Abstract
PURPOSE:
To measure total corneal power using optical coherence tomography (OCT).
SETTING:
Refractive surgery practices at 2 academic eye centers in Cleveland, Ohio, and Los Angeles, California, USA.
METHODS:
Thirty-two eyes of 17 patients having myopic laser in situ keratomileusis (LASIK) were enrolled in a prospective observational study. Manifest refraction, OCT, and Placido ring corneal topography with the Atlas 995 (Carl Zeiss Meditec, Inc.) were performed preoperatively and 3 months after laser in situ keratomileusis (LASIK). A high-speed (2000 axial scans/second) corneal and anterior segment OCT prototype was used. The total corneal power was calculated by summation of the anterior and posterior surface powers, and the value was compared with that determined by simulated keratometry. Two methods of measuring total corneal power were tested: the direct method, which used OCT to measure both corneal surfaces directly, and the hybrid method, which combined OCT with anterior corneal topography.
RESULTS:
The repeatability (pooled standard deviation) of measuring total corneal power using the hybrid method was 3 times better than that using the direct method. It was 0.23 diopter (D) before LASIK and 0.26 D after LASIK. Preoperative total power was 1.13 D (2.6%) lower than the simulated keratometry. Compared to the LASIK-induced change in spherical equivalent refraction, the change in total corneal power was equivalent, while the change in simulated keratometry power was significantly smaller (-18.8%) (P<.001).
CONCLUSIONS:
Keratometry using the traditional index of 1.3375 overestimated the total power in preoperative corneas and underestimated LASIK-induced refractive change. Measuring both corneal surfaces using a combination of OCT and Placido ring topography provided a better measure of total corneal power that closely tracked the refractive change in post-LASIK eyes.
Corneal power is determined by the 2 refractive surfaces at the anterior and posterior boundaries. It is commonly measured by manual keratometry or simulated keratometry output from computerized corneal topography systems. Because keratometry measures only the central 3.0 mm area1 on the anterior corneal surface, 2 assumptions are needed to extrapolate corneal power. First, the corneal surface has uniform curvature and power. Second, the posterior/anterior curvature ratio maintains a fixed value across the normal population. This second assumption is implicit in the keratometric index of 1.3375 that is traditionally used to calculate corneal power. The index itself is lower than the actual corneal refractive index, 1.376,2 to compensate for the negative power of the posterior surface.
After laser in situ keratomileusis (LASIK) or photorefractive keratometry (PRK), however, keratometry no longer provides an accurate measurement of corneal power because the 2 assumptions no longer hold. The anterior corneal surface is modified in the central optical zone by the laser; however, it is not modified in the peripheral cornea, causing the anterior surface power to become non-uniform. As a result of healing effects, even the power within the nominal optical zone can become nonuniform.3 Furthermore, because the posterior surface is not modified by laser ablation, the posterior/anterior curvature ratio is no longer fixed after LASIK and PRK. After laser correction for myopia, the ratio increases; therefore, keratometry overestimates postoperative corneal power and underestimates the surgically induced refractive change.4,5 This can cause a problem in subsequent cataract surgery. The usual keratometry-based intraocular lens (IOL) power formula calculates an IOL with insufficient power, which results in unintended hyperopia after cataract surgery.4-8
Direct measurement of powers of both corneal surfaces avoids making these assumptions and may be more accurate in post-LASIK eyes. Optical coherence tomography (OCT)9 can measure both corneal surfaces and is a possible approach for direct corneal power measurements. Commercial retinal OCT systems have been used to measure central corneal thickness10 and LASIK flap thickness.11 Using OCT to measure corneal power, however, requires a higher scanning speed because surface contour measurement is more susceptible to motion error. Motion error can not be corrected by image processing and can only be reduced by higher scanning speed. As a consequence, it is not possible to use current commercial retinal OCT scanners with scan rates of 100 or 400 axial scans (A-scans)/second to measure corneal power.
In this study, we used a high-speed cornea and anterior segment OCT (CAS-OCT) prototype (Carl Zeiss Meditec, Inc.) to measure total corneal power. Similar high-speed OCT systems have been used to measure anterior chamber width12 and angle.13 Although this OCT system was capable of 2000 A-scans/second, we were not sure whether this speed was enough for direct surface power measurements. Therefore, we developed 2 alternative approaches. The direct method used OCT to measure both corneal surfaces directly, while the hybrid method combined anterior surface measurements by Placido ring corneal topography and corneal thickness maps measured by OCT. We compared the total power before LASIK with the simulated keratometry power. The total and simulated keratometry powers were then compared with manifest refractions to see which measurement performed best in tracking LASIK-induced refractive change.
