Abstract
Background/Objective:
Previous investigations have identified muscular imbalance in the shoulder as a source of pain and injury in manual wheelchair users. Our aim was to determine whether a correlation exists between strength and pushrim biomechanical variables including: tangential (motive) force (Ft), radial force (Fr), axial force (Fz), total (resultant) force (FR), fraction of effective force (FEF), and cadence.
Methods:
Peak isokinetic shoulder strength (flexion [FLX], extension [EXT], abduction [ABD], adduction [ADD], internal rotation [IR], and external rotation [ER]) was tested in 22 manual wheelchair users with a BioDex system for 5 repetitions at 60°/s. Subjects then propelled their own manual wheelchair at 2 speeds, 0.9 m/s (2 mph) and 1.8 m/s (4 mph), for 20 seconds, during which kinematic (OPTOTRAK) and kinetic (SMARTWHEEL) data were collected. Peak isokinetic forces in the cardinal planes were correlated with pushrim biomechanical variables.
Results:
All peak torque strength variables correlated significantly (P ≤ 0.05) with Ft, Fr, and FR, but were not significantly correlated with Fz, FEF, or cadence. Finally, there were no relationships found between muscle strength ratios (for example, FLX/EXT) and Ft, Fr, FR, Fz, or FEF.
Conclusion:
There was a correlation between strength and force imparted to the pushrim among wheelchair users; however, there was no correlation found in wheelchair propulsion or muscle imbalance. Clinicians should be aware of this, and approach strength training and training in wheelchair propulsion techniques separately.
Keywords: Force, Shoulder pain, Wheelchair propulsion, Pushrim biomechanics, Spinal cord injuries, Muscle strength, Strength training, Paraplegia
INTRODUCTION
The high incidence of upper-extremity pain creates a challenge for manual wheelchair users (MWUs) when performing daily activities (work, play, wheeled propulsion, driving, and transferring). Sie and colleagues found that 64% of people with paraplegia reported some sort of upper-extremity pain (1). Of the individuals who reported pain, 32% reported shoulder pain. As MWUs with paraplegia became older, they experienced up to an 85% increase in shoulder pain within 5 to 19 years after injury (1). Forty-two percent of the MWUs with paraplegia in a study by Curtis and colleagues also experienced shoulder pain (2). Weight-bearing and repetitive activities have been thought to alter the shoulder joint's structure (3–5), resulting in upper-extremity pain and injuries. Studies investigating the cause of upper-extremity injuries in MWUs (6–9) show factors that contribute to upper-extremity pathology include increased body weight, higher seat position, and a longer duration of disability. Recent evidence suggests that wheelchair propulsion may contribute to the muscle imbalances observed in the shoulder because of the forces imposed at the joint, selective recruitment of agonist muscles during wheelchair propulsion, and repetitive movement patterns (10–12).
Wheelchair propulsion involves 2 separate phases, the propulsive and the recovery phase (13). The propulsive phase is initiated when the hand comes into contact with the pushrim and continues until the point at which contact is removed at the end of the stroke. The recovery phase involves the motion when the hands disengage from the pushrim until the upper extremities swing back to contact the pushrim once again. As a result of years of wheelchair propulsion, shoulder muscles active during the push phase are believed to become stronger, whereas the muscles that are involved during the recovery phase remain at the same strength (10, 11).
The stabilizing components of the shoulder (rotator cuff, deltoid, and long head of the biceps brachii muscles) may be altered because of the repetitive nature of wheelchair propulsion (10, 12). The muscles active during wheelchair propulsion (internal rotators, adductors, and flexors) (14) may become stronger as new movement patterns are used, creating an imbalance at the shoulder joint. The movement patterns may then be altered, causing the supraspinatus muscle to be impinged between the humeral head and the acromion, creating pain and inflammation (15), and possibly leading to rotator cuff tears.
