Summary
In vertebrates, auditory and vestibular transduction occurs on apical projections (stereocilia) of specialized cells (hair cells). Mutations in myosin VIIA (myo-VIIA), an unconventional myosin, lead to deafness and balance anomalies in humans, mice, and zebra-fish; individuals are deaf, and stereocilia are disorganized [1–3]. The exact mechanism through which myo-VIIA mutations result in these inner-ear anomalies is unknown. Proposed inner-ear functions for myoVIIA include anchoring transduction channels to the stereocilia membrane, trafficking stereocilia linking components, and anchoring hair cells by associating with adherens junctions [4–7]. The Drosophila myoVIIA homolog is crinkled (ck) [8]. The Drosophila auditory organ, Johnston’s organ (JO), is developmentally and functionally related to the vertebrate inner ear. Both derive from modified epithelial cells specified by atonal and spalt homolog expression, and both transduce acoustic mechanical energy ([9] and references therein). Here, we show that loss of ck/myoVIIA function leads to complete deafness in Drosophila by disrupting the integrity of the scolopidia that transduce auditory signals. We demonstrate that ck/myoVIIA functions to organize the auditory organ, that it is functionally required in neuronal and support cells, that it is not required for TRPV channel localization, and that it is not essential for scolopidial-cell-junction integrity.
Results and Discussion
ck/myoVIIA Mutations Lead to Deafness in Drosophila
Usher syndrome Type 1B, owing to myoVIIA mutations, is characterized by sensorineural deafness, vestibular dysfunction, and retinitis pigmentosa [1]. Mammalian myoVIIA localizes along cilia and microvilli in the inner ear, retina, testes, lungs, liver, kidneys, olfactory epithelium, and intestines [10–12]. In the ear, it is located exclusively in hair cells, where it is preferentially localized along the actin-rich stereocilia; in the cuticular plate (a dense network of horizontal filaments); and in the peri-cuticular necklace [10, 11]. Normally, stereocilia are organized in a V-shaped, staircase-arranged cluster. myoVIIA mutations lead to small groups of diversely arranged and incorrectly positioned stereocilia [4]. The exact mechanism through which myoVIIA mutations lead to anomalies in vertebrates is unknown. To further understand myoVIIA roles, we focused on auditory transduction, mediated by the antennae, in Drosophila.
Each antenna consists of three segments. The third segment (a3) inserts into the second (a2) like a key into a keyhole at the a2/a3 junction [13]. Sound-induced vibrations result in a3 rotations in relation to a2 (Figure 1A). The Drosophila auditory organ, Johnston’s organ (JO), is situated within a2. It comprises approximately 200 radially organized units (scolopidia), tethered basally to the cuticle and apically to the a2/a3 joint. a2/a3 joint vibrations stretch the scolopidia to transduce sound. Each scolopidium consists of several cells: two or three ciliated neurons and three or four support cells [9]. The dendrites are encapsulated by an isolated extracellular space (scolopale space) formed by the scolopale cell and shaped by thick, actin-based scolopale rods with interspersed microtubules [9] (Figure 1A). The dendritic cap is an extracellular, tubular/conical structure secreted by the scolopale cell [14] (potentially, other cells contribute). It connects dendrites to the a2/a3 joint by extending through the a2/a3 cuticle. The cap cell apically ensheaths the scolopale cell and most of the cap, aiding in apical juncture and in maintaining scolopale-space ionic concentrations [9] (Figure 1A).
Figure 1. JO Function Is Disrupted by ck/myoVIIA Mutations.