PATIENTS AND METHODS
Thirty-two eyes (17 patients) that had primary myopic LASIK were enrolled in a prospective observational study protocol. The study protocol was approved by the Institutional Review Boards of the Cleveland Clinic and Doheny Eye Institute.
Placido ring topography (Atlas 995 unit, Carl Zeiss Meditec, Inc.) and OCT measurements were taken preoperatively and 3 months after LASIK. Manifest refraction was measured using standard Snellen charts. The LASIK-induced change in manifest refractive spherical equivalent (MRSE) was used as the reference for changes in various corneal power measures. Both OCT and Placido ring topographic measurements were repeated 3 times at each visit to evaluate repeatability.
Optical coherence tomography scans were performed with a CAS-OCT prototype that operated at a 1.3 mm wavelength and had a scanning speed of 2000 A-scans/second. The system had a lateral resolution of 45 mm and an axial resolution of 17 mm (full-width-half-maximum in corneal tissue). A pachymetry map scan pattern was used to measure the anterior and posterior corneal surfaces. The pattern consisted of 8 evenly spaced radial lines (10.0 mm long) centered at the corneal vertex (Figure 1, A). Each radial line consisted of 128 axial scans and provideda meridional cross-sectional image (Figure 1, B). Boundaries of anterior and posterior corneal surfaces were identified from OCT images by a custom computer algorithm written in Matlab 7.0 (The Mathworks, Inc.). Optical coherence tomography anterior and posterior elevation maps were then generated by interpolation. The corneal thickness map was the difference between anterior and posterior elevation maps.
Figure 1.
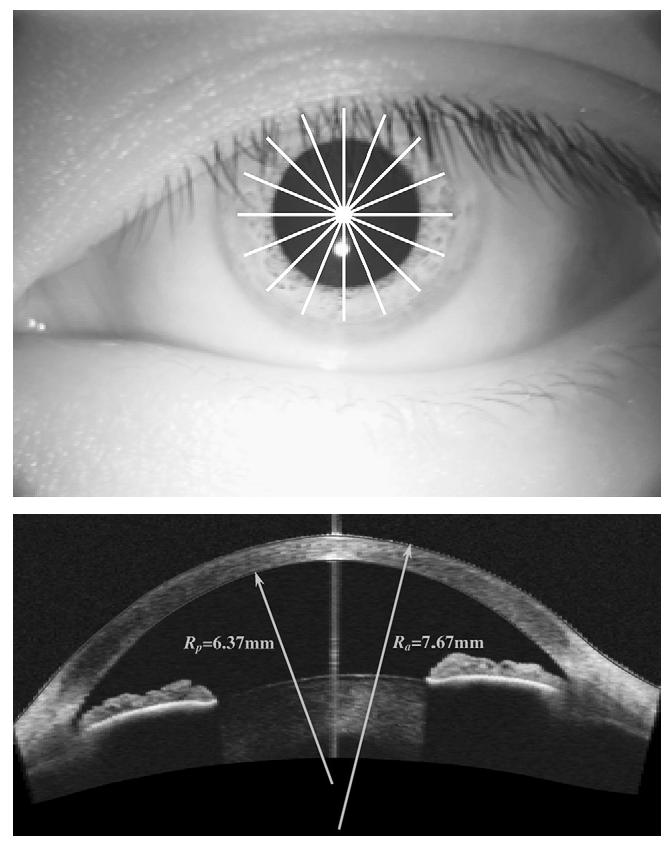
Optical coherence tomography imaging and image processing. Top: Optical coherence tomography topography scan pattern consisting of 8 radial lines. Bottom: Optical coherence tomography meridional crosssectional image from a radial line scan. The best-fit circular curves are overlaid on the anterior and posterior corneal boundaries. The numbers shown are population means of the anterior and posterior radii of curvature.
