Abstract
Superoxide produced by the enzyme NADPH oxidase mediates crucial intracellular signaling cascades in the mNTS, a brain region populated by catecholaminergic neurons, as well as astroglia that play an important role in autonomic function. The mechanisms mediating NADPH oxidase (phox) activity in the neural regulation of cardiovascular processes are incompletely understood, however the subcellular localization of superoxide produced by the enzyme is likely to be an important regulatory factor. We used immunogold electron microscopy to determine the phenotypic and subcellular localization of the NADPH oxidase subunits p47phox, gp91phox, and p22phox in the mNTS. The mNTS contains a large population of neurons that synthesize catecholamines. Significantly, catecholaminergic signaling can be modulated by redox reactions. Therefore, the relationship of NADPH oxidase subunit labeled neurons or glia with respect to catecholaminergic neurons was also determined by dual labeling for the superoxide producing enzyme and tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis. In the mNTS, NADPH oxidase subunits were present primarily in somatodendritic processes and astrocytes, some of which also contained TH, or were contacted by TH labeled axons, respectively. Immunogold quantification of NADPH oxidase subunit localization showed that p47phox and gp91phox were present on the surface membrane, as well as vesicular organelles characteristic of calcium storing smooth endoplasmic reticula in dendritic and astroglial processes. These results indicate that NADPH oxidase assembly and consequent superoxide formation are likely to occur near the plasmalemma, as well as on vesicular organelles associated with intracellular calcium storage within mNTS neurons and glia. Thus, NADPH oxidase-derived superoxide may participate in intracellular signaling pathways linked to calcium regulation in diverse mNTS cell types. Moreover, NADPH oxidase-derived superoxide in neurons and glia may directly or indirectly modulate catecholaminergic neuron activity in the mNTS.
Keywords: autonomic nervous system, blood pressure, catecholamines, reactive oxygen species, superoxide
Introduction
A critical central coordinator of cardiorespiratory processes, the nucleus of the solitary tract (NTS) is known to modulate systemic arterial pressure (Aicher et al., 2000), cerebral blood flow (Agassandian et al., 2003), respiration (Gozal et al., 2000), and energy balance (Glass et al., 2002). This global modulation is directly related to its unique synaptic organization, which includes the first central synapse of glutamatergic mechano- and chemoreceptor, as well as afferents from the circumventricular organ the area postrema, and other areas of the brainstem-forebrain autonomic neuraxis, including the rostral ventrolateral medulla and amygdala (Barraco, 1994).
Within the mNTS, a wide array of signaling molecules, including small amino acid transmitters, as well as catecholamine and peptide modulators, have been implicated in coordinating autonomic, and particularly cardiovascular processes (Lawrence and Jarrott, 1996). Angiotensin II (ANGII)-induced activation of angiotensin AT-1A receptors in the mNTS has been shown to be an important inhibitor of the baroreflex (Diz et al., 2001), an effect that may be mediated by production of reactive oxygen species (ROS). Indeed, in addition to mediating hypertension-induced cellular plasticity in the peripheral vasculature (Zimmerman and Davisson, 2004, Chan et al., 2005), reactive oxygen is also an important molecular substrate of central autonomic regulation (Zimmerman and Davisson, 2004). For example, the production of ROS within central autonomic pathways is associated with sympathetic nervous system activation (Campese et al., 2004), including stress-induced autonomic activation (Mayorov et al., 2004), as well as salt-induced stroke in stroke-prone spontaneously hypertensive rats (Kim-Mitsuyama et al., 2005).
Many detrimental effects of neurogenic hypertension are mediated by superoxide generated via NADPH oxidase (Wang et al., 2004, Zimmerman and Davisson, 2004, Zimmerman et al., 2005). This enzyme was first described in phagocytes (Lassègue and Clempus, 2003), but is now known to be present in diverse cell types (Serrano et al., 2003, Tejada-Simon et al., 2005, Vallet et al., 2005), including NTS neurons (Wang et al., 2004). NADPH oxidase is comprised of two membrane-bound subunits, gp91phox and p22phox, several mobile cytoplasmic subunits including, p40phox, and p67phox, and the essential p47phox, as well as the small GTPase RAC (Lambeth, 2004, Sumimoto et al., 2005). NADPH oxidase activation can be triggered by intracellular calcium, such as that induced by stimulation of the AT-1A receptor subtype, with subsequent phosphorylation and membrane translocation of p47phox to the gp91phox subunit (Cai et al., 2003, Taniyama and Griendling, 2003).
The subcellular location of superoxide generation is an important factor in redox modulation of intracellular signaling pathways (Wolin, 2004). Significantly, the subcellular location of NADPH oxidase subunits is dependent on cell phenotype. For example, phagocyte p47phox is located within the cytosol, and upon phosphorylation it couples with the predominantly plasmalemmal gp91phox subunit to produce superoxide (Lambeth, 2004). In distinction, in endothelial cells, p47phox and gp91phox have an intracellular distribution, including the cytoskeleton and vesicular organelles, where ROS can be produced tonically or in response to an agonist (Li and Shah, 2002). In dissociated rat mNTS neurons, the potentiation of calcium currents induced by activation of the AT-1A receptor is mediated by ROS production via NADPH oxidase (Wang et al., 2004), however, the basal subcellular distribution of NADPH oxidase subunits in neurons of the mNTS is unknown. In addition to neurons, astrocytes, which are abundant in the mNTS, have also been shown to express functional NADPH oxidase (Abramov et al., 2005), however, the subcellular localization of phox proteins in NTS astroglia is also unknown.
Oxidation is an important modulator of catecholaminergic signaling pathways (Smythies and Galzigna, 1998). At intermediate and caudal levels, the mNTS contains many neurons that express tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine biosynthesis (Zigmond et al., 1989). Catecholaminergic neurons in mNTS are sensitive to disturbance of cardiovascular (Chan et al., 1999), respiratory (Buller et al., 1999), fluid (Gordon et al., 1979), and metabolic (Willing and Berthoud, 1997) homeostasis. In addition, catecholamine containing axon terminals within the mNTS play an important modulatory role in the central regulation of systemic arterial pressure (Snyder et al., 1978). There is also an increase in TH activity in the NTS associated with rodent models of hypertension (Misu et al., 1995, Potts et al., 2000., Ferrari et al., 2002). The activity of TH is regulated by NADPH oxidase activation (Kuhn and Geddes, 2002), and by proteins associated with oxidative stress, such as nitrotyrosine (Kuhn et al., 2002). In addition, a major superoxide metabolite, hydrogen peroxide, is accessible to the extracellular milieu (Lambeth, 2004) and thus capable of influencing closely apposed structures. However, there is no evidence that NADPH oxidase subunits are present in TH containing neurons in the mNTS, or in astrocytes contacted by catecholamine containing axon terminals.
Immunogold electron microscopic cytochemistry has the spatial resolution necessary for the detection of protein immunolabeling at distinct subcellular localizations, and is also compatible with dual immunoperoxidase staining for related antigens. We used immunogold electron microscopy to determine the cellular and subcellular distributions of the gp91phox and p47phox subunits in neuronal and glial processes in the mNTS, at the level of the area postrema. To verify this distribution, immunogold labeling of the p22phox subunit was also determined in this brain region. Dual immunoperoxidase labeling for TH and immunogold detection of NADPH oxidase subunits was performed to determine whether catecholamine containing neurons also expressed p47phox or gp91phox, or were apposed to NADPH oxidase subunit containing glia.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Charles River) weighing 300–400 g were individually housed and maintained on a 12-hr light/dark cycle. All rats had unlimited access to water and rat chow in their home cages. The experimental protocols were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at Weill Medical College of Cornell University. All efforts were made to minimize the number of animals used and their suffering.
Tissue preparation
Rats were anesthetized with sodium pentobarbital (150 mg/kg, i.p.), and their brains were fixed by aortic arch perfusion sequentially with: (a) 15 ml of normal saline (0.9%) containing 1000 units/ml of heparin, (b) 50 ml of 3.75% acrolein in 2% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4), and (c) 200 ml of 2% paraformaldehyde in PB, all delivered at a flow rate of 100 ml/minute. The brains were removed and post-fixed for 30 minutes in 2% paraformaldehyde in PB. Coronal sections 40 μm thick were cut with a vibrating microtome from brainstem at the level of the area postrema according to the atlas of Paxinos and Watson (1986). Tissue sections were next treated with 1.0% sodium borohydride in PB and then washed in PB. Sections then were immersed in a cryoprotectant solution (25% sucrose and 2.5% glycerol in 0.05 M PB) for 15 minutes. To enhance tissue permeability, sections were then freeze-thawed in liquid freon and liquid nitrogen. Sections were next rinsed in 0.1 M Tris-buffered saline (TS, pH 7.6) and then incubated for 30 minutes in 0.1% bovine serum albumin (BSA) to minimize nonspecific labeling.
Antisera
To immunolabel NADPH oxidase subunits, polyclonal goat anti-p47phox, -gp91phox, and -p22phox antisera (Santa Cruz Biotechnology, Santa Cruz, CA) were used. For dual labeling of p47phox and gp91phox a mouse anti- gp91phox antibody (BD Transduction Labs) was used in tandem with the goat anti- p47phox antisera in order to prevent species cross-reactivity. These antisera show recognition of their respective epitopes by Western Blotting, immunoprecipitation, and immunocytochemistry (Manufacturer’s data). Preadsorption of each antisera with its respective blocking peptide prevented detection of immunolabeling (Wang et al., 2004). Catecholamine neurons were labeled with a well-characterized monoclonal mouse antibody raised against an epitope within the midportion of TH (ImmunoStar) (Glass et al., 2001). By western blotting, this monoclonal antibody does not cross-react with other major monoaminergic synthetic enzymes including dopamine-ß-hydroxylase, phenylethanolamine-N-methyltransferase, or tryptophan hydroxylase (Manufacturer’s data). By light microscopy, immunolabeling of brain sections with this antisera produces labeling selectively in catecholaminergic cell groups, and deletion of the primary antibody produces no staining.
Immunocytochemical procedures
For single immunogold labeling of NADPH oxidase subunits, brainstem sections were incubated for 48 hours in separate solutions containing one of the phox antisera (1:50). Sections were rinsed in 0.01 M PBS (pH 7.4), and blocked for 10 minutes in 0.5% BSA and 0.1% gelatin in PBS to reduce non-specific binding of gold particles. Sections then were incubated for 2 hours in donkey anti-goat IgG conjugated with 1 nm gold particles (1:50, AuroProbeOne, Amersham, Arlington Heights, IL). The sections were rinsed in PBS and incubated for 10 minutes in 2% glutaraldehyde. The bound gold particles were enlarged by a 6 minute silver intensification using an IntenSE-M kit (Amersham, Arlington Heights, IL). Some sections were also processed for single immunoperoxidase labeling of NADPH oxidase subunits. Sections were incubated in primary antisera (1:100), and after incubation, were then rinsed in TS and prepared for peroxidase identification. Sections were incubated in anti-goat IgG conjugated to biotin, rinsed in TS, and then incubated for 30 minutes in avidin-biotin-peroxidase complex (1:100, Vectastain Elite Kit, Vector Laboratories) in TBS. The bound peroxidase was visualized by reaction for 5–6 minutes in 0.2% solution of 3, 3'-diaminobenzidine and 0.003% hydrogen peroxide in TS.