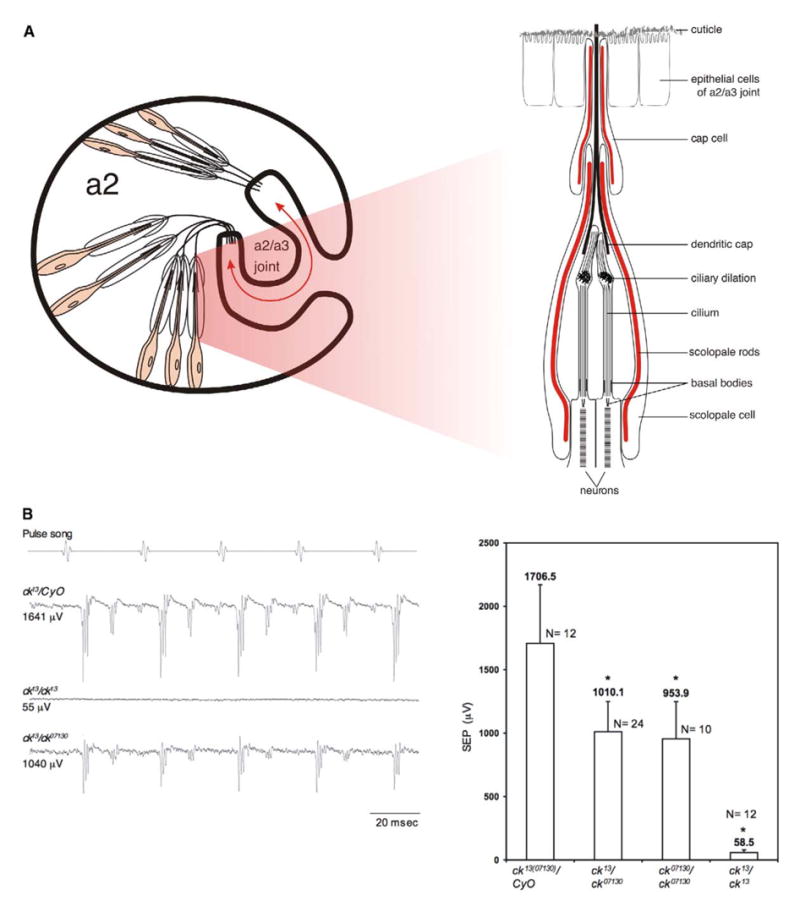
(A) Diagram of JO. The arrow indicates the direction of a2/a3 movement. The drawing is not to scale.
(B) Sound-evoked potentials (SEPs) from the antennal nerve show that ck13/ck07130 flies have lower responses than controls, whereas ck13/ck13 flies have no detectable response. Each trace is the average of ten stimulus/response cycles. Allelic combinations containing ck07130 show SEPs significantly reduced but not absent (p < 0.001), indicating that this is a hypomorphic allele. Error bars indicate the standard deviation.
The Drosophila myoVIIA amino acid sequence, encoded by crinkled (ck), is 61.7% identical to the human homolog [8]. Like vertebrate myoVIIA, it consists of a motor domain, a neck that binds calmodulin and/or specific light chains, and a tail (comprising FERM, MyTH4, MyTH7, and SH3 domains) that can mediate dimerization and interactions with other proteins [8].
We conducted extracellular electrophysiological recordings to determine effects of ck/myoVIIA mutations on auditory transduction. Through sound-evoked potentials (SEPs) [15] recorded from the antennal nerve, we determined that ck/myoVIIA mutations disrupt Drosophila auditory transduction. In response to the pulse song, one component of male fly courtship song, ck/myoVIIA mutations significantly reduced (ck07130 allele; Figure 1B) or completely abolished (ck13 allele) the SEPs. The ck07130 allele has reduced expression because a P element is inserted in the 5′ region of the gene, whereas ck13 causes early truncation [8]. Therefore, similar to the case of vertebrates, ck/myoVIIA is required for auditory transduction in Drosophila. We also tested the response of ck07130 flies to sine frequencies up to 1000 Hz, with results similar to those for the pulse song (not shown). No frequencies were impeded more than others in ck07130 flies. Thus, ck/myo-VIIA is necessary for auditory transduction.