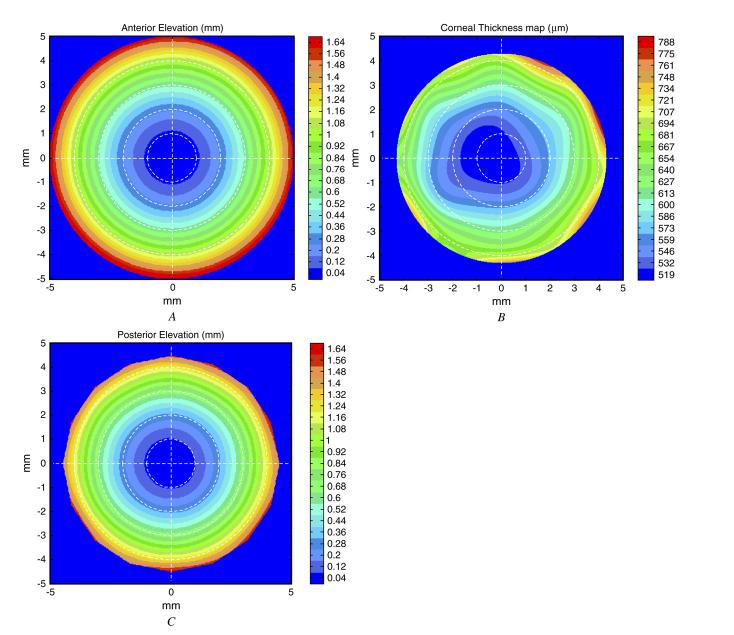
In addition to the above method that used OCT to measure both corneal surfaces directly, a hybrid method combining OCT and Placido ring topography was tested. In this method, the anterior elevation was measured by Placido ring topography (Figure 2, A). The summation of the anterior elevation measured by corneal topography and the OCT-measured thickness map (Figure 2, B) was used to calculate the posterior elevation map (Figure 2, C).
Figure 2.
Hybrid method of corneal power calculation. A: Anterior corneal elevation map from Placido ring topography. B: Optical coherence tomography-derived corneal thickness map. C: Posterior corneal elevation map calculated by adding A and B.
The optical powers of the anterior and posterior surfaces were calculated by the radii of best-fit spheres (BFS) over the central 3.0 mm diameter circle on the elevation maps. The total power was then calculated by
| (1) |
where Pt is the total corneal power, Pa is the anterior surface power, Pp is the posterior surface power, Ra is the anterior surface radius of BFS, Rp is the posterior surface radius of the BFS, n0 is the refractive index of air, n1 is the refractive index of the cornea, and n2 is the refractive index of the aqueous humor. The values for n0, n1, and n2 were 1, 1.376,2 and 1.336,2 respectively.
The mean central keratometric power (central K) was calculated based on the anterior surface only rather than on both surfaces as follows:
| (2) |
where Pk is the central K and nk is the keratometric index. The value for nk was 1.3375, and the values for n0 and Ra were as defined above.
As a keratometric measure, central K was different from simulated keratometry because simulated keratometry only averaged power along the 3.0 mm ring, neglecting the region within. In contrast, central K averaged the entire 3.0 mm region. Because an earlier study showed that corneal power measurements over the 4.0 mm diameter better correlated with LASIK-induced refractive change,14 the total power and central K power over the central 4.0 mm diameter were also calculated.
The repeatability of corneal power measurements was evaluated by pooled standard deviation. Generalized estimating equations were used to calculate the error margins (standard error, P value) of statistical tests. Because both eyes from the same patient were highly correlated, treating the eyes as independent data points may overestimate the degree of freedom and lead to invalid statistical inference. The generalized estimating equations approach developed by Zeger et al.15 use a generalized linear model and account for within-subject correlation of dependent variables. This allowed the use of data from both eyes without overstating statistical inferences.16 Statistical analysis was perform using SAS 9.0 (SAS Institute, Inc.).
RESULTS
Twenty-seven eyes (14 patients) were enrolled at the Cleveland Clinic from October 22, 2003, to April 22, 2004, and 5 eyes (3 patients) were enrolled at Doheny Eye Institute from May 6, 2005, to September 1, 2005. The LADARVision excimer laser system (Alcon Laboratories, Inc.) was used at the Cleveland Clinic and the Visx S4 laser at the Doheny Eye Institute. The optical zone diameter was 6.5 mm in 25 eyes, 6.0 mm in 5 eyes, and 5.5 mm in 1 eye. Six eyes had conventional LASIK without a transition zone. Others had wavefront-guided LASIK, which included a transition area that increased the total ablation zone diameter to 8.0 mm. The mean laser setting was -4.46 diopters (D) ± 1.87 (SD) (range -1.75 to -8.75 D) sphere with 0.62 ± 0.54 D (range 0 to 2.25 D) cylinder.
The OCT-only method yielded repeatability in posterior power measurements comparable to those using the hybrid method (topography + OCT); however, the repeatability of the anterior power measurement using the OCT-only method was worse than that of the posterior power measurement by a factor of 9. As a result, the repeatability of total corneal power based on the OCT-only method was worse than the hybrid approach (Table 1). The hybrid method produced a repeatability (approximately 0.25 D) similar to that of conventional simulated keratometry. The hybrid approach will be discussed in this article.
Table 1.