Previous investigations into upper-extremity isometric strength in individuals with spinal cord injuries reported that men with paraplegia displayed no significant maximum isometric shoulder strength differences when compared with the able-bodied controls (16). The authors suggested that muscle imbalance did not play a significant role in secondary upper-extremity injuries in MWUs.
Bernard and colleagues investigated the isokinetic strength of 7 athletic men with paraplegia (11). Internal rotation (IR) and external rotation (ER) of both shoulders were tested at 60, 180, and 300°/s for 5, 5, and 10 repetitions, respectively. They found IR/ER ratios of 1.40 to 1.60, with internal rotators generally being stronger than the external rotators. In general, the rotator cuff muscles (supraspinatus, subscapularis, infraspinatus, and teres minor) are thought to provide dynamic stabilization to the humeral head on the glenoid fossa (17, 18). Other authors believe that the stronger internal rotators relative to their antagonists may predispose MWUs to secondary injuries in the shoulders (11).
Burnham and colleagues investigated the upper-extremity strength of 19 male wheelchair athletes and matched able-bodied controls (10). Shoulder examinations were performed on each wheelchair athlete to determine whether impingement was present. If 2 of the following 5 clinical signs were present, the subjects were deemed to have shoulder impingement: (a) painful arc of abduction (ABD); (b) pain in the “impingement position” (15, 19); (c) pain with resisted shoulder ABD, ER, or flexion (FLX); (d) tenderness to palpation over the greater tuberosity, lesser tuberosity, or bicipital groove; or (e) wasting of the supraspinous or infraspinous fossae. Twenty-six percent had shoulder impingement and displayed weakness in adduction (ADD), IR, and ER when comparing the affected to the nonaffected (no signs of impingement) shoulder. When the wheelchair athletes were compared with the able-bodied controls, Burnham and colleagues discovered that the wheelchair athletes displayed a larger ABD/ADD strength ratio (10). Weakness of shoulder adductor muscles was observed in the wheelchair athletes. They further suggest that this weakness may heighten the wheelchair athlete's risk of shoulder impingement, because the stronger abductors may pull the humeral head further up into the subacromial space (10).
The purpose of this study was to examine the peak shoulder isokinetic torque and muscle ratios in individuals who use a manual wheelchair, and to determine how the shoulder strength and muscle ratios relate to wheelchair biomechanics. Our aim was to determine whether a correlation exists between strength and pushrim biomechanical variables including tangential (motive) force (Ft), radial force (Fr), axial force (Fz), total (resultant) force (FR), fraction of effective force (FEF), and cadence. Further, we aimed to establish whether a correlation exists between muscle imbalance, as determined by muscle ratios, and pushrim biomechanics/efficiency. We hypothesized that pushrim forces (Ft, Fr, and Fz) would correlate with shoulder isokinetic peak torque and isokinetic peak torque ratios at both slow (0.9 m/s) and fast speeds (1.8 m/s).
METHODS
Subject Recruitment
The subjects were recruited via flyers and an in-house database containing previously tested subjects (6,13,20–23). The Investigational Review Board approved the protocol, and informed consent was obtained before any testing. The subjects qualified for the study on the following basis: (a) a manual wheelchair is propelled for mobility; (b) a manual wheelchair has been the primary means of mobility for at least the last 6 months; (c) a blood pressure below 160/90 mmHg and a resting heart rate between 50 and 100 bpm; (d) no known heart or blood vessel disease or abnormalities; (e) not taking a muscle-enhancing supplement (ie, creatine monohydrate) within the last 30 days; (f) no participation in a sporting event that could affect upper-extremity muscle strength; (g) no signs or diagnosis of gout; (h) no history of a hernia in the last 2 months; (i) not currently suffering with an illness; (j) no major illness, surgery, or hospitalization in the last 2 months; (k) not pregnant at time of testing; (l) no cardiopulmonary (heart/lung) condition that may be exacerbated by lifting, pulling, or pushing an object; (m) subject's stated age in the range of 18 to 65 years; and (n) subject has a complete or incomplete spinal cord injury (SCI) at or below T2.