For dual detection of immunogold labeling of phox proteins and immunoperoxidase detection of TH, brainstem sections were incubated for 48 hours in a solution containing a mixture of either p47phox or gp91phox antisera (1:50) and the TH (1:25,000) antibody. After incubation, sections were rinsed in TS and prepared first for peroxidase identification of TH. Sections were incubated in anti-mouse IgG conjugated to biotin, rinsed in TS, and then incubated for 30 minutes in avidin-biotin-peroxidase complex (1:100, Vectastain Elite Kit, Vector Laboratories) in TBS. The bound peroxidase was visualized by reaction for 5–6 minutes in 0.2% solution of 3, 3'-diaminobenzidine and 0.003% hydrogen peroxide in TS. Sections were then processed for immunogold labeling. Sections were rinsed in 0.01 M PBS (pH 7.4), and blocked for 10 minutes in 0.5% BSA and 0.1% gelatin in PBS to reduce non-specific binding of gold particles. Sections then were incubated for 2 hours in donkey anti-goat IgG conjugated with 1 nm gold particles (1:50, AuroProbeOne, Amersham, Arlington Heights, IL). The sections were rinsed in PBS and incubated for 10 minutes in 2% glutaraldehyde. The bound gold particles were enlarged by a 6 minute silver intensification using an IntenSE-M kit (Amersham, Arlington Heights, IL). Alternate sections were processed for immunoperoxidase labeling of phox proteins (1:100) and immunogold detection of TH (1:4000). For dual labeling of NADPH oxidase subunits, goat anti-p47phox (1:50) and mouse anti-gp91phox (1:100) antisera were labeled by immunogold and immunoperoxidase methods, respectively. Each of the primary antisera was omitted during labeling conditions to determine the specificity of labeling. There is the possibility that ABC/DAB reaction product will be intensified by silver enhancement. In our dual labeling experiments, the primary antisera labeled with a gold secondary was omitted as a control for this possibility (Chan et al., 1990). It should also be pointed out that even with the punctate immunoperoxidase staining frequently obtained when labeling for receptors and some other signaling proteins, the resultant reaction product is typically not particulate, but rather somewhat diffuse in nature, and thus with careful visual inspection not easily confused with silver enhanced immunogold.
For light microscopy, sections were mounted on glass slides, dehydrated, and covered in resin beneath a glass coverslip. For electron microscopy, sections were postfixed in 2% osmium tetroxide in PB for one hour, and dehydrated in a series of alcohols, through propylene oxide, and embedded in EM BED 812 (EMS, Fort Washington, PA) between 2 sheets of Aclar plastic. Ultrathin sections from the surface of the tissue were cut with a diamond knife using an ultramicrotome (Ultratome, NOVA, LKB, Bromma, Sweden). These sections were collected on grids, and then counterstained with Reynold's lead citrate and uranyl acetate.
Ultrastructural analysis
Electron microscopic sampling and analysis was conducted on a total of at least 62,500 μm2 of thin sectioned mNTS sections obtained from no less than 6 animals. For the analysis of gold particle distributions, we used an established procedure that successfully shows regional differences in subcellular distribution of surface proteins in other brain regions (Haberstock-Debic et al., 2003). From the mNTS of each of three animals, two ultrathin sections at the tissue-surface interface were selected for analysis. Digital images were captured and analyzed to determine: (1) the number of labeled dendritic profiles, and (2) the number of gold-silver particles present in the cytoplasm, or in contact with the plasma membrane. Gold particles in contact with any portion of the surface membrane were considered as plasmalemmal. The classification of labeled dendrites was based upon descriptions by Peters et. al. (Peters et al., 1991). Dendrites were identified by the presence of postsynaptic densities, as well as ribosomes and both rough and smooth endoplasmic reticulum. However, profiles were also considered dendritic whenever postsynaptic densities were observed, independent of endoplasmic reticulum. Synapses were defined as either symmetric or asymmetric, according to the presence of either thin or thick postsynaptic specializations, respectively. Appositions were distinguished by closely spaced plasma membranes that lacked recognizable specializations, without the presence of interposing astrocytic processes. At least two gold-silver particles per profile were imposed as evidence of positive labeling in a neuropil in which comparable areas of epon, myelin or other tissues, not expected to express phox proteins, were largely devoid of gold-silver deposits. Under similar conditions of low background, we have recently shown that 1–2 gold particles in small profiles, such as dendritic spines, is equivalent to four or more in dendritic profiles with a larger surface area (Wang et al., 2003). Data were analyzed by t-tests, or one-or two-way factorial ANOVA, where applicable. For analysis of the ratio of plasmalemmal to surface immunogold labeling, the data were converted by arcsine prior to analysis to allow for comparison of proportions. Differences in means were analyzed by Fisher’s PLSD.
Light Microscopy
For light microscopy, the mounted sections were viewed with a Nikon Microphot-FX microscope (Nikon, Garden City, NY) equipped with a digital CoolSNAP camera (Photometrics, Huntington Beach, CA). The light microscopic images were acquired through an interface between the camera and a Macintosh computer. These images were adjusted for contrast and brightness using Photoshop 6.0 software, and imported into Powerpoint 2001, to add lettering and prepare the composite light microscopic figure.
Electron Microscopy
Electron microscopic images were using a digital camera (Advanced Microscopy Techniques) interfaced with a transmission electron microscope (Technai 12 BioTwin, FEI, Hillsboro, OR). For preparation of figures, images were adjusted for contrast and brightness using Photoshop 6.0 software, and imported into QuarkXpress 4.0, to add lettering. For quantification of cross-sectional area, surface area, as well as minor and major axis length digital images were analyzed with Microcomputer Imaging Device software (MCID, Imaging Research Inc., Ontario, Canada).
Results
In the dorsal vagal complex (Figure 1 top left) of brainstem sections processed for light microscopic single immunoperoxidase labeling for p47phox (Figure 1), there were apparent neuronal somata showing staining within the mNTS (Fig.1 bottom). In addition, there were many instances of elongated processes, which were frequently present near blood vessels (not shown). A similar labeling pattern was seen for the other NADPH oxidase subunits (data not shown).
Figure 1.
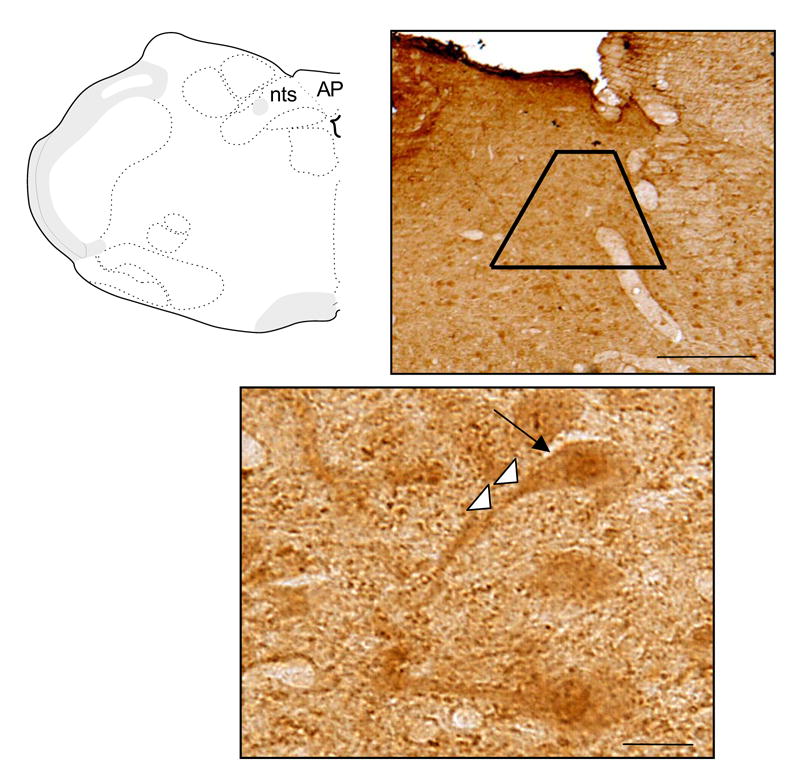
Light microscopic immunoperoxidase labeling for p47phox in the dorsal vagal complex. At the top left, a schematic figure representing the brainstem level sampled in the present study is provided for orientation. At the top right, p47phox immunoperoxidase labeling in the mNTS and adjacent dorsal motor nucleus and area postrema are shown in a 40 μm coronal section through the dorsal medulla. The trapezoid illustrates the relative size and location of the area in which ultrathin sections were obtained for electron microscopic analysis in separately processed brainstem sections. A high power light micrograph shows a neuron (large arrow) with immunoperoxidase labeling for p47phox in the soma and primary dendrite (small white arrows). AP= area postrema, nts= nucleus of the solitary tract. Scale bars = 0.5 mm (top), 10 μm (bottom).
Electron microscopic imaging of single p47phox immunogold labeled profiles in the mNTS
Within the mNTS, immunogold labeling of p47phox was present in somata and dendritic profiles. Within somata, p47phox was frequently present in association with rough endoplasmic reticula (rER) and round vesicular organelles (Fig. 2A). Less frequently, p47phox immunogold particles were present near the plasmalemma of these cell bodies. Within dendritic profiles, immunogold-silver deposits for p47phox were often associated with intracellular organelles, particularly endomembrane-like tubular organelles characteristic of smooth endoplasmic reticula (sER) (Fig. 2B). Immunolabeled dendrites were present in the neuropil as well as near the neurovasculature (Fig. 2B). Immunogold particles for p47phox were also frequently found in association with the surface membrane of dendritic profiles when labeled with immunogold (Fig. 2C) or immunoperoxidase methods (Fig. 2D).
Figure 2.
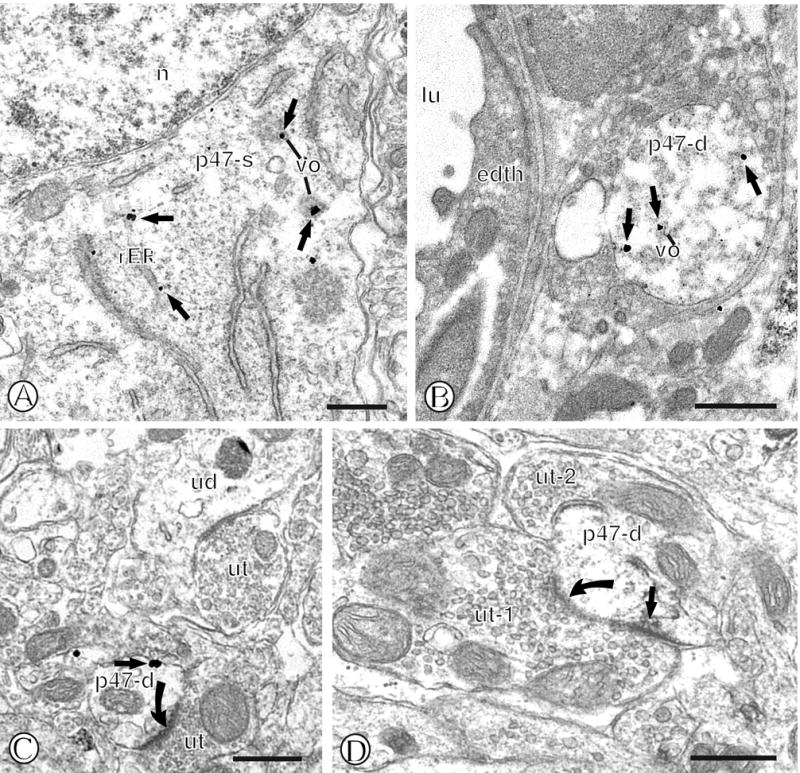
Immunogold labeling of p47phox in mNTS neurons. (A). Immunogold particles (small closed arrows) for p47phox are present in diverse intracellular compartments of a single labeled soma (p47-s). Gold-silver deposits can be seen near rough endoplasmic reticula (rER) just beneath the nucleus (n), as well as nearby small vesicular organelles (vo). (B). A single immunogold labeled dendritic profile (p47-d) contains gold labeling for p47phox (small closed arrow) near vesicular organelles (vo) and the cytoplasm. This dendritic profile is present in proximity to the neurovasculature as indicated by a vessel lumen (lu) and an associated endothelial cell (edth). (C). A single gold labeled small dendritic profile (p47-d) is present within the neuropil. This dendritic profile contains immunogold labeling on the extrasynaptic surface membrane adjacent to an unlabeled axon terminal (ut) that forms an excitatory-type asymmetric synapse (closed curved arrow). Unlabeled dendritic (ud) and axon terminal (ut) profiles are seen in the nearby neuropil. (D). A small single immunoperoxidase labeled dendritic profile (p47-d) is present within the neuropil containing unlabeled axon terminals (ut1 and ut-2). Immunoperoxidase reaction product is seen near the plasmalemma beneath an asymmetric synapse (closed curved arrow) formed by an unlabeled axon terminal (ut-1). Scale Bars = 0.5 μm.