Repeatability of corneal power measurements (17 patients, 32 eyes).
| Preop Corneal Power (D) |
Postop Corneal Power (D) |
|||
|---|---|---|---|---|
| Measurement and Method | Mean | Repeatability | Mean | Repeatability |
| Keratometric | ||||
| SimK | 43.85 | 0.19 | 40.95 | 0.24 |
| Central K | 43.99 | 0.22 | 40.96 | 0.25 |
| Anterior | ||||
| OCT only | 51.66 | 0.79 | 47.84 | 0.73 |
| Topography | 49.00 | 0.24 | 45.63 | 0.28 |
| Posterior | ||||
| OCT only | 6.56 | 0.10 | 6.49 | 0.087 |
| OCT + topography | 6.29 | 0.068 | 6.27 | 0.079 |
| Total | ||||
| OCT only | 45.11 | 0.71 | 41.35 | 0.66 |
| OCT + topography | 42.72 | 0.24 | 39.36 | 0.26 |
OCT = optical coherence tomography; SimK = simulated keratometry
A comparison between different types of corneal powers in normal preoperative eyes found a central K power slightly higher than the simulated keratometry power (Figure 3, A); the mean difference was 0.13±0.18 D (P<.001). However, the difference was less than the measurement repeatability (Table 1) for simulated keratometry (0.19 D) and central K (0.22 D). On the other hand, the total corneal power was highly significantly lower than the simulated keratometry power (Figure 3, B); the mean difference was -1.13 ± 0.21 D (P<.0001).
Figure 3.
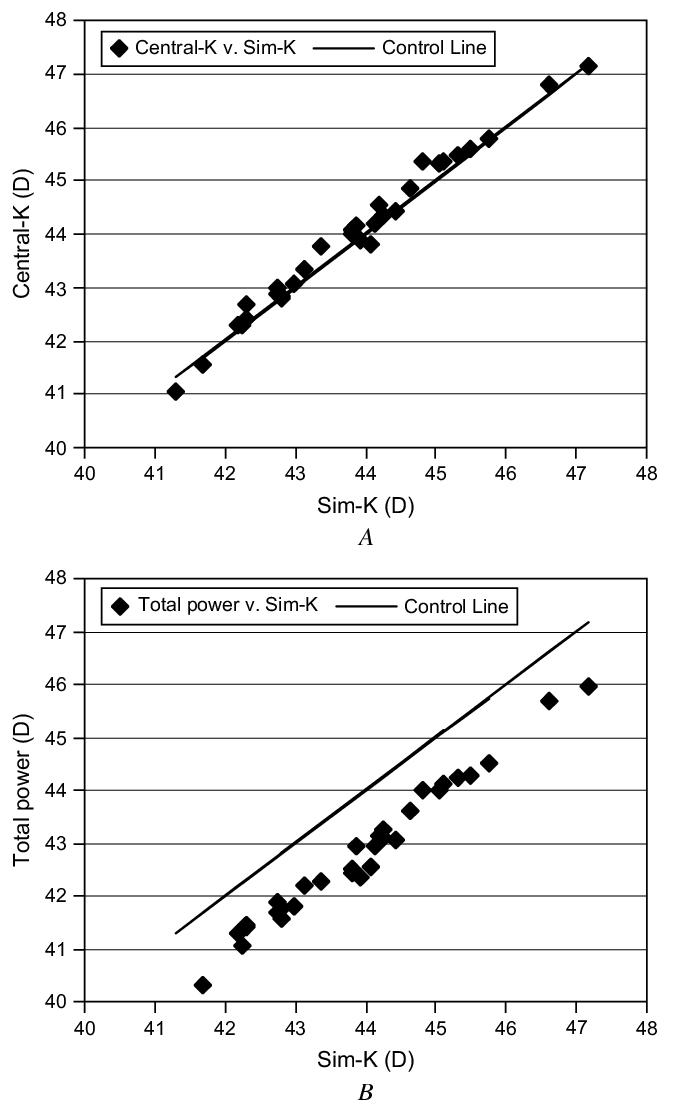
Relationship between different types of corneal power measured preoperatively. A: Central K versus simulated keratometry B: Total power versus simulated keratometry.