Data Collection
Isokinetic Procedures
The isokinetic testing protocol has been previously described (23), and, therefore, an abbreviated version is presented. All isokinetic data were collected using a BioDex System 3 (FP_version 1.00 1.03; BioDex Medical Systems, Shirley, NY) to measure the concentric shoulder strength of each subject at a constant speed of 60°/s. Each subject had his or her blood pressure and resting heart rate measured before testing. The upper-extremity strength of each subject was assessed bilaterally in a randomized order. Each subject was instructed to perform 5 repetitions of the following movements with maximum voluntary effort: (a) shoulder FLX/extension (EXT) in the sagittal plane from 0 to 50°; (b) shoulder ABD/ADD in the frontal plane from 25 to 75°; and (c) shoulder IR/ER in the transverse plane from 0 to 45° (22). From strength testing, FLX, EXT, ABD, ADD, ER, and IR peak shoulder isokinetic torques were calculated. Although wheelchair propulsion is multiplanar, strengthening exercises are usually conducted in isolated planes of motion. We, therefore, measured uniplanar strength in this protocol.
From this testing, muscle ratios (FLX/EXT, ABD/ADD, and IR/ER) were also calculated. The subjects rested for 5 minutes between each exercise to ensure fatigue was not a confounding factor. A demonstration of the exercises was provided before testing. The subjects then performed 2 submaximal repetitions to become familiarized with the testing. After the practice repetitions, the subjects rested for 5 minutes, and maximal repetition testing for each of the muscle groups followed. After all of the exercises were performed, the subjects rested again for 5 minutes. Blood pressure and resting heart rate were then rechecked. A rest period of 30 to 60 minutes was taken before wheelchair biomechanics testing took place.
Wheelchair Biomechanics Testing
A 4-belt tie down system was used to secure each subject's own manual wheelchair onto a 2-drum dynamometer, centering the subjects evenly between 2 camera systems on either side (24). The resistance of the dynamometer was set to emulate the rolling resistance of a tiled surface (25). All wheelchair testing was completed in the same order. The subjects were acclimated to the testing environment and procedures by propelling their wheelchair at a self-selected, comfortable speed for 3 to 5 minutes. The subjects then performed 2 trials of wheelchair propulsion, one at 0.9 m/s and the second at 1.8 m/s. Each trial was approximately 60-seconds long. Once a steady speed was achieved, data were collected for 20 seconds. Between each trial, the subjects had a rest period of 3 to 5 minutes. A monitor was placed in front of the subjects to provide visual speed feedback, and verbal feedback was scripted. All strokes during the 20-second trials were analyzed at the 2 speeds.
Experimental Protocol
Kinematic System
Kinematic data were collected in real time by a three-dimensional (3-D) motion analysis system (OPTOTRAK; Northern Digital Inc, Waterloo, Canada). Each subject's movements were obtained by placing a camera system on the right and left sides of the person. Both cameras were synchronized and calibrated to view the same markers in real time. Infrared markers were placed bilaterally over the third metacarpophalangeal joint, the ulnar styloid, and the radial styloid. The kinematic data were collected for 20 seconds at 60 Hz for each trial (22). The kinematic data were filtered with a low-pass, eighth-order Butterworth filter, with an 8-Hz cut-off (26).
Kinetic System
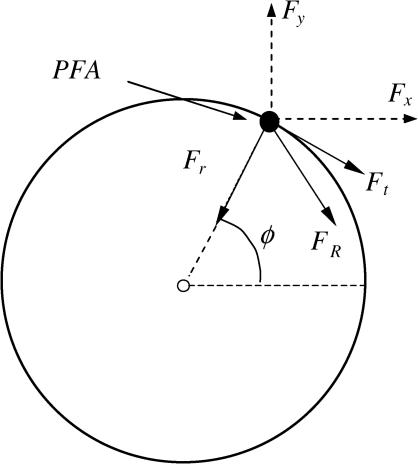
The subject's wheelchair wheels were removed and replaced by instrumented SMARTWheels (Three Rivers Holdings LLC, Phoenix, AZ (27). The wheels are capable of measuring the amount of 3-D forces (Fx, Fy, and Fz) and moments (Mx, My, and Mz) exerted on the wheel rim during wheelchair propulsion (27). The forces in the global coordinate system (Fx and Fy) were placed into a pushrim coordinate system consisting of Ft, Fr, and Fz (Figure 1) (27).