Immunogold labeling for p47phox was also frequently present in non-neuronal compartments, particularly astrocytes. Immunogold-silver deposits were present in the cell bodies of astrocytes, often in association with Golgi complexes (GC) and small round vesicular organelles (Fig. 3A). More frequently, however, immunogold particles for p47phox were found in thin elongated processes present within the neuropil (Fig. 3B). These small astrocytic profiles frequently surrounded unlabeled axons or dendrites, many of which formed appositions or synapses. In addition, p47phox immunolabeling was often present in perivascular astrocytes. In these processes, p47phox immunogold labeling was conspicuously present beneath the surface membrane in contact with the basal lamina (Fig. 3C).
Figure 3.
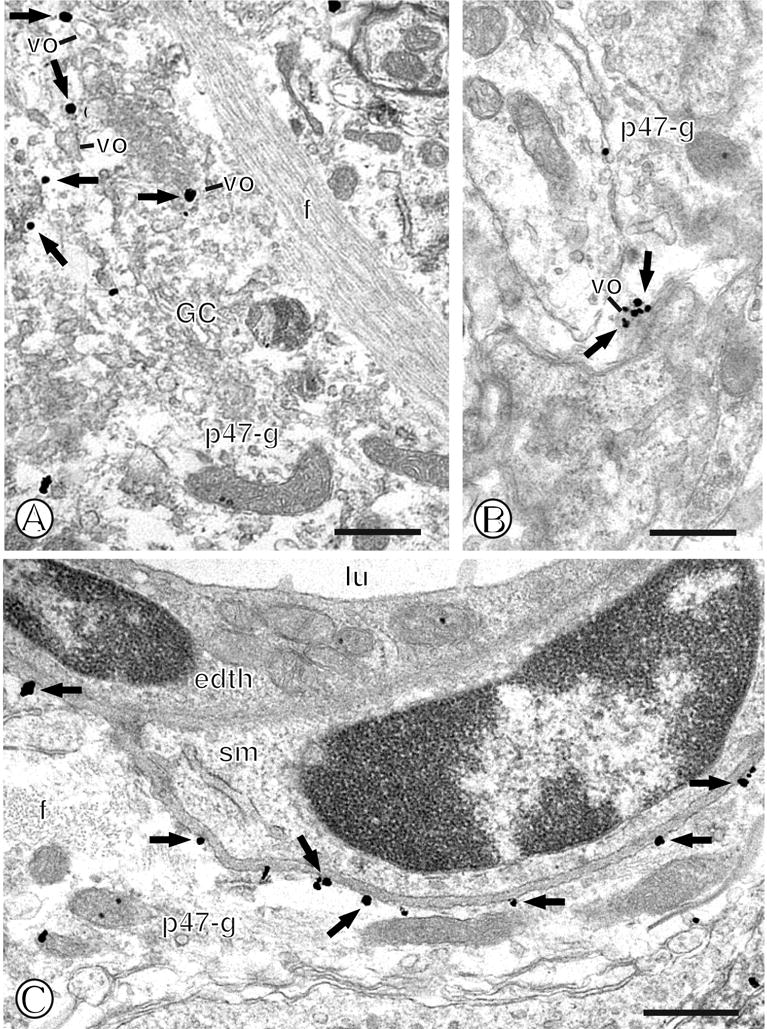
Immunogold labeling of p47phox in mNTS astroglia. (A). A large single labeled fibrous astroglial profile (p47-g) contains immunogold particles (small closed arrows) associated with small vesicular organelles (vo). This glial process contains extensive fiber bundles (f), a Golgi complex (GC), and numerous vesicular organelles (vo). (B). A single labeled glial process (p47-g) contains a cluster of gold particles (small closed arrows) near a dense vesicular organelle (vo). (C). A single labeled astrocytic process (p47-g) contains plasmalemmal immunogold labeling (small closed arrows). This profile, which contains a bundle of cross-sectional fibers (f), is present near a blood vessel lumen (lu) and its associated endothelial cell (edth) and a smooth muscle cell (sm). Scale Bars= 0.5 μm.
Electron microscopic imaging of gp91phox immunogold labeling in the mNTS
Within the mNTS, gp91phox immunogold labeling was present in both neuronal and glial profiles. Similar to what was described for p47phox, immunogold-silver deposits for gp91phox were found in Golgi complexes and the rER of neuronal somata (Fig. 4A). Dendritic profiles containing gp91phox immunolabeling were present within the neuropil containing unlabeled small unmyelinated axons and axon terminals (Fig. 4B). Within these dendrites, gold-silver deposits for gp91phox were present near the surface membrane (Fig. 4B) as well as vesicular organelles (Fig. 4B) and mitochondria (Fig. 4C). Labeling near the surface membrane and vesicular organelles was also seen with an immunoperoxidase marker (Fig. 4D). Labeling for gp91phox was also present in the cell bodies of astrocytes (Fig. 5A) where immunogold particles were frequently located near Golgi complexes and vesicular organelles, while sporadic labeling near fibers was also seen. Gold-silver deposits were also seen in small glial processes within the neuropil containing phox labeled dendritic profiles (Fig. 5B). Small elongated gp91phox labeled glial processes were also often present in the neuropil when labeled for immunoperoxidase detection (Fig. 5C).
Figure 4.
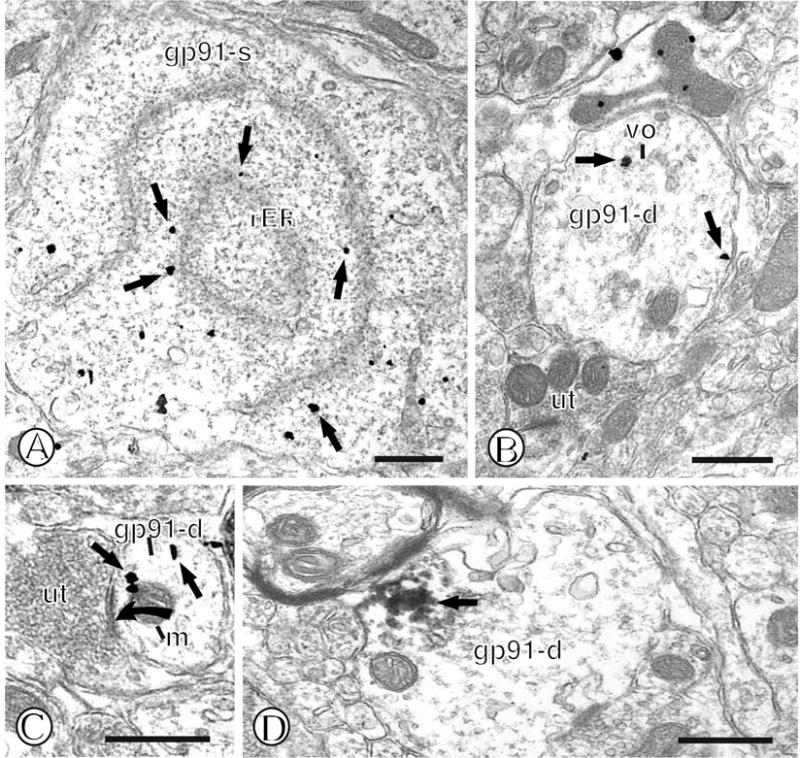
Immunogold labeling of gp91phox in mNTS neurons. (A). A single labeled neuronal cell body (gp91-s) contains gold particles (small closed arrows) near rough endoplasmic reticula (rER). (B). A single labeled dendritic profile (gp91-d) contains gold particles (small closed arrows) intracellularly, as well as near the extrasynaptic plasma membrane. The dendrite is apposed to an unlabeled axon terminal (ut). (C). A small dendritic profile (gp91-d) contains gold particles (small closed arrow) in diverse intracellular sites, including the cytoplasm and near a mitochondrion (m). This dendritic profile receives an asymmetric synapse (closed curved arrow) from an unlabeled axon terminal (ut). (D). A single labeled dendritic profile (gp91-d) contains a dense intracellular aggregate of immunoperoxidase reaction product (small closed arrow). Scale Bars = 0.5 μm.
Figure 5.
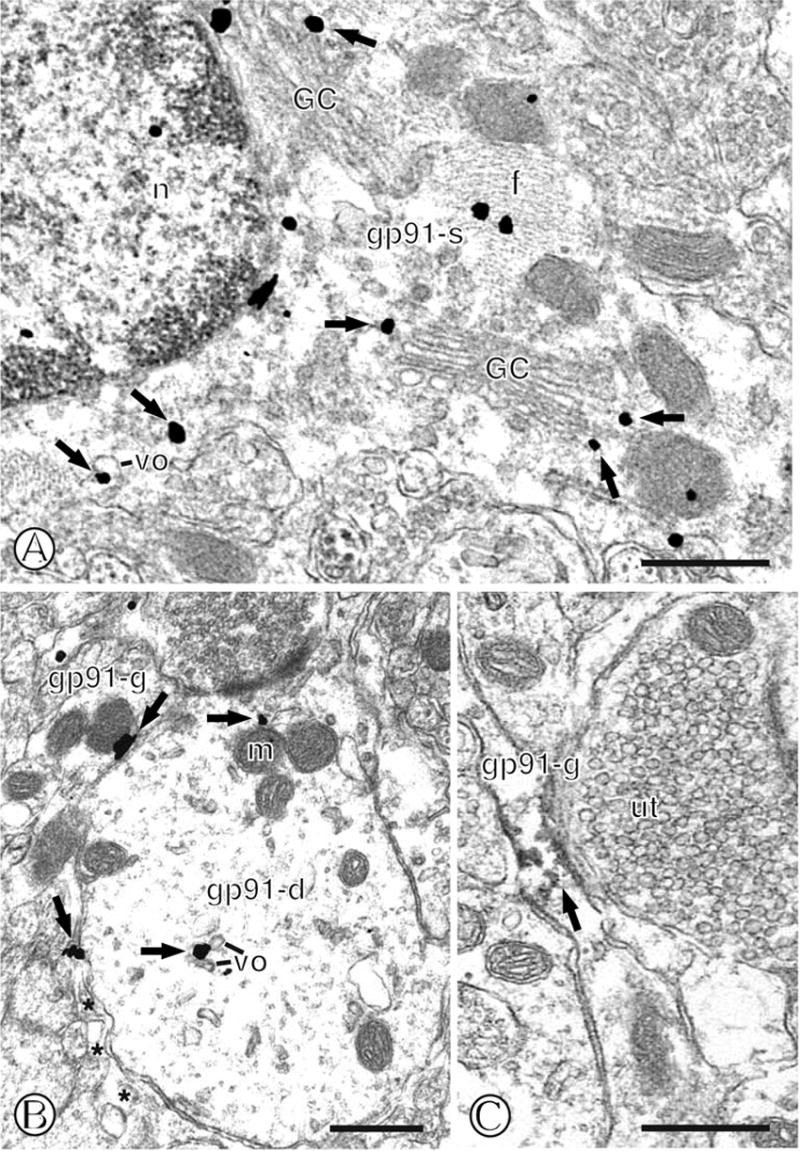
Immunogold labeling of gp91phox in mNTS astroglia. (A). The cell body of an astrocyte (gp91-s) containing numerous cross-sectioned fibers (f) displays immunogold particles (small closed arrows) near diverse intracellular organelles. These include Golgi complex’s (GC) and vesicular organelles (vo) that surround the nucleus (n). (B). A single labeled glial process (gp91-g) contains immunogold particles (small closed arrows) near the surface membrane. This glia is apposed to a single labeled dendritic profile (gp91-d) that displays gold labeling near small vesicular organelles (vo) and a mitochondrion (m) located just beneath the postsynaptic density. (C). A small elongated glial process (gp91-g) shows immunoperoxidase labeling (small closed arrow) intracellularly and near the surface membrane. The surrounding neuropil contains unlabeled profiles, including an axon terminal (ut). Scale Bars = 0.5 μm.