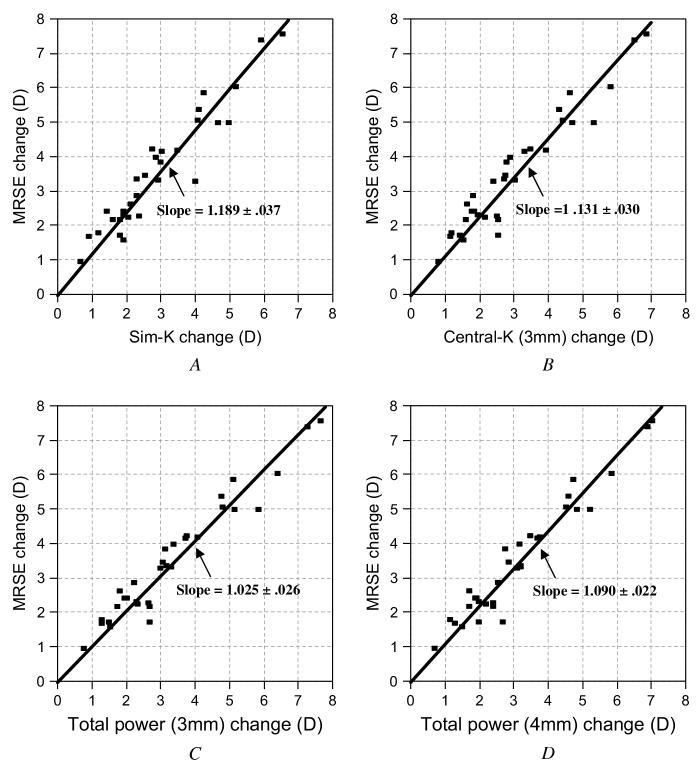
Changes in simulated keratometry, central K, and total power induced by LASIK were compared with the MRSE. Based on the slopes (Figure 4), all corneal power measures underestimated the change in MRSE (slope O1). Keratometric measures by simulated keratometry and central K, which are based on the anterior corneal surface alone, produced the greatest underestimations. Simulated K (Figure 4, A) underestimated by 18.9%, and central K(Figure 4, B) underestimated by 13.1%. The total corneal power tracked the MRSE more closely. The total corneal power change in the 3.0 mm zone (Figure 4, C) and the 4.0 mm zone (Figure 4, D) underestimated the MRSE change by 2.5% and 9.0%, respectively. The total corneal power change over the central 3.0 mm was statistically equivalent to the MRSE change based on the linear regression (slope = 1.025; P =.33 compared with a slope of 1). All other corneal power measures had slopes that were significantly different from 1 (P<.001).
Figure 4.
Relationship between LASIK-induced changes in corneal power measures and MRSE changes. The linear regression was performed with the intercept set to zero. The slope and its standard error are shown with the plots. A: MRSE versus. simulated keratometry. B: The MRSE versus central K (3.0 mm). C: The MRSE versus Total power (3.0 mm) D: The MRSE versus total power (4.0 mm).
The same pattern of underestimation emerged when the comparison was made in terms of diopters rather than slope (Table 2). All measures of corneal power change correlated well with the MRSE change but produced underestimations. Simulated K and central K produced worse underestimations than total cornea power. Measurements based on the central 4.0 mm zone produced greater underestimation than those based on the central 3.0 mm zone. Although all differences between corneal power change and MRSE change were statistically significant (P<.05), the total power in the 3.0 mm zone came closest to tracking refraction, with a mean difference of 0.16 D.
Table 2.
Mean difference between LASIK-induced changes in corneal power measures and MRSE change (17 patients, 32 eyes).
| Parameter | Mean Difference±SD | P Value* | Correlation (r2) |
|---|---|---|---|
| SimK | -0.60±0.55 | <.001 | 0.91 |
| Total power (3.0 mm) | -0.16±0.44 | .04 | 0.94 |
| Total power (4.0 mm) | -0.33±0.43 | <.001 | 0.94 |
| Central K (3.0 mm) | -0.48±0.49 | <.001 | 0.92 |
| Central K (4.0 mm) | -0.65±0.50 | <.001 | 0.93 |
MRSE = manifest refractive spherical equivalent; SimK = simulated keratometry
P values are for the null hypothesis that the difference between corneal power change and MRSE change is zero. Generalized estimation equations were used to calculate the P values.
The mean LASIK-induced change in posterior power was 0.03±0.12 D. It was not significantly different from zero (P =.16).
DISCUSSION
The CAS-OCT system used in this study is 5 times faster than the current standard commercial retinal OCT system, the Stratus OCT (Carl Zeiss Meditec); however, the repeatability of corneal power measurements by the OCT-only method remains below standard keratometry. The variability is most likely a result of motion error. Surface contour measurement is exquisitely sensitive to axial eye movement. A 10 mm axial sag error within the central 3.0 mm diameter can produce up to 3.00 D of error in corneal power. This amount of movement is plausible within the 19 msec required for our CAS-OCT system to scan across 3.0 mm. Thickness measurement, on the other hand, is much more robust. Axial eye motion between A-scans moves the anterior and posterior surfaces equally and therefore has little effect on the thickness measurement. Axial motion within each A-scan could affect thickness measurements. But because each A-scan takes only 0.5 msec and only 15% of that time (0.075 msec) is required to scan across 600 mm of corneal thickness, there is virtually no motion on this time scale. Thus, the repeatability of corneal power measurements by the hybrid method is much better than that using the OCT-only method. We found the repeatability of the hybrid method was generally comparable to that of simulated keratometry.