Figure 1. Forces in a global (Fx and Fy) and pushrim coordinate system (Ft, Fr, and Fz). The PFA is also shown. Fz is not shown. The direction of Fz is out of the page.
The FEF (Ft squared divided by the FR applied to the pushrim squared), and cadence (number of strokes per minute) were also calculated. The kinetic data were collected at 240 Hz, and synchronized to the 20 seconds of kinematic data. An eighth-order, low-pass Butterworth filter with a 25-Hz cut-off was used to filter the kinetic data (26).
Statistical Analysis
The peak shoulder isokinetic torque (FLX, EXT, ABD, ADD, IR, and ER) values obtained during isokinetic testing were averaged for each side. Because data were normally distributed, a Pearson correlation was performed on all of the isokinetic peak torque variables to determine whether the right and left sides could be combined to reduce the number of variables under investigation.
The force parameters measured from the SmartWHEEL were transformed from the global coordinate system to the pushrim coordinate system for Ft, Fr, and Fz, based on the method of point of force application (PFA). PFA is calculated by synchronizing kinematic and kinetic data (36). Briefly, the PFA is the point on the pushrim that best represents the location where force is applied by the hand (28). Previous studies have shown that this point lies between the third metacarpophalangeal joint and the midpoint of the radial and ulnar styloid processes (29). A 2-tailed Pearson bivariate correlations test was performed on all of the pushrim forces and moments variables to determine whether the right and left side could be combined to further reduce the number of variables.
A 2-tailed Pearson bivariate correlation test was used to establish whether peak shoulder isokinetic torque (FLX, EXT, ABD, ADD, IR, and ER) and shoulder isokinetic peak torque ratios (FLX/EXT, ABD/ADD, and IR/ER) were related to pushrim variables (Ft, Fr, Fz, FR, FEF, and cadence). Because, in this data set, we had previously determined that some peak shoulder isokinetic strength variables (EXT, ABD, ADD, IR, and ER) are significantly correlated with neurological level of SCI (23), a partial correlation controlling for level of injury was performed for correlating these variables to pushrim variables. All data analyses were done using the MatLab v5.3 (Math Works Inc, Natick, MA) mathematical program. All statistical calculations were performed by SPSS v10.1 statistical package (SPSS Inc, Chicago, IL). Variables were considered statistically significant at P ≤ 0.05, and a trend was present at P ≤ 0.10.
RESULTS
Subject
A total of 22 subjects (6 female) with a median SCI level of T8.8 (range = T2 to L1) participated in this study. The average age, weight, height, and years after SCI, respectively, are as follows: 43.0 ± 9.5 years, 732.1± 166.9 N (74.7 ± 17.0 kg), 174.0 ± 9.8 cm, and 16.6 ± 7.4 years.
Reduction of Data
The results of the Pearson correlation displayed a strong relationship between the right and left sides (r = 0.86; average P-value = 0.001) when investigating peak shoulder isokinetic torque values, thus, the sides were combined into a single value. Similarly, for the pushrim forces, the right and left sides were correlated for all variables (average r = 0.731; average P-value = 0.001); therefore, right and left sides were also combined. We followed this methodology in previous papers (6,13). Because the data points are not independent, they could not be considered separately. Along these lines, there was a significant correlation between the pushrim variables for the 0.9 m/s trial and the 1.8 m/s trial (P < 0.001 for FR, Ft, Fr, and Fz). Therefore, for correlations with the peak shoulder isokinetic torque data, the pushrim variables at the 2 speeds data were averaged.