Dual electron microscopic imaging of p47phox and gp91phox labeling in the mNTS
Since functional NADPH oxidase requires the formation of a p47phox - gp91phox complex, brainstem sections were also processed for dual immunogold and immunoperoxidase labeling for these proteins, respectively. In the mNTS, immunogold and immunoperoxidase reaction product for these proteins were sometimes found in separate subcellular sites, but were also frequently found to overlap. In dendritic profiles aggregates of immuoperoxidase reaction product for gp91phox were present near endosomal-like vesicular organelles that also contained immunogold-silver deposits for p47phox (Fig. 6A). In addition, overlapping phox protein immunolabeling was also present in intracellular sites near mitochondria (Fig. 6B) or near the surface membrane of dendritic profiles (Fig. 6C). Similarly, profiles of small astrocytic processes also showed overlapping gp91phox and p47phox immunoreactivity (Fig. 6D).
Figure 6.
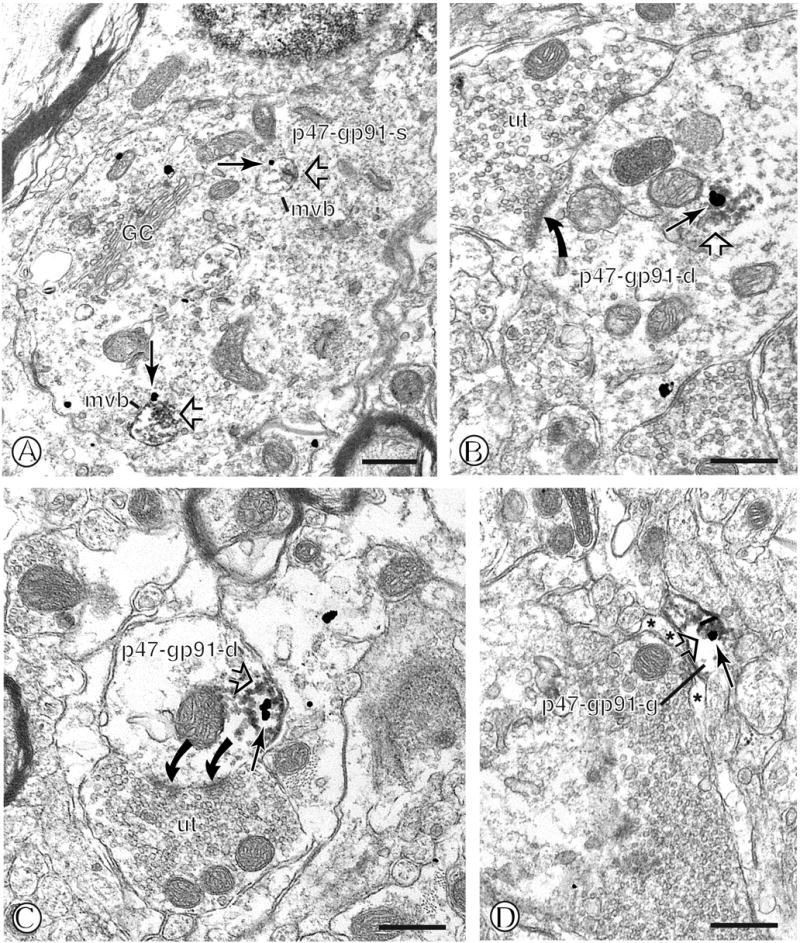
Dual immunogold labeling of p47phox and immunoperoxidase labeling of gp91phox in mNTS neurons and astroglia. (A). A dual labeled neuronal soma (p47-gp91-s) contains large vesicular organelles characteristic of multiple vesicular bodies (mvb). These organelles contain immunogold labeling for p47phox (large arrow) and aggregates of peroxidase reaction product for gp91phox (open arrow) in the inner lumen. (B). A large dendritic profile (p47-gp91-d) contains overlapping intracellular immunogold p47phox (large arrow) labeling and gp91phox immunoperoxidase reaction product (open arrow). This dendrite is contacted by multiple axon terminals, one of which forms an asymmetric synapse (closed curved arrow) (C). A small dendritic profile (p47-gp91-d) contains overlapping p47phox (large arrow) gold and gp91phox immunoperoxidase labeling (open arrow) just beneath the surface membrane. This dendritic profile is contacted by an unlabeled axon terminal (ut) forming a perforated asymmetric synapse (curved closed arrows). (D). A small glial process (p47-gp91-g) shows overlapping p47phox immunogold (large arrow) and gp91phox immunoperoxidase labeling (open arrow) near the surface membrane. Asterisks= unlabeled glia. found Scale Bars = 0.5 μm.
Electron microscopic imaging of p22phox immunogold labeling in the mNTS
Separate brainstem sections were processed for single immunogold labeling of p22phox. Similar to the observed immunogold labeling of p47phox and gp91phox, the p22phox subunit was present in somata and dendritic profiles in mNTS neurons. Gold labeling of p22phox was also frequently present in association with Golgi complexes and small round vesicular organelles in somata (Fig. 7A). Immunogold labeling was also found in intracellular sites including mitochondria in large dendritic profiles that were apposed by unlabeled axon terminals (Fig. 7B). In addition, p22phox immunolabeling was also present near the surface membrane of small dendritic profiles that were contacted by unlabeled axon terminals (Fig. 7C). p22phox labeled dendrites were also frequently seen near the neurovasculature (Fig. 7D). Compared to the other phox proteins, there was also a comparable degree of p22phox labeling in astrocytic processes. Within the cell bodies of glia, single immunogold particles were present in association with Golgi complexes and vesicular organelles (Fig. 8A), whereas in small astrocytic processes p22phox formed aggregates near vesicular organelles (Fig. 8B). During the course of visual inspection of sampled tissue, numerous phox labeled astrocytic profiles were observed near the neurovasculature, frequently on the surface membrane apposed to the basal lamina (Fig. 8C), although these relationships were not quantified.
Figure 7.
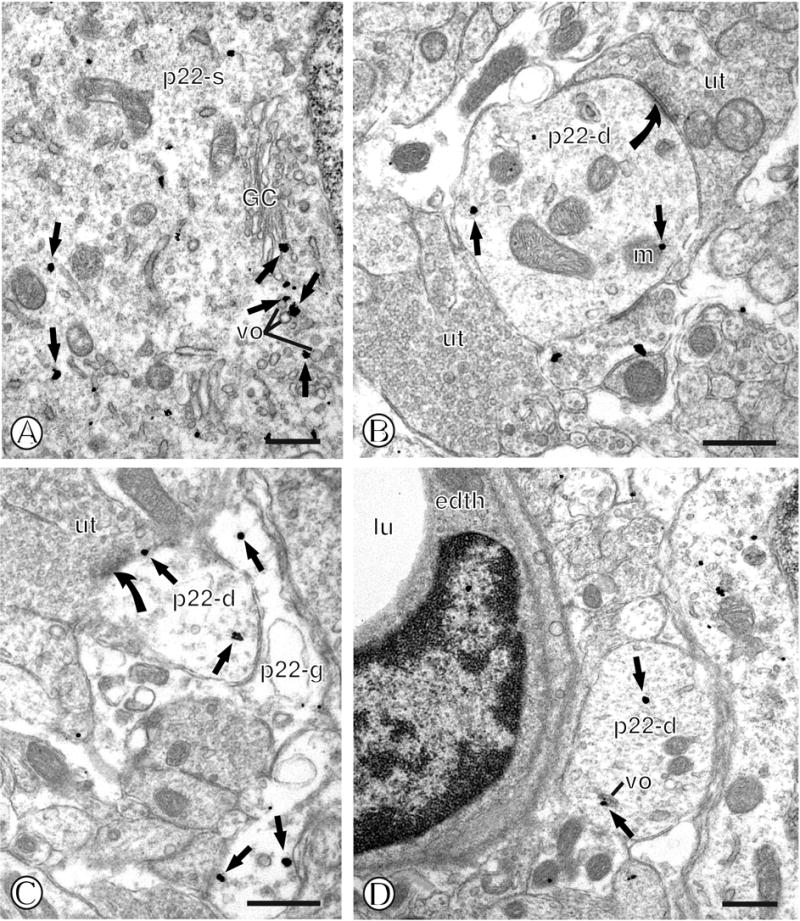
Immunolabeling of p22phox in mNTS neurons. (A). A neuronal cell body (p22-s) contains immunogold labeling (small closed arrows) in association with diverse intracellular organelles, including a Golgi complex (GC), and small round vesicles (vo). (B). A dendritic profile (p22-d) contains an intracellular gold particle (small closed arrow) near a mitochondrion (m). This profile is contacted by unlabeled axon terminals (ut), one of which forms an asymmetric synapse (closed curved arrow). (C). A small dendritic profile (p22-d) contains gold particle at diverse sites, including the perisynaptic plasmalemma (small closed arrow) near the postsynaptic density of an asymmetric synapse (closed curved arrow) formed by an unlabeled axon terminal (ut). This dendritic profile is also contacted by an immunogold labeled glial process (p22-g). (D). A single labeled dendritic profile (p22-d) contains an intracellular gold particle (small closed arrow) near a small vesicular organelle (vo). This dendritic process is located near a blood vessel, as seen by the vessel lumen (lu) and associated endothelial cell (edth). Scale Bars = 0.5 μm.
Figure 8.