We have not reached the intrinsic speed limit of OCT, however. Current high-speed Fourier-domain OCT technology has a scanning speed of 29 kHz17 using spectrometric detection and 115 kHz using a swept-source design.18 Thus, at least a further 57-fold improvement in speed is possible. If motion error decreases linearly with scanning speed, it is possible for a faster OCT system (115000 A-scan/second) to achieve excellent repeatability of 0.02 D using the OCT-only method. This would be significantly better than the repeatability of the simulated keratometry power estimate (0.19 to 0.24 D) in the current study.
We were surprised to find that the simulated keratometry power was higher than the total power in normal preoperative eyes. We wondered whether this discrepancy could be attributed to the traditional keratometric index of 1.3375. To investigate the anatomic assumptions for the keratometric index mentioned earlier, we performed the following analysis:Define c as the ratio of anterior and posterior radii of curvature
then total corneal power (equation 1) is described by
| (3) |
Comparison of equation 3 and equation 2 clearly shows that for the keratometric index to be 1.3375, c must have a value of 0.963. This implies that the posterior surface has a larger radius than the anterior surface and that the cornea is thicker in the center than in the periphery. We know this is not true for a normal cornea.19 Therefore, the traditionally accepted keratometric value does not agree with normal anatomy. Our data indicate that in the normal (preoperative) eye, the mean radius of curvature for the anterior surface and posterior surface is 7.67 mm and 6.37 mm, respectively. Thus, the mean anterior/posterior curvature ratio c is 1.204. When this value is used in equations 2 and 3, the mean keratometric index for pre-LASIK eyes is 1.3278 rather than 1.3375. A theoretical study20 also suggests a lower keratometric index of 1.3315 should be used to provide an accurate estimate of corneal power.
However, we do not advocate simply changing the keratometric index to 1.3278 in clinical practice. In the United States, clinical systems for calculating IOL power have built-in compensations for the bias introduced by using the traditional keratometric index of 1.3375. Contact lenses and topography systems are calibrated using the traditional keratometric index. A whole new system of instrument calibration and clinical software is needed to convert to a correct evidence-based keratometric index.
After myopic LASIK, we expected the keratometric power to overestimate corneal power for 2 theoretical reasons. First, the anterior surface becomes nonuniform and oblate with the central cornea flatter than the periphery. Second, the anterior/posterior radii ratio increases.
The first reason that simulated keratometry would overestimate post-LASIK corneal power is that it measures only the 3.0 mm ring and neglects the flatter central region. This expectation was confirmed by the finding that central K, which averaged power over the central 3.0 mm zone, more closely tracked refractive change than simulated keratometry. Empirically, we found that measurements made over the central 3.0 mm area agreed better with refraction than those over 4.0 mm diameter, although corneal powers measured at both diameters correlated well with refraction (r2 Z 0.94). The theoretical reason for this is complex. We speculate that after myopic LASIK, the central 3.0 mm area of the cornea is significantly more uniform in power than 4.0 mm or wider areas. The uniform refractive power provides a sharper peak on the retinal point-spread function and a better endpoint for clinical refraction.
The ratio between the radii of anterior and posterior corneal surfaces changes after LASIK. Myopic LASIK flattens the anterior surfaces while retaining the shape of the posterior surface. This means the anterior/posterior radius ratio c becomes larger. Because this ratio depends on the magnitude of the laser correction and is variable, no single keratometric index can provide accurate keratometry in all post-LASIK corneas. Thus, to estimate the effective keratometric index after myopic LASIK, Jarade et al.21 divided post-LASIK corneas into subgroups based on the magnitude of laser correction. In their study, the effective keratometric index after myopic LASIK ranged from 1.3355 for the subgroup with ablations less than 4.00 D to 1.3172 in eyes with ablations greater than 12.00 D. To avoid this problem, both the anterior and posterior corneal surfaces must be measured to calculate the corneal power in post-LASIK eyes.
Total power measures both corneal surfaces and avoids use of the keratometric index. Therefore, we expected it would measure post-LASIK corneal power more accurately, and our results agreed. Total power was in better agreement with refraction changes than simulated keratometry or central K power. The difference between total power changes and LASIK-induced refraction change is 0.16 D, 2.5% by linear regression, while simulated keratometry underestimated refraction change by 0.60 D, 18.9% by linear regression.