Shoulder Isokinetic Peak Torque
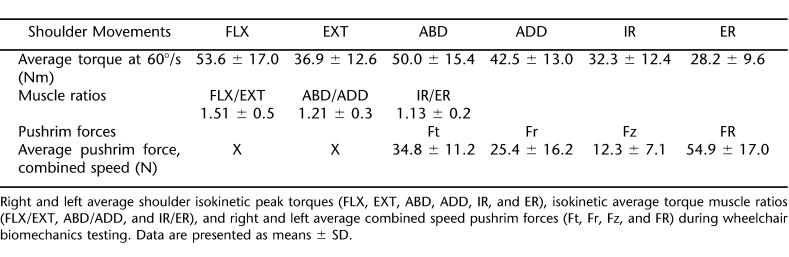
The major motion involved with manual wheelchair propulsion involves FLX, ADD, and IR. Therefore, it was not surprising that greatest peak shoulder isokinetic torque value would be FLX, which was 51% greater when compared with EXT (Table 1). In addition, the peak shoulder IR was observed to be 13% greater when compared with ER. However, when examining the peak shoulder isokinetic torque values of ABD and ADD, a 15% difference was observed, with ABD producing the larger value of the two. Muscle ratios are displayed in Table 1.
Table 1.
Average Peak Torques, Muscle Ratios, and Pushrim Kinetics
Shoulder Isokinetic Peak Torque and Pushrim Force Correlations
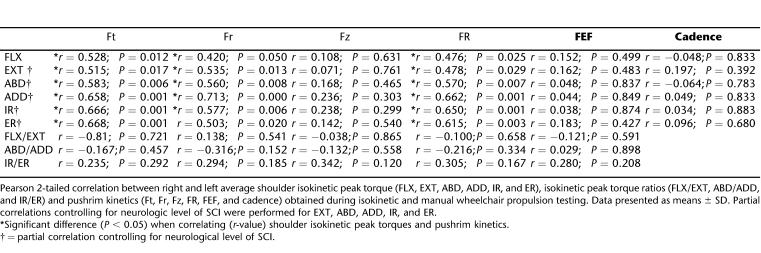
The Ft was the greatest pushrim force, followed by the Fr and Fz, respectively, for the averaged values of the 2 speed trials (Table 1). Ft, Fr, and FR were significantly correlated with all muscle strength variables (Table 2). On the other hand, Fz, FEF, and cadence were not correlated with any of the strength variables. Finally, none of the muscle ratios were significantly correlated to pushrim variables.
Table 2.
Shoulder Torque and Pushrim Forces; Pearson 2-Tailed Correlations; Right and Left Sides Combined; 0.9 m/s and 1.8 m/s Speeds Combined
DISCUSSION
Researchers have investigated various biomechanical aspects of wheelchair propulsion to find what factors may cause secondary upper-extremity injuries in MWUs. This study concentrated on muscle strength and its relationship to wheelchair propulsion biomechanics.
Peak Shoulder Isokinetic Torque
The primary motion for wheelchair propulsion is shoulder FLX, along with accessory motions of shoulder ADD and IR. Therefore, it was not surprising that the greatest peak isokinetic torque production came from FLX. Along these lines, we expected that the strength of muscles used predominantly during wheelchair propulsion would be highly correlated with pushrim variables, whereas antagonist muscles would not be highly correlated with pushrim variables. In fact, we did find that FLX, ADD, and IR were significantly correlated with Ft, Fr, and FR (Table 2), but that none of the strength variables correlated with Fz, FEF, or cadence. Therefore, stronger individuals do, in fact, exert a higher motive force on the pushrim; however, this increase in force does not necessarily result in a higher FEF (Ft2/FR), because they are also exerting a higher total force on the pushrim. Furthermore, not only were FLX, ADD, and IR significantly correlated with Ft, Fr, and FR, but so were the muscle antagonist torques, EXT, ABD, and ER, indicating that the correlation between pushrim variables and strength is not a direct result of isolated strengthening of the muscles predominantly recruited during wheelchair propulsion.