Morphological Effects of ck/myoVIIA Mutations in JO
Vertebrate studies have indicated a role for myoVIIA in stereocilia organization and morphology [4]. We investigated JO morphology in mutants through electron microscopy. Compared to the wild-type (Canton S), ck13 leads to scolopidial apical detachment and overall JO disorganization (Figure 2A) that in some ways mimics the stereocilia disarray previously described in mutant vertebrate homologs [4]. Scolopidial basal attachments to a2 cuticle remain unaffected (Figure 2A). A substantial number of scolopidia were detached in ck07130/ck07130 and ck07130/ck13 flies as well (Figure 2A). Thus, the degrees of disorganization/detachment correlate with the degrees of deafness in different alleles. Therefore, ck/myoVIIA must function in apical attachment and, hence, in physical stimulus propagation from cuticle to scolopidia.
Figure 2. ck/myoVIIA Mutations Lead to JO Disorganization.
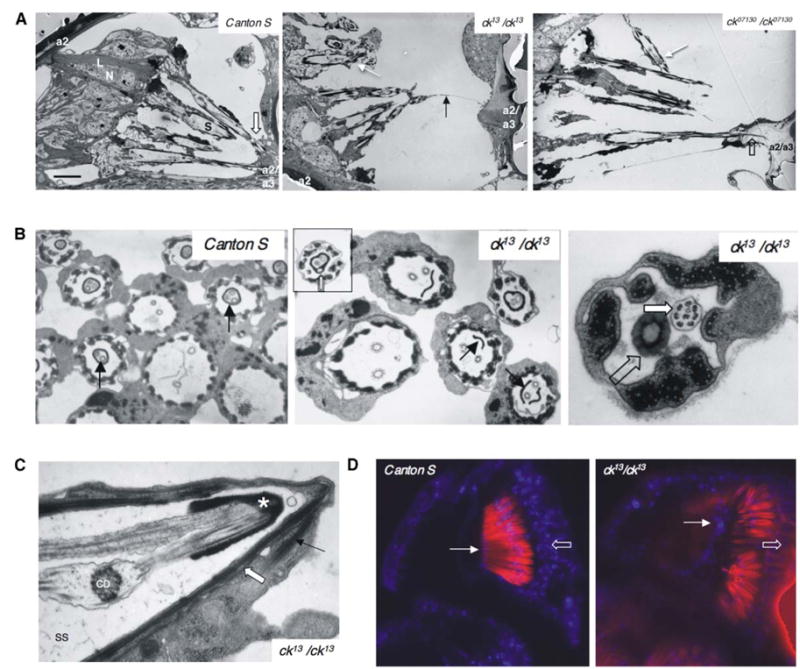
(A) ck13 and ck07130 scolopidia, compared to controls (Canton S), show apical detachment (white arrows). In one occasion, out of 14 specimens examined, we observed a residual link (black arrow). The following abbreviations were used: N, neuron; S, scolopale space; L, basal ligament; block arrow, scolopidial attachment to a2/a3 joint; and open block arrow, dendritic cap extending into a2/a3 joint cuticle. The scale bar represents 5 μm.
(B) Unlike controls (Canton S, arrow), ck13 shows an incomplete dendritic cap (arrow) and subcompartmentalization (inset, block arrow). When the cap of ck13 completes a profile (open block arrow), it may fail to enclose one of the cilia (block arrow).
(C) In detached scolopidia, the cap cell (arrow) detaches from the joint and remains with the scolopidium (block arrow demarcates cell junctions). The following abbreviations were used: SS, scolopale space; CD, ciliary dilation; and asterisk, cap.
(D) Nuclear labeling with TO-PRO 3 (blue). a2/a3 joint epithelial cells (arrow) were stained in controls (ck13/CyO) and mutants. Red: actin filaments labeled with Texas Red-Phalloidin; open block arrow: neuronal nuclei. Sections in (D) are equivalent.