Slit-scan-based corneal topography systems such as the Orbscan (Orbtek, Inc.), Orbscan II (Bausch & Lomb, Inc.), and Pentacam (Oculus GmbH) can also measure both surfaces of the cornea and calculate total corneal power. Srivannaboon et al.14 compared Orbscan-derived total optical power, which is based on measurement of both surfaces, with the mean axial power, which is based on measurement of the anterior surface alone. The total optical power was similar to the total corneal power and the mean axial power was similar to the central K in our study. They showed total optical power is much better correlated with LASIK-induced MRSE change than with the mean axial power. In their study of 20 eyes, the correlation coefficient between the total optical power and MRSE change was 0.853 for the 4.0 mm analytical zone and 0.841 for the 3.0 mm zone. They drew no conclusion on the optimum zone in terms of agreement between total optical power and MRSE, but their data showed an analytic zone diameter between 3.0 and 4.0 mm would produce the best agreement.
In another study, Sónego-Krone et al.22 found that MRSE change after LASIK was best estimated by 4.0 mm total optical power from Orbscan. For the 4.0 mm total optical power, the mean difference was -0.08 ± 0.53 D and the correlation coefficient 0.87. In our study, OCT-measured total powers for the 3.0 mm and 4.0 mm zones had an even higher correlation coefficient of 0.94. This suggests that the combination of the Atlas topography system and the high-speed CAS-OCT prototype may be more accurate than the Orbscan. We did not find published data on total corneal power measured by the Pentacam slit-scanning Scheimpflug camera system, perhaps because it is a relatively new product. Theoretically, OCT has the advantage of higher axial resolution and therefore might be more accurate and robust than the slit-scanning systems.
Studies using Orbscan show a forward shift of the posterior corneal surface relative to the BFS after myopic LASIK. The mean shift ranged from 17.2 mm23 to 54.3 mm.24 A forward shift corresponds to an increase in the curvature and power of the posterior surface. The shifts were detected in normal post-LASIK eyes, not in cases of keratectasia.23-25 Therefore, they must represent a normal elastic response of the cornea or an artifact of Orbscan measurement. In our study, we found a slight decrease in posterior curvature after LASIK, but it was not statistically significant. Our result supports the hypothesis that the forward shifts found by Orbscan were measurement artifacts. However, we measured posterior curvature within the central 3.0 mm only while the Orbscan defined forward shift over a much wider area; thus, the comparison is not conclusive.
In this study, we developed 2 methods to measure total corneal power using OCT. To our knowledge, this is the first demonstration of both the OCT-only and the hybrid OCT-Placido ring methods. The critical elements of the more successful hybrid method are that it (1) combines the anterior corneal map from Placido ring corneal topography and corneal thickness map from OCT; (2) calculates corneal power by the BFS over the central area rather than measuring along a ring, as in simulated keratometry; and (3) uses an analytical zone of 3.0 mm diameter, which provides the best agreement with refraction.
In conclusion, the hybrid method provided repeatable measurements of total corneal power that closely tracked refractive change after LASIK. This means corneal power can now be measured accurately before and after LASIK and other keratorefractive surgeries. Thus, OCT may be a key element in providing the data needed for accurate IOL power calculation in eyes after refractive surgery.
Footnotes
Drs. Huang and Tang receive grant support from Carl Zeiss Meditec, Inc. Dr. Huang receives patent royalties related to optical coherence tomography technology. Drs. Li and Avila have no financial or proprietary interest in any material or method mentioned.
Supported by grants from NIH (R01 EY013516 and P30 EY03040), Carl Zeiss Meditec, Inc., and Research to Prevent Blindness, Inc.