It has been shown that individuals who are more effective at wheelchair propulsion push the wheel with a increased push time, decreased cadence, and greater push angle (the angle created by the scalar with an origin wheelchair hub extending to point of contact on the pushrim at the beginning of a propulsive stroke and the scalar with an origin wheelchair hub, extending to the point of hand release of the pushrim at the end of a stroke (30,31). Boninger et al have shown that cadence is related to an increased predisposition for median nerve injury (6), as is the case with most repetitive strain injuries (32). In our study, although strength was positively correlated with the FR applied to the pushrim, this was not correlated with a decreased cadence. Clinicians should, therefore, implement 2 types of rehabilitation strategies for individuals with SCI: (a) stretching and strengthening of the shoulder muscle complex, and (b) emphasis on proper wheelchair propulsion techniques. To address shoulder impingement in individuals with an SCI who use a manual wheelchair, strengthening and stretching exercises should be encouraged to maintain proper glenohumeral alignment and to increase the shoulder complex's resistance to fatigue. In addition, because upper-extremity strengthening alone does not seem to result in an increased effectiveness of propulsion, clinicians should emphasize proper propulsion techniques during the early stages of rehabilitation after SCI. Such techniques include, as indicated before, using increased push angles with a greater amount of time spent on the pushrim, allowing for a decreased cadence.
Muscle Ratios
When considering muscle ratios, based on the muscles predominantly recruited during wheelchair propulsion, as expected, FLX was stronger than EX, and IR was stronger than ER. What was not expected was the fact that the ABD was greater than the ADD of the subjects. This phenomenon may be attributed to the fact that so many movements of daily activities for individuals with SCI are performed at or above shoulder height, which would have a tendency to strengthen shoulder abductors and shoulder forward flexors. In theory, an imbalance in the strength of the abductor muscles when compared with shoulder adductors may lead to shoulder impingement because the abductors cause an upward displacement of the humeral head within the glenoid cavity, thereby decreasing the subacromial space (33). However, it is still unknown whether these muscle imbalances lead to injury.
The muscle ratio most highly considered to be indicative of a predisposition for shoulder pathology is the ratio between internal rotators and external rotators. Some have found that a modified ratio may be related to impingement and instability of the shoulder (34); however, most of the research that exists to date relating to muscle imbalance at the shoulder has been conducted in unimpaired athletes, such as baseball players and tennis players. The few studies that do exist in MWUs have demonstrated conflicting results regarding the presence of muscle imbalance in this population (10–12). Yet again, a link between the imbalance and pain or predisposition for upper-extremity injury has never been established. Along these lines, our study found no significant correlation between muscle ratios and push-rim biomechanics variables.
Our study is limited by the fact that this study considers only maximal torque production over a short period. Future studies should consider correlations between pushrim variables to muscle properties in the mode in which they operate. That is, because wheelchair propulsion is a repetitive and continuous task, it would be beneficial to conduct the same correlations considering the ability of the individuals to maintain a maximal torque output over an extended period of time or over a large number of repetitions. Rodgers et al have shown that there is a power shift in wheelchair propulsion with increasing fatigue, and that the predominant source of joint power during propulsion in a fatigued state shifts from the shoulder to the elbow and wrist (35). It is possible that in endurance-trained individuals, this shift is not as predominant. Our results revealed that maximal torque output is not correlated with effective force; however, it is possible that the ability to sustain a constant shoulder force output over time is, in fact, correlated with effective force. Future studies should investigate the effectiveness of wheelchair propulsion as individuals propel the wheelchair predominantly with shoulder muscles, as in a fresh state, vs propulsion using predominantly elbow and wrist muscles, as in a fatigued state.
Also, keeping in mind the fact that individuals who weigh more generally have an increased strength, it is understandable that they would need to push the chair with increased forces. According to Boninger et al, weight is related to the peak FR applied to the pushrim and to median nerve function (6), and an increased weight is associated with increased forces required to propel a wheelchair. They found that an increased weight of MWUs may increase the likelihood of carpal tunnel syndrome. From this study, authors suggested that a combination of weight loss and changes in pushrim biomechanics may help minimize the risk for median nerve injury.
