraminidase genes. Avian Pathol. 2003;32:25–32. doi: 10.1080/0307945021000070688. [DOI] [PubMed] [Google Scholar]
- Karaca K, Swayne DE, Grosenbaugh D, Bublot M, Robles A, et al. Immunogenicity of fowlpox virus expressing the avian influenza virus H5 gene (TROVAC AIV-H5) in cats. Clin Diagn Lab Immunol. 2005;12:1340–1342. doi: 10.1128/CDLI.12.11.1340-1342.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veits J, Wiesner D, Fuchs W, Hoffmann B, Granzow H, et al. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc Natl Acad Sci U S A. 2006;103:8197–8202. doi: 10.1073/pnas.0602461103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Deng G, Wen Z, Tian G, Wang Y, et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81:150–158. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodihalli S, Goto H, Kobasa DL, Krauss S, Kawaoka Y, et al. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J Virol. 1999;73:2094–2098. doi: 10.1128/jvi.73.3.2094-2098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein SL, Tumpey TM, Misplon JA, Lo CY, Cooper LA, et al. DNA vaccine expressing conserved influenza virus proteins protective against H5N1 challenge infection in mice. Emerg Infect Dis. 2002;8:796–801. doi: 10.3201/eid0808.010476. [DOI] [PMC free article] [PubMed] [Google Scholar]