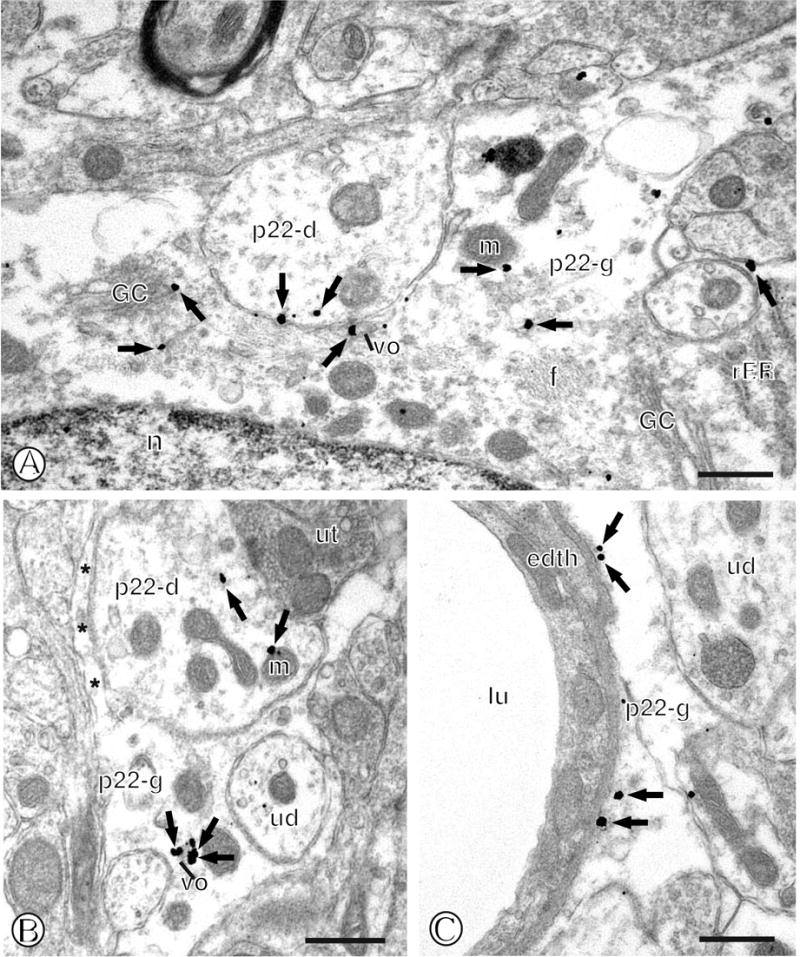
Immunolabeling of p22phox in mNTS astroglia. (A). The cell body of an astroglia (p22-g) contains gold particles (small closed arrows) in association with a Golgi complex (GC), vesicular organelles (vo) and the surface membrane. A dendritic profile (p22-d) apposes this glial profile. (B). A glial process (p22-g) contains an aggregation of gold particles (small closed arrows) near a vesicular organelle (vo). A single labeled dendritic profile (p22-d) is directly apposed to this glial process. (C). A single labeled glial process (p22-g) contains gold particles near the surface membrane just beneath the basal lamina surrounding an endothelial cell (edth) and vessel lumen (lu). Asterisks= unlabeled glia. Scale Bars = 0.5 μm.
Quantification of phox proteins in neuronal or astrocytic compartments in the mNTS
The majority of p47phox immunogold labeled profiles were cell bodies (n=92), dendrites (n=216) or glial processes (n=236), only a small number were axons (n=26) or axon terminals (n=26; Fig. 9A). In addition, most of the gp91phox immunogold labeled profiles were cell bodies (n=45), dendrites (n=162) or glial processes (n=199), only a small number were axons (n=3) or axon terminals (n=13; Fig. 9B). Similar to the other phox proteins, p22phox was present to a higher degree in dendrites (n=72), soma (n=23), and glia (n=74), and to a much lesser extent axon terminals (n=16) and axons (n=3 ; Fig. 9C).
Figure 9.
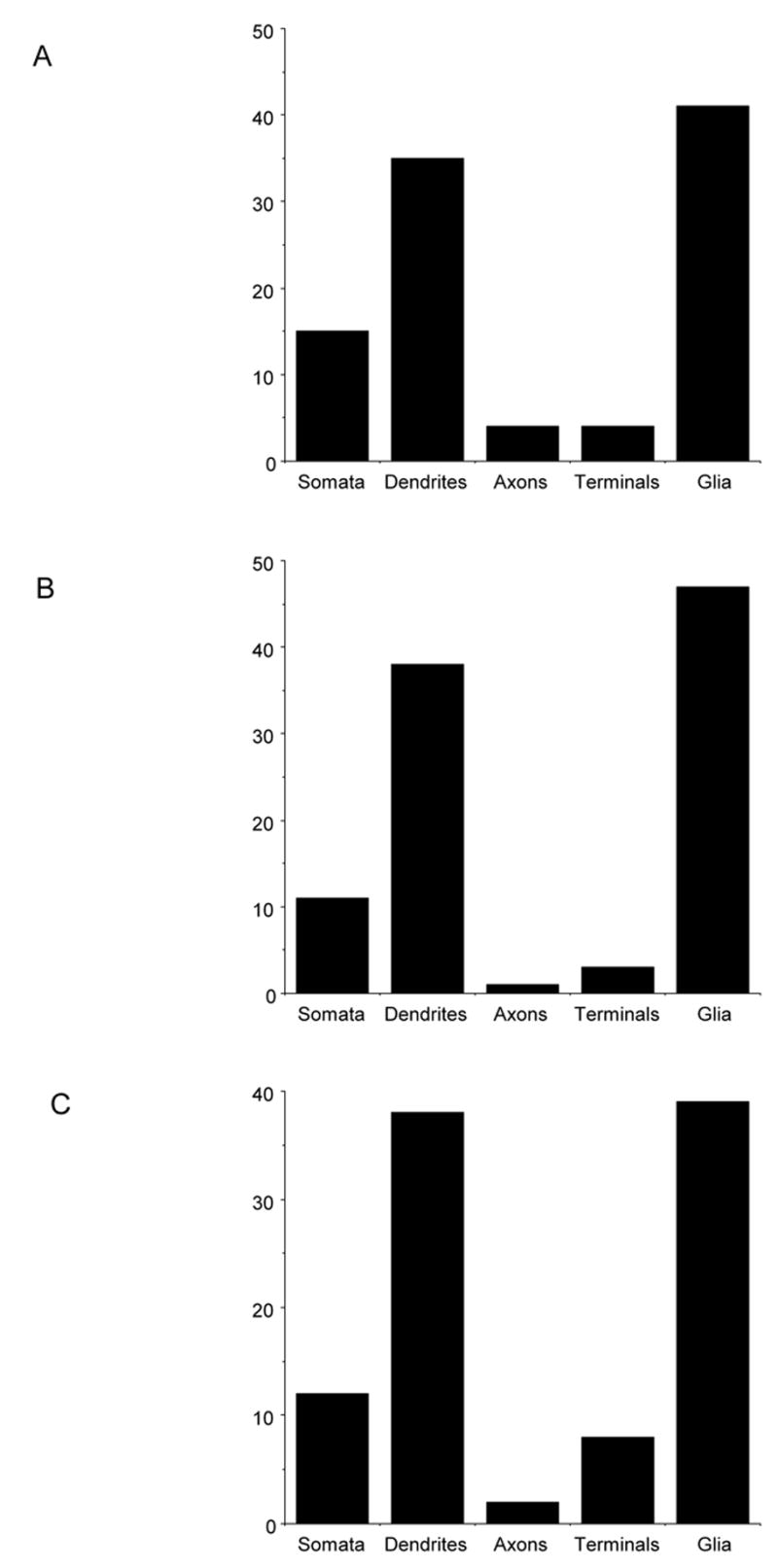
Quantification of NADPH oxidase subunits in mNTS neuronal and astroglial profiles. (A). There is a differential distribution of p47phox immunogold labeling in neuronal compartments and in astroglia in the mNTS. In neuronal profiles, labeling of p47phox was most prominent in somata and dendrites. There was also substantial labeling within glial processes. (B and C). Similar to p47phox, labeling of gp91phox and p22phox in the mNTS is mainly present in somatodendritic profiles, as well as astroglia.
Comparison of subcellular p47phox and gp91phox gold labeling in mNTS neurons and astrocytes
Morphometric comparison of p47phox and gp91phox immunolabeled dendritic profiles indicated that there were no differences in the cross-sectional (2.3±.29 μm versus 2.2±.36 μm, p=.93) or surface (5.8±.4 versus 5.8±.5, p=.96) areas between each group of processes. There was also no difference in the total number of gold particles in p47phox or gp91phox labeled dendritic profiles (2.8±.16 versus 2.5±.2, p=.25). In addition, the intracellular distribution of p47phox or gp91phox in mNTS labeled dendritic processes was comparable (p=.89; Fig. 10A) (Table 1A). When gold labeling was categorized by type of intracellular organelle, there were still no differences in cytosolic (p=.9), vesicular (p=.5) or mitochondrial (p=.24) labeling (Table 1A). There was, however, a significantly higher frequency of dendritic plasmalemmal immunogold labeling of p47phox as compared to gp91phox in the mNTS (p=.04; Table 1A; Fig 10B).
Figure 10.
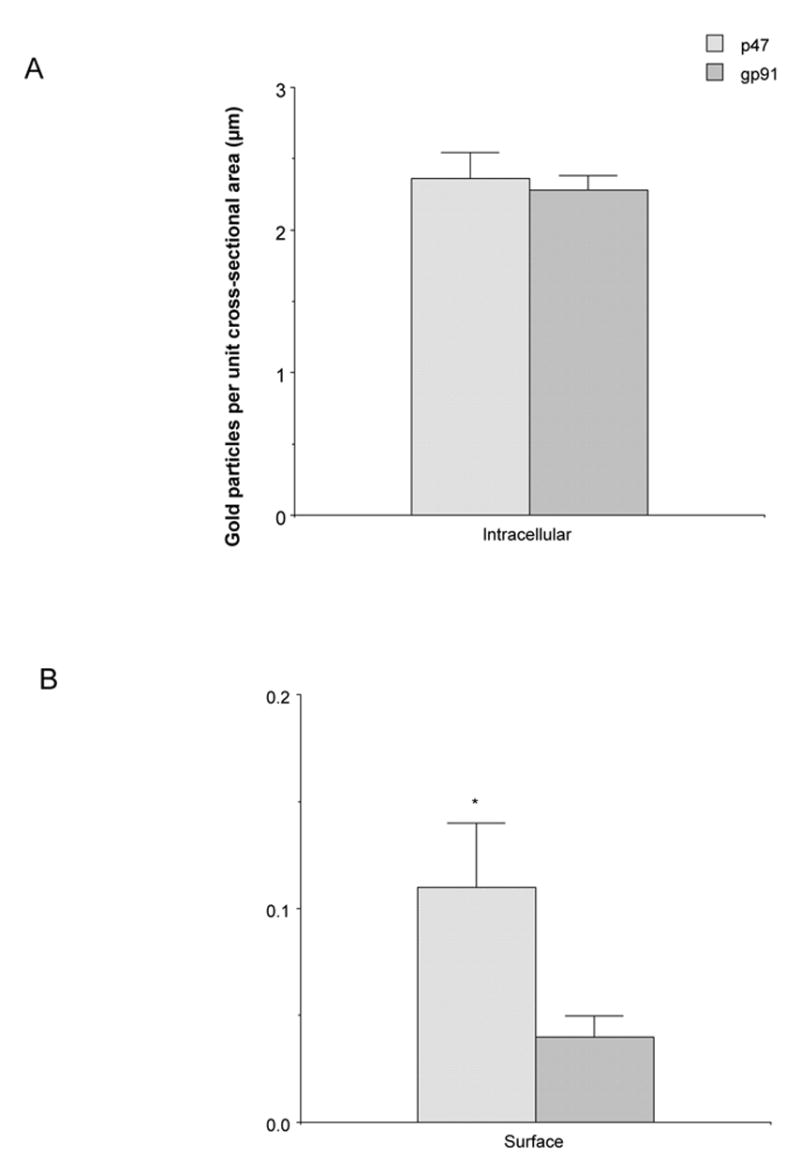
Quantification of the subcellular localization of NADPH oxidase subunits in mNTS neuronal profiles. (A). The number of p47phox and gp91phox immunogold particles per unit cross-sectional area is similar in mNTS neurons. (B). There is a higher density of p47phox gold particles per unit surface area relative to gp91phox gold labeling in mNTS neurons. Data presented as mean±SEM. *=p<0.05.