We examined scolopidial ultrastructure and detected morphological anomalies associated only with the dendritic cap. The cap has a circular cross-sectional profile enclosing the tips of both dendrites (Figure 2B, Canton S). In ck13 flies, the cap often encloses the cilia incompletely, with one cilium remaining outside it (Figure 2B). Mutant caps may also be divided into several compartments, each hosting a cilium or being empty (Figure 2B). Similar defects are observed in ck07130 flies (not shown). Such anomalies could perturb sound transduction in ck07130 flies by affecting scolopidia that remain attached to the a2/a3 joint. Furthermore, because the apical juncture is believed to occur through the cap and the cap cell [9] and because the cap cell in detached scolopidia remains associated with the scolopidium (Figure 2C) rather than with the joint cuticle, detachment is likely due to morphological anomalies that are in the cap and weaken apical juncture.
We also investigated the possibility that a2/a3 joint epithelium leads to detachment by cellular degeneration. Nuclear staining (Figure 2D) showed cells present and positioned at the joint, indicating that they are not lost in ck/myoVIIA mutants. Thus, apical detachment is most likely due to cap morphological defects as a result of ck/myoVIIA mutations.
Scolopales in ck/myoVIIA mutants were shorter in length and wider at the base. Measurements (not shown) from attached and detached scolopales indicated that these are most likely secondary effects of loss in tension from detachment. Thus, ck/myoVIIA does not play an active role in shaping the scolopale solely through cell-autonomous effects on cytoskeletal structure per se. In conclusion, ck/myoVIIA is important for JO organization by functioning in scolopidial apical attachment; detachment most likely occurs from abnormal dendritic cap morphology as a result of ck/myo-VIIA mutations.
ck/myoVIIA Is Necessary for Neuronal and Scolopale Cell Function
Anti-ck/myoVIIA antibody [8] showed staining throughout the cytoplasm of scolopale cells and neurons (Figure 3A). In scolopale cells, ck/myoVIIA protein is concentrated along actin-rich scolopale rods and more apically, near scolopale cell-cap cell junctions. In neurons, ck/myoVIIA protein is concentrated near the basal body, where neurons are tethered to the scolopale cell. In the vertebrate inner ear, myoVIIA is expressed by hair cells alone, not by support cells [10]. That both neuronal and support cells express ck/myoVIIA in fly indicates that although the inner ear and JO are related, there are also structural and functional differences. Nevertheless, the data suggest that the scolopale cell and neuron are more closely evolutionarily related to inner-ear hair cells than to support cells. This conclusion suggests a model in which the scolopale cell and the neuron result from an additional cell division in comparison to the hair cell so that in flies, cytoskeletal features that are split into neuronal and scolopale cells (i.e., cilium and actin rods) are in vertebrates together in the same cell (kinocilium and stereocilia). In this scenario, ck/myoVIIa continues to be expressed in both daughter cells.
Figure 3. ck/myoVIIA Functions in Scolopale Cells and Neurons.