Finally, we considered only concentric/concentric muscle ratios. This has been a limiting factor in previous studies considering muscle imbalance in MWUs (10–12). It has been reported that concentric strength ratios of these muscles are not as important as the “dynamic control ratio” that considers the concentric action of the internal rotators as it relates to the eccentric action of the external rotators (34). External rotators are not only important for maintaining glenohumeral positioning, but also act to decelerate the arm during various activities through eccentric contraction. Therefore, future studies should investigate the relationship between the dynamic control ratio of the shoulder and wheelchair pushrim biomechanics.
CONCLUSION
From these data, we conclude that increased shoulder strength does not necessarily indicate a more optimal manual propulsion strategy. Clinicians should consider principles of specificity in strength training for manual wheelchair propulsion, as well as proper propulsion techniques to maximize the effectiveness of the task. Future research should account for differences in the fatigue resistance of muscles used during wheelchair propulsion because they correlate with pushrim biomechanics. Furthermore, a longitudinal study examining the effects of muscle strength and its relationship to manual wheelchair propulsion may help to determine structural changes that the body undergoes over time. A longitudinal study comparing the kinematic and kinetic changes over time could identify the change in movement patterns that are suspected to be the root cause of muscle impingement and pain in individuals who use manual wheelchairs for their primary means of locomotion.
Footnotes
Partial funding for this research was provided by the U.S. Department of Veterans Affairs Rehabilitation Research & Development Services (Project B3057R), The National Institutes for Disability and Rehabilitation Research (H133A011107 and H133N407019), and the Paralyzed Veterans of America provided partial funding for this research.
REFERENCES
- Sie IH, Walters RL, Adkins RH, Gellman H. Upper extremity pain in the post-rehabilitation spinal cord injured patient. Arch Phys Med Rehabil. 1992;73:44–48. [PubMed] [Google Scholar]
- Curtis KA, Drysdale GA, Lanza D, Kolber M, Vitolo RS, West R. Shoulder pain in wheelchair users with tetraplegia and paraplegia. Arch Phys Med Rehabil. 1999;80:453–457. doi: 10.1016/s0003-9993(99)90285-x. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Hussey RW, Ha CY. Surgical decompression of impingement in the weight-bearing shoulder. Arch Phys Med Rehabil. 1993;74:324–327. [PubMed] [Google Scholar]
- Curtis KA, Dillon DA. Survey of wheelchair athletic injuries: common patterns and prevention. Paraplegia. 1985;23:170–175. doi: 10.1038/sc.1985.29. [DOI] [PubMed] [Google Scholar]
- Bayley JC. The weight-bearing shoulder. The impingement syndrome in paraplegics. J Bone Joint Surg Am. 1987;69(5):676–678. [PubMed] [Google Scholar]
- Boninger ML, Cooper RA, Baldwin MA, Shimada SD, Koontz A. Wheelchair pushrim kinetics: body weight and median nerve function. Arch Phys Med Rehabil. 1999;80:910–915. doi: 10.1016/s0003-9993(99)90082-5. [DOI] [PubMed] [Google Scholar]
- Masse LC, Lamontagne M, O'Riain MD. Biomechanical analysis of wheelchair propulsion for various seating positions. J Rehabil Res Dev. 1992;29:12–28. doi: 10.1682/jrrd.1992.07.0012. [DOI] [PubMed] [Google Scholar]