TABLE 1A.
Subcellular distribution of phox immunogold labeling in mNTS dendritic profiles
| Surface | Intracellular | vesicular | mitochondrial | cytosolic | |
|---|---|---|---|---|---|
| p47phox | 0.5±.1* | 2.2±.2 | 1.5±.1 | 0.2±.1 | 0.6±.1 |
| gp91phox | 0.2±.1 | 2.3±.2 | 1.3±.2 | 0.3±.1 | 0.6±.2 |
In glial profiles, there were no differences in total (5.0±.5 versus 6.7±.8, p=.08), intracellular (p=.09), or surface (p=.6) immunogold labeling of p47phox or gp91phox in the mNTS (Table 1B). When intracellular gold labeling was categorized by type of intracellular organelle, there were significant differences in phox protein labeling in mNTS glia. There was a higher incidence of p47phox labeling in the cytosol (p<.05, Fig. 11) compared to gp91phox. However, there was a significantly higher number of gp91phox gold particles in association with vesicular organelles as compared to p47phox (p=.03; Table 1B, Fig. 11).
TABLE 1B.
Subcellular distribution of phox immunogold labeling in mNTS glial profiles
| Surface | Intracellular | vesicular | mitochondrial | cytosolic | |
|---|---|---|---|---|---|
| p47phox | 0.8±.3 | 4.2±.4 | 3.0±.3 | 19±.1 | 1.0±.2 |
| gp91phox | 1.0±.2 | 5.5±.7 | 4.5±.6* | 0.4±.1 | 0.6±.2 |
Figure 11.
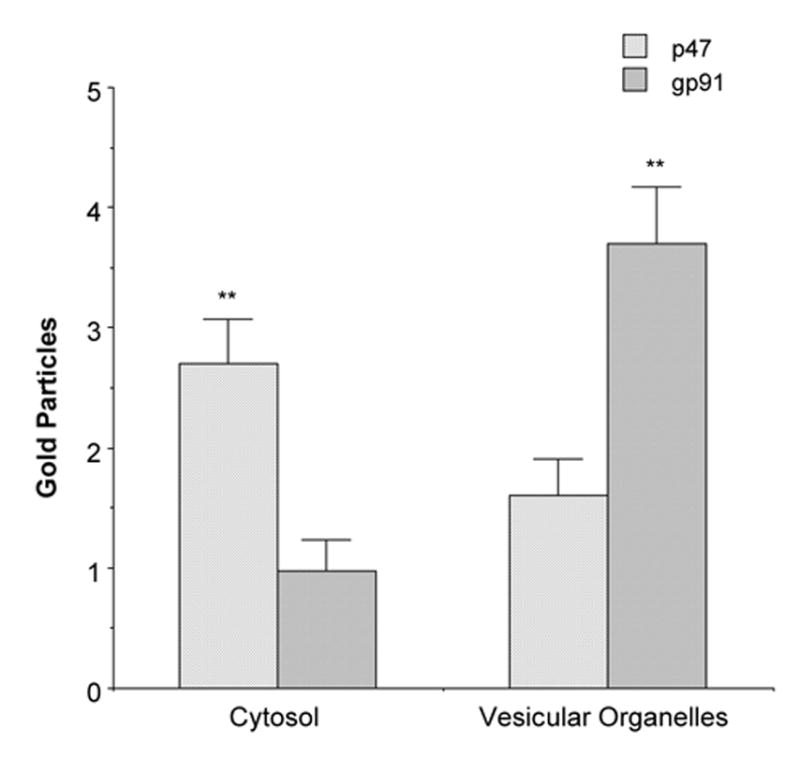
Quantification of the subcellular localization of NADPH oxidase subunits in mNTS astroglial profiles. Immunogold labeling of NADPH oxidase subunits are differentially distributed in intracellular compartments in mNTS glia. There is a higher level of p47phox labeling associated with the cytosol compared to gp91phox. In contrast, gp91phox is more prevalent in association with vesicular organelles. **=p<0.01.
Electron microscopic imaging of p47phox immunogold and TH immunoperoxidase labeled profiles in the mNTS
Immunogold-silver deposits for p47phox were found in somatodendritic profiles that also contained immunoperoxidase reaction product for the catecholamine synthesizing enzyme TH. Similar to single labeled profiles, p47phox immunolabeling was often present near Golgi complexes and rER of cell bodies also labeled for TH (Fig. 12A). Further, gold particles were also frequently seen near vesicular organelles and the surface membrane in catecholaminergic dendritic profiles (Fig. 12B). In addition to colocalization with TH in neurons, p47phox was seen in thin elongated glial processes that were apposed to TH immunolabeled axons or axon terminals (Fig. 12C).
Figure 12.
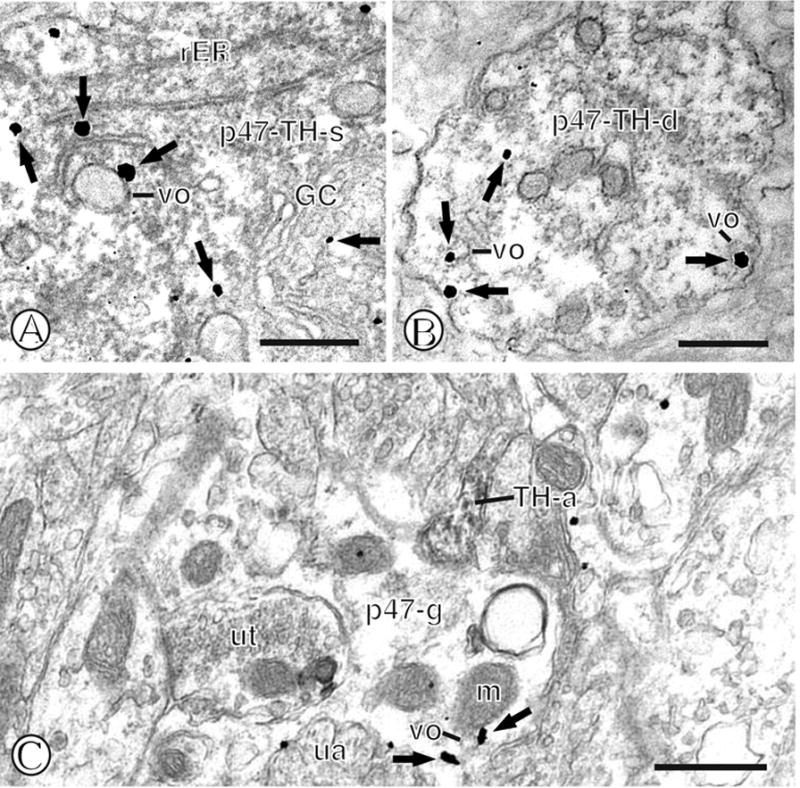
Dual immunolabeling of p47phox and TH in mNTS neurons or astroglia. (A). A dual labeled soma (p47-TH-s) containing diffuse immunoperoxidase reaction product for TH also shows immunogold p47phox labeling (small closed arrows) in association with diverse intracellular organelles, including rough endoplasmic reticula (rER) and a Golgi complex (GC). (B). A dual labeled dendritic profile (p47-TH-d) shows dispersed immunoperoxidase immunoreactivity for TH and immunogold labeling of p47phox (small closed arrows) near vesicular organelles (vo) and the plasma membrane. (C). A single labeled glial process (p47-g) contains immunogold-silver deposits (small closed arrows) near a mitochondrion (m) a vesicular organelle (vo) and the surface membrane. This glial profile is contacted by a small axon (TH-a) containing TH immunoperoxidase labeling. Scale Bars = 0.5 μm.
Quantification of p47phox immunogold and TH immnoperoxidase labeling in the mNTS
Of all TH immunoreactive somata or dendrites, approximately 21% (73/339) also showed labeling for p47phox, whereas approximately 24% (73/308) of p47phox profiles were labeled for TH. In addition, there were many instances of appositions between p47phox labeled somatodendritic profiles or glia and TH labeled axons or axon terminals. Approximately 10% (25/236) of all p47phox labeled glia and 26% (81/308) of somatodendrites were apposed by TH immunoreactive axons.
Electron microscopic imaging of gp91phox immunogold and TH immunoperoxidase labeled profiles in the mNTS
Within the mNTS, gp91phox immunoreactivity was frequently present in neurons immunolabeled for TH. In somatodendritic profiles that contained immunoperoxidase reaction product for the catecholamine synthesizing enzyme, gp91phox immunogold particles were often present near vesicular organelles or the surface membrane (Fig. 13A). Dendritic profiles containing gp91phox were sometimes apposed by axon terminals containing TH (Fig. 13B). In addition to colocalization with TH in neurons, gp91phox was often located in thin elongated glial processes that were apposed to TH immunolabeled axons or axon terminals (Fig. 13C).
Figure 13.
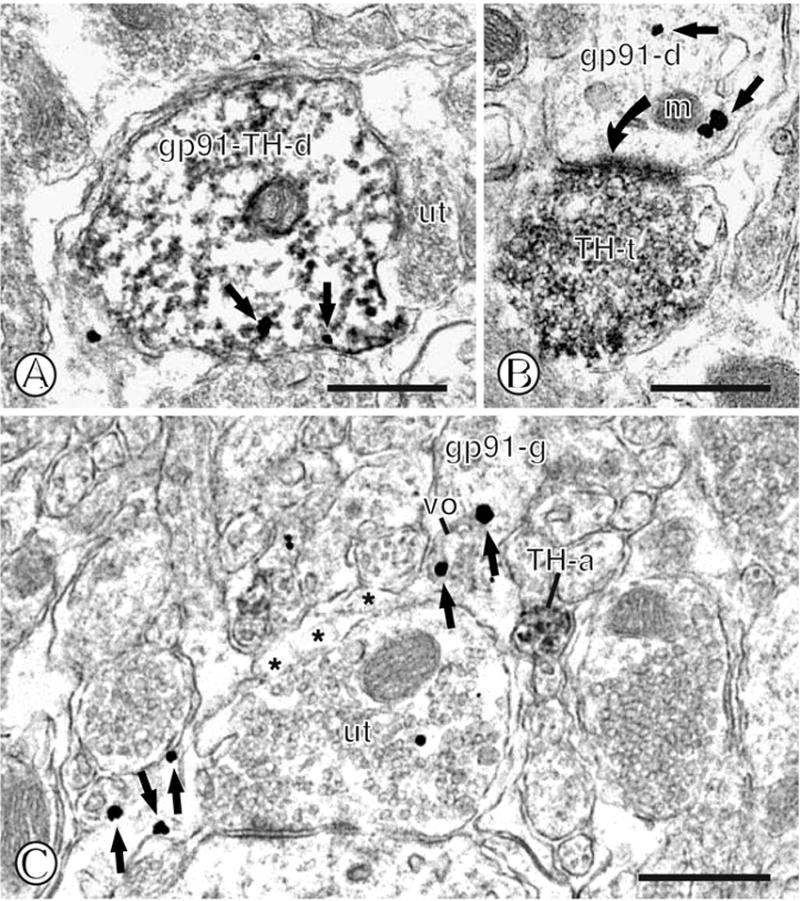
Dual immunolabeling of gp91phox and TH in mNTS neurons or astroglia. (A). A dual labeled dendritic profile (gp91-TH-d) displays diffuse immunoperoxidase reaction product for TH. Immunogold particles (small closed arrows) are present intracellularly and near the surface membrane adjacent to an unlabeled axon terminal (ut). (B). A single gp91phox labeled dendritic profile (gp91-d) contains intracellular immunogold labeling (small closed arrows), including near a mitochondria (m). This dendrite receives an asymmetric type synapse (curved closed arrow) from an axon terminal (TH-t) showing dense immunoperoxidase reaction product for TH. (C). A single labeled glial process (gp91-g) contains dispersed immunogold particles (small closed arrows) near a vesicular organelle (vo) and the plasmalemma. This glia is contacted by numerous neuronal profiles, including an unlabeled axon terminal (ut) and a small immunoperoxidase labeled unmyelinated axon (TH-a). Scale Bars = 0.5 μm.