REFERENCES
- 1.Henson DB. Optometric Instrumentation. Butter-worths; London, Boston, MA: 1983. [Google Scholar]
- 2.Rabbetts RB. Bennett and Rabbetts’ Clinical Visual Optics. 3rd ed. Butterworth-Heinemann; Oxford, Boston, MA: 1998. [Google Scholar]
- 3.Huang D, Tang M, Shekhar R. Mathematical model of corneal surface smoothing after laser refractive surgery. Am J Ophthalmol. 2003;135:267–278. doi: 10.1016/s0002-9394(02)01942-6. [DOI] [PubMed] [Google Scholar]
- 4.Leyland M. Validation of Orbscan II posterior corneal curvature measurement for intraocular lens power calculation. Eye. 2004;18:357–360. doi: 10.1038/sj.eye.6700659. [DOI] [PubMed] [Google Scholar]
- 5.Seitz B, Langenbucher A, Nguyen NX, et al. Underestimation of intraocular lens power for cataract surgery after myopic photorefractive keratectomy. Ophthalmology. 1999;106:693–702. doi: 10.1016/S0161-6420(99)90153-7. [DOI] [PubMed] [Google Scholar]
- 6.Gimbel H, Sun R, Kaye GB. Refractive error in cataract surgery after previous refractive surgery. J Cataract Refract Surg. 2000;26:142–144. doi: 10.1016/s0886-3350(99)00327-2. [DOI] [PubMed] [Google Scholar]
- 7.Gimbel HV, Sun R, Furlong MT, et al. Accuracy and predictability of intraocular lens power calculation after photorefractive keratectomy. J Cataract Refract Surg. 2000;26:1147–1151. doi: 10.1016/s0886-3350(00)00480-6. [DOI] [PubMed] [Google Scholar]
- 8.Ladas JG, Boxer Wachler BS, Hunkeler JD, Durrie DS. Intraocular lens power calculations using corneal topography after photorefractive keratectomy. Am J Ophthalmol. 2001;132:254–255. doi: 10.1016/s0002-9394(01)00894-7. [DOI] [PubMed] [Google Scholar]
- 9.Huang D, Swanson EA, Lin C-P, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechmann M, Thiel MJ, Neubauer AS, et al. Central corneal thickness measurement with a retinal optical coherence tomography device versus standard ultrasonic pachymetry. Cornea. 2001;20:50–54. doi: 10.1097/00003226-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado MJ, Ruiz-Oblitas L, Munuera JM, et al. Optical coherence tomography evaluation of the corneal cap and stromal bed features after laser in situ keratomileusis for high myopia and astigmatism. Ophthalmology. 2000;107:81–87. doi: 10.1016/s0161-6420(99)00022-6. discussion by DR Hardten, 88. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith JA, Li Y, Chalita MR, et al. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology. 2005;112:238–244. doi: 10.1016/j.ophtha.2004.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–1059. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 14.Srivannaboon S, Reinstein DZ, Sutton HFS, Holland SP. Accuracy of Orbscan total optical power maps in detecting refractive change after myopic laser in situ keratomileusis. J Cataract Refract Surg. 1999;25:1596–1599. doi: 10.1016/s0886-3350(99)00286-2. [DOI] [PubMed] [Google Scholar]
- 15.Zeger SL, Liang K-Y, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. correction1989; 45:347. [PubMed] [Google Scholar]
- 16.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113:586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 17.Nassif NA, Cense B, Park B, et al. In vivo high-resolution video-rate spectral-domain optical coherence tomography of the human retina and optic nerve. Opt Express. 2004;12:367–376. doi: 10.1364/opex.12.000367. [DOI] [PubMed] [Google Scholar]
- 18.Oh WY, Yun SH, Tearney GJ, Bouma BE. 115 kHz tuning repetition rate ultrahigh-speed wavelength-swept semiconductor laser. Opt Lett. 2005;30:3159–3161. doi: 10.1364/ol.30.003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martola EL, Baum JL. Central and peripheral corneal thickness; a clinical study. Arch Ophthalmol. 1968;79:28–30. doi: 10.1001/archopht.1968.03850040030009. [DOI] [PubMed] [Google Scholar]
- 20.Gobbi PG, Carones F, Brancato R. Keratometric index, videokeratography, and refractive surgery. J Cataract Refract Surg. 1998;24:202–211. doi: 10.1016/s0886-3350(98)80201-0. [DOI] [PubMed] [Google Scholar]
- 21.Jarade EF, Abi Nader FC, Tabbara KF. Intraocular lens power calculation following LASIK: determination of the new effective index of refraction. J Refract Surg. 2006;22:75–80. doi: 10.3928/1081-597X-20060101-15. [DOI] [PubMed] [Google Scholar]
- 22.Sónego-Krone S, López-Moreno G, Beaujon-Balbi OV, et al. A direct method to measure the power of the central cornea after myopic laser in situ keratomileusis. Arch Ophthalmol. 2004;122:159–166. doi: 10.1001/archopht.122.2.159. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Chen J, Yang B. Posterior corneal surface topographic changes after laser in situ keratomileusis are related to residual corneal bed thickness. Ophthalmology. 1999;106:406–409. doi: 10.1016/S0161-6420(99)90083-0. discussion by RK Maloney, 409-410. [DOI] [PubMed] [Google Scholar]
- 24.Kamiya K, Oshika T. Corneal forward shift after excimer laser keratorefractive surgery. Semin Ophthalmol. 2003;18:17–22. doi: 10.1076/soph.18.1.17.14070. [DOI] [PubMed] [Google Scholar]
- 25.Twa MD, Roberts C, Mahmoud AM, Chang JS., Jr. Response of the posterior corneal surface to laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2005;31:61–71. doi: 10.1016/j.jcrs.2004.09.032. [DOI] [PubMed] [Google Scholar]