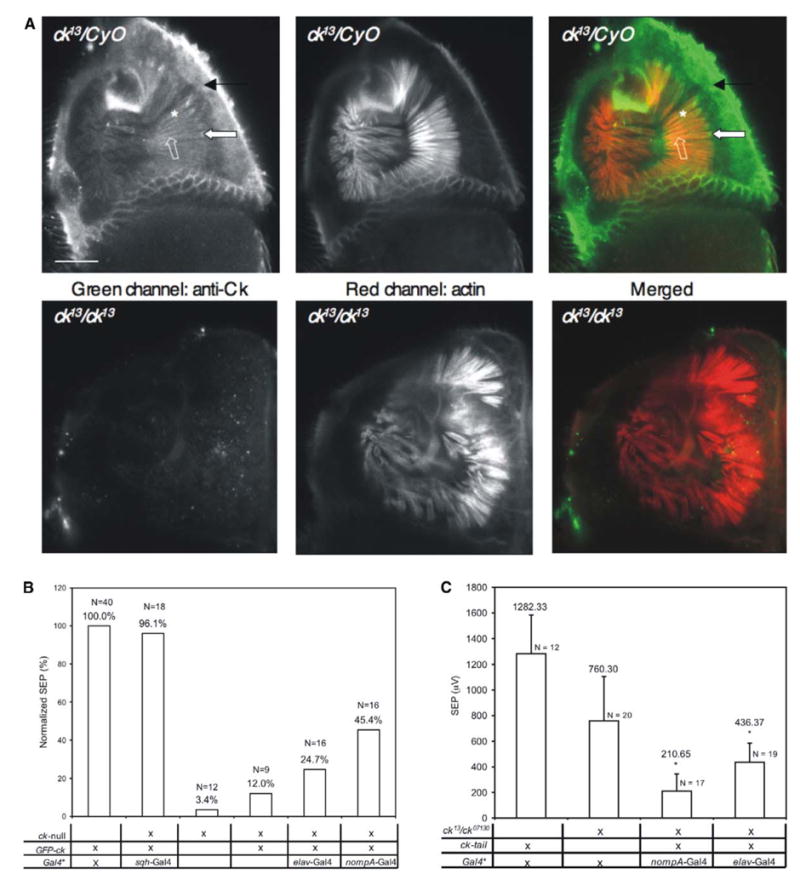
(A) Anti-ck/myoVIIA protein antibody labeling endogenous ck/myoVIIA protein in adult ck13/CyO antenna. Rhodamine-phalloidin-stained actin is shown. The arrow indicates neuronal expression; the asterisk, scolopale-cell expression; the block arrow, basal body accumulation; and the open block arrow, apical accumulation. No specific labeling was detected in mutants. Cuticular autofluorescence is observed in both channels. The scale bar represents 20 μm.
(B) Full rescue was obtained with sqh-Gal4 (ubiquitous expression). Partial rescue was obtained with nompA-Gal4 (scolopale cells) and elav-Gal4 (neurons). Basal rescue was observed in the absence of any driver, but significantly less than with elav-Gal4 (p < 0.001).
(C) myoVIIA/ck tail significantly reduced transduction when expressed in either neurons or scolopale cells (p < 0.001). Error bars indicate standard deviations. For (B) and (C), Gal4*: Gal4 is either elav-Gal4 or nompA-Gal4; no significant difference was found between them. “X” denotes the presence of mutant/construct/driver in genotype.
To examine the requirement for ck/myoVIIA in different cell types of JO, we generated an N terminus, GFP-linked ck/myoVIIA rescue construct (GFP-Ck) selectively expressed with the Gal4-UAS system. Expression in scolopale cells (with nompA-Gal4 [14]) or neurons (with elav-Gal4) alone led to significant, albeit incomplete, rescue of the auditory phenotype (Figure 3B). Ubiquitous expression (sqh-Gal4), on the other hand, provided full rescue (Figure 3B). Confocal imaging indicated some attachment in both scolopale-cell- and neuronal-rescued flies and full attachment with ubiquitous rescue (not shown), further supporting a correlation between degrees of deafness and degrees of detachment/disorganization in JO. Preliminary results from experiments in which we manipulate temporal expression of rescue with Gal80ts, a temperature-sensitive inhibitor of Gal4, suggest that ck/myoVIIA is required for ongoing maintenance of JO organization even in mature flies (not shown).
Rescue results were supported by experiments conducted with a ck/myoVIIA tail construct that functions as a dominant negative (J.D.F. and D.P.K., unpublished data). GFP replaced the motor head and part of the neck of ck/myoVIIA. Expressing this construct in scolopale cells or neurons in a sensitized ck/myoVIIA background (ck07130/ck13) significantly reduced SEPs, consistent with ck/myoVIIA functional requirement in both cell types (Figure 3C).