Quantification of gp91phox immunogold and TH immnoperoxidase labeling in the mNTS
In tissue processed for dual labeling, approximately 76% (58/76) of all TH labeled somatodendritic profiles were also immunoreactive for gp91phox, and approximately 28% (58/207) of all gp91phox somatodendrites were also labeled for TH. Approximately 10% (20/207) of all labeled somatodendrites, and 3% (6/199) of all gp91phox labeled glia and were apposed by TH immunoreactive axons.
Discussion
The present study provides the first immunocytochemical evidence that p47phox, gp91phox, and p22phox are present in neuronal and astrocytic processes in the rat mNTS. Immunolabeling of each NADPH oxidase subunit was predominantly present in somatodendritic profiles as well as perineuronal and perivascular astrocytic processes. These results, in conjunction with overlapping p47phox and gp91phox immunolabeling, suggest that NADPH oxidase mediated superoxide production in the mNTS is likely to impact postsynaptic responses of neurons, as well as the activity of glia. In addition, many mNTS neurons were dually labeled for phox proteins and TH, while many catecholaminergic axons frequently apposed phox labeled astrocytes. Thus, of the many neuronal phenotypes present in the mNTS, catecholaminergic neurons are likely to play a significant role in the actions of superoxide produced by NADPH oxidase.
Somatodendritic labeling of phox proteins in mNTS neurons
Within the mNTS, immunogold labeling of p47phox, gp91phox, or p22phox was present in somata and dendrites. Within somata, phox protein immunoreactivity was associated with intracellular organelles that are involved in protein synthesis and transport, including rER and Golgi complexes. NADPH oxidase subunit labeling was also found near mitochondria as well as the surface membrane in cell bodies. Thus, the subcellular distribution of phox proteins as revealed by immunogold labeling is consistent with active synthesis and transport within mNTS neuronal somata, as also shown in non-neuronal cell types (Yu et al., 1999).
Within dendritic profiles, NADPH oxidase subunit immunolabeling was frequently found in intracellular sites, including small tubulovesicular organelles and mitochondria. The tubulovesicular organelles exhibited the morphology of smooth ER, some of which were also associated with the outer mitochondrial membrane. Phox immunolabeling in dendritic smooth ER, known sites for storage and intracellular release of calcium, provides ultrastructural evidence for the regulation of calcium levels in mNTS neurons by superoxide produced by NADPH oxidase (Wang et al., 2004, Kimura et al., 2005). In addition to intracellular sites, phox protein immunolabeling was also seen at synaptic and peri-synaptic areas of the plasmalemma. This is in accord with previous findings that NADPH oxidase has a synaptic distribution in other brain regions (Tejada-Simon et al., 2005).
Phox proteins in mNTS neurons: Functional considerations
Production of ROS within central autonomic circuitry is emerging as an important substrate of neural cardiovascular regulation (Wang et al., 2004). Our ultrastructural evidence for phox labeling in association with membranous organelles, including endoplasmic reticula, is in agreement with physiological results indicating that the potentiation of calcium currents induced by AT-1A receptor activation is mediated by NADPH dependent superoxide production in mNTS neurons (Wang et al., 2004). Together these findings suggest that superoxide production and increased calcium levels induced by AT-1A receptor activation in mNTS neurons occurs near diverse cellular membranes, including the plasmalemma and endoplasmic reticula. Thus, one cellular mechanism by which ROS production may modulate the activity of mNTS neurons is by redox regulation of cell surface or endomembrane proteins (Wolin, 2004), leading to increased intracellular calcium. Thus, within the mNTS, NADPH oxidase-induced superoxide production may be an important link between extracellular signals, such as ANGII, and intracellular events related to calcium signaling.
Astroglial labeling of phox proteins in mNTS
In addition to neurons, mNTS glia were also frequently immunoreactive for NADPH oxidase subunits. The larger glial processes had the morphology of fibrous astrocytes, as indicated by ovoid nuclei, the presence of dense glial fibrillary acidic protein-like fibers within the cytosol of cell bodies, the latter of which gave off small thin organelle-sparse processes that traversed the surrounding neuropil (Peters et al., 1991). Within astrocytic somata, immunogold particles for phox proteins were associated with Golgi complexes and rough ER. In the thin processes, gold particles for NADPH oxidase subunits often formed aggregates near the outer membrane of vesicular organelles. Phox protein immunolabeling was also frequently observed in association with the plasma membrane of small astrocytic profiles that were apposed to unlabeled or phox labeled dendritic profiles or axons. In addition, glial profiles showing NADPH oxidase subunit labeling were often present near blood vessels. When present near the vasculature, immunolabeling for phox proteins was mainly located on the surface membrane apposed to other glia, or beneath the basal lamina beneath endothelial or smooth muscle cells. The finding that NADPH oxidase subunit labeling is prominent in astrocytes of the mNTS is consistent with a number of recent reports providing evidence for astrocytic phox proteins in other brain regions (Abramov et al., 2005).
Phox proteins in mNTS astrocytes: Functional considerations
Astrocytes are emerging as an important component of neural autonomic regulatory circuits. For example, astrocytes are the primary source of angiotensinogen in brain autonomic pathways, and are thus a crucial component of the central renin-angiotensin system (Morimoto et al., 2001). In addition, systemic hypertension is associated with increased mNTS angiotensinogen (Sangaleti et al., 2004) and brain ROS (Zimmerman and Davisson, 2004), whereas expression of the angiotensinogen gene is regulated by factors known to be sensitive to ROS production (Agarwal et al., 2004). These findings, together with the present results, suggest that mNTS astrocytes may couple hypertension with oxidative stress and ANGII activity.
Many of the astrocytes labeled for phox proteins were present in association with the neurovasculature. These labeled astroglia were often found in close proximity to endothelial cells, frequently in direct contact with the basal lamina. Thus, NADPH oxidase activation may be sensitive to circulating or endothelial derived factors, including ANGII or nitric oxide (NO) produced by endothelial nitric oxide synthase (eNOS). In addition to ANGII, NO produced by eNOS is a known neuromodulator of cardiovascular processes by acting on mNTS baroreflex circuitry (Paton et al., 2001). The close coupling of phox proteins with neurovascular structures in the mNTS suggests that NADPH oxidase-induced superoxide production is strategically targeted for the modulation of signals derived from endothelial cells, astrocytes, and the adjacent neuropil.
Tyrosine hydroxylase and phox proteins in neurons and astrocytes in mNTS
Within the mNTS, p47phox and gp91phox proteins were often co-localized with TH in somatodendritic processes. NADPH oxidase subunits were present in somata showing various intensities of immunoperoxidase reaction product for TH. However, phox labeling was independent of the levels of TH labeling in somata, where it was frequently present in association with rER, Golgi complexes, and endomembrane organelles. The presence of NADPH oxidase subunit labeling in TH containing neurons that are also known to express the alpha-2A adrenergic receptor (Glass et al., 2001) or the AT-1A receptor (Glass et al., 2005.) indicates that superoxide production may play a role in catecholamine and angiotensin signaling in mNTS neurons.
Phox protein immunolabeled astrocytic profiles were frequently apposed to TH immunoreactive dendrites and axons in the mNTS. Glial cells of similar morphology to those currently described, have been shown to contain surface and intracellular adrenergic receptors (Glass et al., 2001). In addition, the extraneuronal monoamine transporter expressed in glia is known to be present in high density within the brainstem, suggesting that NADPH oxidase subunit labeled glia apposed to TH expressing neurons are capable of catecholamine binding and uptake.
Tyrosine hydroxylase and phox proteins in mNTS neurons and glia: Functional Considerations
It is known that TH is sensitive to cardiorespiratory and other forms of stress (Chan and Sawchenko, 1995, Chan and Sawchenko, 1998). In addition, free radicals can modulate the activity of TH (Kuhn and Geddes, 2002, Kuhn et al., 2002), while the oxidation of catecholamines, such as norepinephrine, results in reactive molecules that impact a variety of intracellular processes including mitochondrial respiration and regulation of metabolic enzymes (Xin et al., 2000). It is also important to note that hydrogen peroxide, a major superoxide metabolite, is accessible to the extracellular milieu (Lambeth, 2004) and thus may influence closely apposed structures. Thus, catecholamine signaling in the mNTS, which is known to play a role in the regulation of systemic arterial pressure (Snyder et al., 1978), may be directly modulated by superoxide generated by NADPH oxidase in neurons, or indirectly in apposed astroglia.
NADPH oxidase subunit localization in the mNTS: Conclusions
The subcellular localization of NADPH oxidase-mediated superoxide production has important consequences for intracellular signaling (Lambeth, 2004). Within the framework of the classic neutrophil model, the basally quiescent NADPH oxidase requires stimulation for assembly and superoxide production at the surface membrane. However the precise spatial location of active phox proteins is now known to depend upon cellular phenotype. For example, in endothelial cells functional NADPH oxidase is present intracellularly, frequently in association with the perinuclear cytoskeleton, where it also produces ROS at low basal levels (Li and Shah, 2002). The present results demonstrate that phox subunits in mNTS neurons and astroglia are present near the surface membrane, as well as near endoplasmic reticula-like vesicular organelles, known sites for storage and release of intracellular calcium. These findings therefore indicate further cell phenotypic diversity of NADPH oxidase function. In addition, the presence of phox proteins in diverse cellular compartments of mNTS neurons and glia supports a structural basis for the emerging role of redox modulation of autonomic, and particularly cardiovascular processes. Moreover, given the important role of ROS, dorsal vagal pathology, and autonomic dysfunction in neurological disease, aberrant free radical production in the NTS may be a potential candidate substrate of dorsal vagal damage accompanying sudden infant death syndrome (Sparks et al., 1996, Biondo et al., 2004), myocardial infarction (De Caro et al., 2000), as well as several major neurological disorders (Saper et al., 1991, Schroder and Linke, 1999, Dewey, 2004).