Because neurons are not known to play a role in apical attachment, ck/myoVIIA’s function in neurons may be directly or indirectly linked to the transduction process. ck/myoVIIA may strengthen neuron-scolopale cell junctions from the neuronal side to ionically isolate the scolopale space, anchor membrane elements to underlying structures, and/or transport/localize elements such as transduction channels.
In scolopale cells, ck/myoVIIA may participate in transporting or localizing proteins that are involved in generating or maintaining the cap by moving along scolopale rods, or it may strengthen cell-cell junctions from the scolopale-cell side. These functions are consistent with our observations that ck/myoVIIA protein is enriched near these junctions. Its requirement in scolopale cells is most likely tied to ck/myoVIIA function in apical attachment and therefore to acoustic stimulus propagation from the a2/a3 joint to transducing elements.
ck/myoVIIA Is Not Necessary for Cell-Cell Junction Integrity in JO
Our data indicate that scolopidial detachment occurs apical to the cap cell. Thus, the scolopidium itself remains as a unit. Within this scolopidial unit, ck/myoVIIA protein concentrates near adherens junctions between neurons and scolopale cells and between scolopale cells and cap cells (Figure 3A). Previous reports indicate that myoVII plays a role during initial steps of cell adhesion necessary for phagocytosis, cell-cell interactions, and filopodia formation in Dictyostelium discodeum [16, 17]. However, evidence from vertebrates indicates that myoVIIA does not play a role in cell adhesion in early-stage phagocytosis in the retinal pigmented epithelium, although it is important for proper ingested phagosome processing [18]. Also, although vertebrate myoVIIA associates with adherens junctions in the auditory organ [5], no data have elucidated an exact role for myoVIIA here. Thus, myoVIIA may play organism-wide roles in cell adhesion and association of extracellular elements to actin filaments, at least in some taxa.
To investigate whether ck/myoVIIA is important for scolopidial cell-cell junction integrity, we used Rhodamine-Dextran (10 kDa; see [19, 20]; see Figure S1A in the Supplemental Data available with this article online) or the much smaller Lucifer Yellow (MW: 457.23; Figure S1B) to show that junctions are not detectably compromised in ck/myoVIIA mutants. The markers are excluded to the same extent from the scolopale space in mutant and control antennae (ck13/CyO; Figures S1A and S1B). However, if ck/myoVIIA protein is involved in regulating adherens junction strength, it could affect tensile stretch on structures, such as the scolopale rods, associated with adherens junctions and affect the dendrite (like the string on a bow [9]) while not affecting junctional integrity per se. This may affect the overall sensitivity of JO neurons to physical stimulation. In conclusion, ck/myoVIIA is not important for cell-cell junction integrity in detached scolopidia.
ck/myoVIIA Is Not Important for Transporting Two Unrelated Proteins
To explore the possibility that ck/myoVIIA is involved in protein transport/localization, we examined localization of the vanilloid-related transient receptor potential (TRPV)-channel subunit Inactive (Iav). Iav and Nanchung transduce sounds in JO and require each other for localization along the ciliated dendrite [21, 22]. We reasoned that ck/myoVIIA protein concentrated in the inner dendritic segment may shuttle ion channels into the cilium because myoVIIA reportedly is involved in opsin transport in vertebrate photoreceptors [23] and may transport harmonin 1b in inner-ear hair cells [6]. We used a GFP- tagged iav genomic construct in ck/myoVIIA mutants and found that its localization is similar to controls (ck13/CyO; Figure 4A). Thus, ck/myoVIIA is not necessary for Iav transport/localization in JO. Nonetheless, it remains possible that ck/myoVIIA transports other neuronal proteins.
Figure 4. Localization of Iav-GFP and NompA-GFP in ck/myoVIIA Mutants.
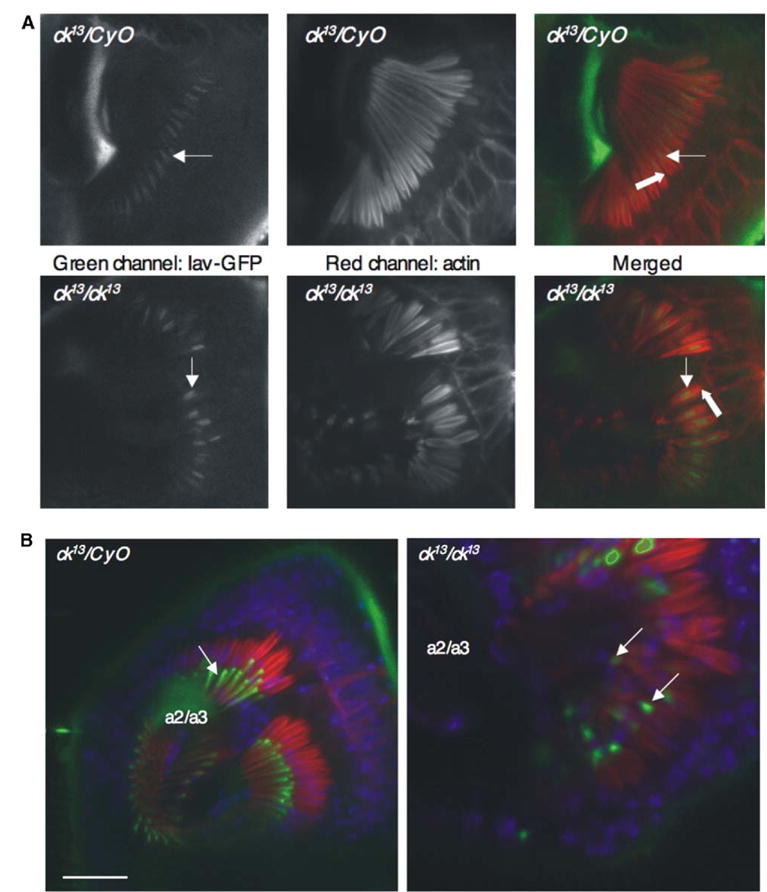
(A) Both control (ck13/CyO) and mutant antennae show similar localization of Iav-GFP at the dendritic cilium (arrows) above the location where ck/myoVIIA protein presence is concentrated at the basal body (block arrow).
(B) NompA-GFP localization (arrows, green) is unperturbed in ck/myoVIIA antennae in comparison to controls (ck13/CyO), although the cap is fully detached from a2/a3. Blue, TO-PRO3 nuclear labeling; red, actin filaments labeled with Texas Red-phalloidin. The scale bar represents 20 μm.
We also examined the possibility that ck/myoVIIA transports NompA, a zona pellucida (ZP) domain protein secreted by the scolopale cell into the dendritic cap [14]. As summarized in Figure 4B, NompA-GFP protein localizes at the dendritic cap in both control (ck13/CyO) and ck/myoVIIA mutant antennae; it does not accumulate within scolopale cells of ck/myoVIIA mutants as it would in the case of faulty transport. Thus, ck/myoVIIA is unnecessary for NompA transport. Nonetheless, it may still be necessary for transport of other cap components that could explain the detachment phenotype. Finally, this marker confirms that the cap is fully detached from the a2/a3 joint.
In conclusion, we have shown that Drosophila ck/myo-VIIA plays important structural, organizational, and physiological roles in auditory transduction by functioning in support and neuronal cells. ck/myoVIIA is expressed by JO neuronal and scolopale cells. In scolopale cells, ck/myoVIIA may be involved in dendritic-cap secretion (though not of NompA) through intracellular transport of cap components and thus become involved in apical scolopidial attachment. Finally, although ck/myoVIIA protein is enriched near JO cell junctions, it is not crucial for their integrity. Near the basal body area, ck/myoVIIA protein may be involved instead in recruiting components other than the TRPV-channel subunits, whose localization does not appear to be ck/myoVIIA dependent. At the apical location, it may also provide tensile forces crucial to the operation of scolopidia by tightening cell junctions. Future studies in this excellent, versatile model organism will define developmental roles for ck/myoVIIA in JO organization and physiological roles in auditory transduction.