Acknowledgments
This work is supported by NIH grant HL18974
LIST OF ABBREVIATIONS USED IN MAIN TEXT
- ABC
avidin biotin peroxidase complex
- ANGII
Angiotensin II
- AT-1A
Angiotensin II receptor subtype 1A
- AP
area postrema
- BSA
bovine serum albumin
- GC
Golgi complex
- IgG
immunoglobulin G
- m
mitochondria
- mNTS
medial nucleus of the solitary tract
- NADPH
oxidase nicotinamide adenine dinucleotide phosphate-oxidase
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- phox
phagocyte oxidase
- RAC
Rho-related C3 botulinum toxin substrate
- ROS
reactive oxygen species
- rER
rough endoplasmic reteicula
- sER
smooth endoplasmic reteicula
- sol
solitary tract
- TH
tyrosine hydroxylase
- TS
Tris-buffered saline
- vo
vesicular orgenelle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Campbell RC, Warnock DG. Oxidative stress in hypertension and chronic kidney disease: role of angiotensin II. Seminars in Nephrology. 2004;24:101–114. doi: 10.1016/j.semnephrol.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Agassandian K, Fazan VP, Margaryan N, Dragon DN, Riley J, Talman WT. A novel central pathway links arterial baroreceptors and pontine parasympathetic neurons in cerebrovascular control. Cell Mol Neurobiol. 2003;23:463–478. doi: 10.1023/A:1025059710382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull. 2000;51:107–110. doi: 10.1016/s0361-9230(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Barraco IRA, editor. Nucleus of the Solitary Tract. CRC; Boca Raton: 1994. [Google Scholar]
- Biondo B, Magagnin S, Bruni B, Cazzullo A, Tosi D, Matturri L. Glial and neuronal alterations in the nucleus tractus solitarii of sudden infant death syndrome victims. Acta Neuropathologica. 2004;108:309–318. doi: 10.1007/s00401-004-0895-2. [DOI] [PubMed] [Google Scholar]
- Buller KM, Smith DW, Day TA. NTS catecholamine cell recruitment by hemorrhage and hypoxia. Neuroreport. 1999;10:3853–3856. doi: 10.1097/00001756-199912160-00024. [DOI] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Campese VM, Ye S, Zhong H, Yanamadala V, Ye Z, Chiu J. Central ROS play a role in regulation of systemic blood pressure, possibly by modulating SNS activity. Am J Physiol. 2004;287:H695–703. doi: 10.1152/ajpheart.00619.2003. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JY, Chen WC, Lee HY, Chang TJ, Chan SH. Phosphorylation of transcription factor cyclic-AMP response element binding protein mediates c-fos induction elicited by sustained hypertension in rat nucleus tractus solitarii. Neurosci. 1999;88:1199–1212. doi: 10.1016/s0306-4522(98)00273-5. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Hemodynamic regulation of tyrosine hydroxylase messenger RNA in medullary catecholamine neurons: a c-fos-guided hybridization histochemical study. Neurosci. 1995;66:377–390. doi: 10.1016/0306-4522(94)00600-a. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Differential time- and dose-related effects of haemorrhage on tyrosine hydroxylase and neuropeptide Y mRNA expression in medullary catecholamine neurons. Eur J Neurosci. 1998;10:3747–3758. doi: 10.1046/j.1460-9568.1998.00387.x. [DOI] [PubMed] [Google Scholar]
- Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circulation Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- De Caro R, Parenti A, Montisci M, Guidolin D, Macchi V. Solitary tract nuclei in acute heart failure. Stroke. 2000;31:1187–1193. doi: 10.1161/01.str.31.5.1187. [DOI] [PubMed] [Google Scholar]
- Dewey RB. Autonomic dysfunction in Parkinson's disease. Neurologic Clinics. 2004;22(3 Suppl):S127–139. doi: 10.1016/j.ncl.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Diz DI, Westwood B, Averill DB. AT(1) antisense distinguishes receptors mediating angiotensin II actions in solitary tract nucleus. Hypertension. 2001;37:1292–1297. doi: 10.1161/01.hyp.37.5.1292. [DOI] [PubMed] [Google Scholar]
- Ferrari MF, Almeida RS, Chadi G, Fior-Chadi DR. Acute changes in 3H-PAC and 125I-PYY binding in the nucleus tractus solitarii and hypothalamus after a hypertensive stimulus. Clinical Experimental Hypertension. 2002;24:169–186. doi: 10.1081/ceh-120003199. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Briggs JE, Billington CJ, Kotz CM, Levine AS. Opioid receptor blockade in rat nucleus tractus solitarius alters amygdala dynorphin gene expression. Am J Physiol. 2002;283:R161–167. doi: 10.1152/ajpregu.00480.2001. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Aicher SA, Milner TA, Pickel VM. Subcellular localization of alpha-2A-adrenergic receptors in the rat medial nucleus tractus solitarius: regional targeting and relationship with catecholaminergic neurons. J Comp Neurol. 2001;433:193–207. doi: 10.1002/cne.1135. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Huang J, Speth RC, Iadecola C, Pickel VM. Angiotensin II AT-1A receptor immunolabeling in rat medial nucleus tractus solitarius neurons: subcellular targeting and relationships with catecholamines. Neurosci. 2005;130:713–723. doi: 10.1016/j.neuroscience.2004.08.057. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Brody MJ, Fink GD, Buggy J, Johnson AK. Role of central catecholamines in the control of blood pressure and drinking behavior. Brain Res. 1979;178:161–173. doi: 10.1016/0006-8993(79)90095-7. [DOI] [PubMed] [Google Scholar]
- Gozal D, Gozal E, Simakajornboon N. Signaling pathways of the acute hypoxic ventilatory response in the nucleus tractus solitarius. Respiration Physiol. 2000;121:209–221. doi: 10.1016/s0034-5687(00)00129-8. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J of Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Mitsuyama S, Yamamoto E, Tanaka T, Zhan Y, Izumi Y, Izumiya Y, Ioroi T, Wanibuchi H, Iwao H. Critical role of angiotensin II in excess salt-induced brain oxidative stress of stroke-prone spontaneously hypertensive rats. Stroke. 2005;36:1083–1088. doi: 10.1161/01.STR.0000163084.16505.e3. [DOI] [PubMed] [Google Scholar]
- Kimura S, Zhang G, Nishiyama A, Shokoji T, Yao L, Fan Y, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H Oxidase- and Mitochondria-Derived Reactive Oxygen Species in Cardioprotection of Ischemic Reperfusion Injury by Angiotensin II. Hypertension. 2005;45:860–866. doi: 10.1161/01.HYP.0000163462.98381.7f. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. Reduced nicotinamide nucleotides prevent nitration of tyrosine hydroxylase by peroxynitrite. Brain Res. 2002;933:85–89. doi: 10.1016/s0006-8993(02)02307-7. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Sadidi M, Liu X, Kreipke C, Geddes T, Borges C, Watson JT. Peroxynitrite-induced nitration of tyrosine hydroxylase: identification of tyrosines 423, 428, and 432 as sites of modification by matrix-assisted laser desorption ionization time-of-flight mass spectrometry and tyrosine-scanning mutagenesis. J Biol Chem. 2002;277:14336–14342. doi: 10.1074/jbc.M200290200. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Lassègue B, Clempus RE. Vascular NAD(P)H oxidase specific features, expression, and regulation. Am J Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Prog Neurobiol. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- Mayorov DN, Head GA, De Matteo R. Tempol attenuates excitatory actions of angiotensin II in the rostral ventrolateral medulla during emotional stress. Hypertension. 2004;44:101–106. doi: 10.1161/01.HYP.0000131290.12255.04. [DOI] [PubMed] [Google Scholar]
- Misu Y, Yue JL, Okumura Y, Miyamae T, Ueda H. Altered basal release and depressor effect of L-DOPA in the nucleus tractus solitarii of spontaneously hypertensive rats. Clin Exper Pharmacol Physiol. 1995;22:S34–36. doi: 10.1111/j.1440-1681.1995.tb02946.x. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circulation Research. 2001;89:365–372. doi: 10.1161/hh1601.094988. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster H. The fine structure of the nervous system. Oxford University Press; New York: 1991. [Google Scholar]
- Potts PD, Ludbrook J, Gillman-Gaspari TA, Horiuchi J, Dampney RA. Activation of brain neurons following central hypervolaemia and hypovolaemia: contribution of baroreceptor and non-baroreceptor inputs. Neurosci. 2000;95:499–511. doi: 10.1016/s0306-4522(99)00426-1. [DOI] [PubMed] [Google Scholar]
- Sangaleti CT, Crescenzi A, Michelini LC. Endogenous angiotensin and pressure modulate brain angiotensinogen and AT1A mRNA expression. Hypertension. 2004;43:317–323. doi: 10.1161/01.HYP.0000112030.79692.21. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sorrentino DM, German DC, de Lacalle S. Medullary catecholaminergic neurons in the normal human brain and in Parkinson's disease. Annals of Neurology. 1991;29:577–584. doi: 10.1002/ana.410290602. [DOI] [PubMed] [Google Scholar]
- Schroder R, Linke RP. Cerebrovascular involvement in systemic AA and AL amyloidosis: a clear haematogenic pattern. Virchows Archiv. 1999;434:551–560. doi: 10.1007/s004280050383. [DOI] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- Smythies J, Galzigna L. The oxidative metabolism of catecholamines in the brain: a review. Biochimica et Biophysica Acta. 1998;1380:159–162. doi: 10.1016/s0304-4165(97)00131-1. [DOI] [PubMed] [Google Scholar]
- Snyder DW, Nathan MA, Reis DJ. Chronic lability of arterial pressure produced by selective destruction of the catecholamine innervation of the nucleus tractus solitarii in the rat. Circ Res. 1978;43:662–671. doi: 10.1161/01.res.43.4.662. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Davis DG, Bigelow TM, Rasheed K, Landers TM, Liu H, Coyne CM, Hunsaker JC. Increased ALZ-50 immunoreactivity in sudden infant death syndrome. J of Child Neurol. 1996;11:101–107. doi: 10.1177/088307389601100208. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Comm. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu G, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neurosci. 2005;132:233–238. doi: 10.1016/j.neuroscience.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci. 2004;24:5516–5524. doi: 10.1523/JNEUROSCI.1176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cuzon VC, Pickel VM. Postnatal development of mu-opioid receptors in the rat caudate-putamen nucleus parallels asymmetric synapse formation. Neurosci. 2003;118:695–708. doi: 10.1016/s0306-4522(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol. 1997;272:R59–R67. doi: 10.1152/ajpregu.1997.272.1.R59. [DOI] [PubMed] [Google Scholar]
- Wolin MS. Subcellular localization of Nox-containing oxidases provides unique insight into their role in vascular oxidant signaling. Arteriosclerosis, Thrombosis and Vascular Biology. 2004;24:625–627. doi: 10.1161/01.ATV.0000117201.14603.5d. [DOI] [PubMed] [Google Scholar]
- Xin W, Shen XM, Li H, Dryhurst G. Oxidative metabolites of 5-S-cysteinylnorepinephrine are irreversible inhibitors of mitochondrial complex I and the alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes: possible implications for neurodegenerative brain disorders. Chem Res Toxicol. 2000;13:749–760. doi: 10.1021/tx990170t. [DOI] [PubMed] [Google Scholar]
- Yu L, DeLeo FR, Biberstine-Kinkade KJ, Renee J, Nauseef WM, Dinauer MC. Biosynthesis of flavocytochrome b558. gp91(phox) is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J Biol Chem. 1999;274:4364–4369. doi: 10.1074/jbc.274.7.4364. [DOI] [PubMed] [Google Scholar]
- Zigmond RE, Schwarzschild MA, Rittenhouse AR. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophysics Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–723. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]