Supplemental Data
Acknowledgments
Thanks to M. Kernan and S. Sweeney for fly stocks. We thank Grace Boekhoff-Falk for suggesting wider scolopale bases in detached scolopidia, D. Abel for electron microscopy, and C. Faber for her help with Dextran and Lucifer Yellow labeling. Thanks to the Central Microscopy Research Facilities (CMRF) at the University of Iowa for use of confocal and electron microscopes. This work was supported by American Heart Association grant 0410077Z to S.V.T. and National Institutes of Health grants #GM07184 to J.D.F., #GM33830 to D.P.K., and #DC04848 to D.F.E.
Footnotes
Detailed Experimental Procedures and a supplemental figure are available at http://www.current-biology.com/cgi/content/full/15/9/862/DC1/.
References
- 1.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 2.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SD. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 3.Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: A zebra-fish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- 4.Self T, Mahoney M, Fleming J, Walsh J, Brown SDM, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- 5.Küssel-Andermann P, El-Amraoui A, Safieddine S, Nouaille S, Perfettini I, Lecuit M, Cossart P, Wolfrum U, Petit C. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 2000;19:6020–6029. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SDM, Richardson GP, Steel KP. Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- 8.Kiehart DP, Franke JD, Chee MK, Montague RA, Chen TL, Roote J, Ashburner M. Drosophila crinkled, mutations of which disrupt morphogenesis and cause lethality, encodes fly myosin VIIA. Genetics. 2004;168:1337–1352. doi: 10.1534/genetics.104.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todi SV, Sharma Y, Eberl DF. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech. 2004;63:388–399. doi: 10.1002/jemt.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc. Natl Acad Sci USA. 1995;92:9815–9819. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y-d, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–1307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfrum U, Liu X, Schmitt A, Udovichenko I, Williams D. Myosin VIIa as a common component of cilia and microvilli. Cell Motil Cytoskeleton. 1998;40:261–271. doi: 10.1002/(SICI)1097-0169(1998)40:3<261::AID-CM5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Göpfert MC, Robert D. Turning the key on Drosophila audition. Nature. 2001;411:908. doi: 10.1038/35082144. [DOI] [PubMed] [Google Scholar]
- 14.Chung YD, Zhu J, Han Y, Kernan MJ. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron. 2001;29:415–428. doi: 10.1016/s0896-6273(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 15.Eberl DF, Hardy RW, Kernan M. Genetically similar transduction mechanisms for touch and hearing in Drosophila. J Neurosci. 2000;20:5981–5988. doi: 10.1523/JNEUROSCI.20-16-05981.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Titus MA. A class VII unconventional myosin is required for phagocytosis. Curr Biol. 1999;9:1297–1303. doi: 10.1016/s0960-9822(00)80051-2. [DOI] [PubMed] [Google Scholar]
- 17.Tuxworth RI, Weber I, Wessels D, Addicks GC, Soll DR, Gerisch G, Titus MA. A role for myosin VII in dynamic cell adhesion. Curr Biol. 2001;11:318–329. doi: 10.1016/s0960-9822(01)00097-5. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs D, Kitamoto J, Williams DS. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc Natl Acad Sci USA. 2003;100:6481–6486. doi: 10.1073/pnas.1130432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb RS, Ward RE, Schweizer L, Fehon RG. Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells. Mol Biol Cell. 1998;9:3505–3519. doi: 10.1091/mbc.9.12.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J Cell Biol. 2003;161:991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Chung YD, Park DY, Choi S, Shin DW, Soh H, Lee HW, Son W, Yim J, Park CS, et al. A TRPV family ion channel required for hearing in Drosophila. Nature. 2003;424:81–84. doi: 10.1038/nature01733. [DOI] [PubMed] [Google Scholar]
- 22.Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Udovinchenko IP, Brown SDM, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