- Veeger HE, van der Woude LHV, Rozendal RH. Load on the upper extremity in manual wheelchair propulsion. J Electromyogr Kinesiol. 1991;1(4):270–280. doi: 10.1016/1050-6411(91)90014-V. [DOI] [PubMed] [Google Scholar]
- Burnham RS, Steadward RD. Upper extremity peripheral nerve entrapments among wheelchair athletes: prevalence, location, and risk factors. Arch Phys Med Rehabil. 1994;75:519–524. [PubMed] [Google Scholar]
- Burnham RS, May L, Nelson E, Steadward R, Reid DC. Shoulder pain in wheelchair athletes. The role of muscle imbalance. Am J Sports Med. 1993;21(2):238–242. doi: 10.1177/036354659302100213. [DOI] [PubMed] [Google Scholar]
- Bernard PL, Codine P. Isokinetic shoulder of paraplegics: observation of global and specific muscle ratio. Int J Rehabil Res. 1997;20(1):91–98. [PubMed] [Google Scholar]
- Miyahara M, Sleivert GG, Gerrard DF. The relationship of strength and muscle balance to shoulder pain and impingement syndrome in elite quadriplegic wheelchair rugby players. Int J Sports Med. 1998;19(3):210–214. doi: 10.1055/s-2007-971906. [DOI] [PubMed] [Google Scholar]
- Boninger ML, Baldwin MA, Cooper RA, Koontz AM, Chan LC. Manual wheelchair pushrim biomechanics and axle position. Arch Phys Med Rehabil. 2000;81:608–613. doi: 10.1016/s0003-9993(00)90043-1. [DOI] [PubMed] [Google Scholar]
- Mulroy SJ, Gronley JK, Newsam CJ, Perry J. Electromyographic activity of the shoulder muscles during wheelchair propulsion by paraplegic persons. Arch Phys Med Rehabil. 1996;77(2):187–193. doi: 10.1016/s0003-9993(96)90166-5. [DOI] [PubMed] [Google Scholar]
- Hawkins RJ, Kennedy JC. Impingement syndrome in athletes. Am J Sports Med. 1980;8:151–158. doi: 10.1177/036354658000800302. [DOI] [PubMed] [Google Scholar]
- Kotajarvi BR, Basford JR, An KN. Upper-extremity torque production in men with paraplegia who use wheelchairs. Arch Phys Med Rehabil. 2002;83(4):441–446. doi: 10.1053/apmr.2002.6685. [DOI] [PubMed] [Google Scholar]
- Lippitt S, Matsen F. Mechanisms of glenohumeral joint stability. Clin Orthop. 1993;291:20–28. [PubMed] [Google Scholar]
- Lippitt SB, Vanderhooft E, Harris SL, Sidles JA, Harryman DT, Matsen FA. Glenohumeral stability from concavity-compression: a quantitative analysis. J Shoulder Elbow Surg. 1993;2(1):27–35. doi: 10.1016/S1058-2746(09)80134-1. [DOI] [PubMed] [Google Scholar]
- Neer CS, Welsh RP. The shoulder in sports. Orthop Clin North Am. 1977;8:583–591. [PubMed] [Google Scholar]
- Koontz AM, Boninger ML, Towers J, Cooper RA, Baldwin MA. Pittsburgh, PA: 1999. Wheelchair propulsion forces and MRI evidence of shoulder impairment: Proceedings of the 23rd Annual Meeting of the American Society of Biomechanics; pp. 21–23. [Google Scholar]
- Boninger ML, Dicianno BE, Towers JD, Koontz AM, Cooper RA. Wheelchair propulsion forces and MRI evidence of shoulder injury. Arch Phys Med Rehabil. In press. [DOI] [PubMed]
- Koontz AM, Cooper RA, Boninger ML, Souza AL, Fay BT. Shoulder kinematics and kinetics during two speeds of wheelchair propulsion. J Rehabil Res Dev. 2002;39(6):635–649. [PubMed] [Google Scholar]
- Souza A, Boninger ML, Fitzgerald SG, Shimada SD, Cooper RA, Ambrosio F. Upper limb strength in individuals with spinal cord injury who use manual wheelchairs. J Spinal Cord Med. 2005;28(1):26–32. doi: 10.1080/10790268.2005.11753795. [DOI] [PubMed] [Google Scholar]
- Vosse AJ, Cooper RA, Dhaliwal B. Washington, DC: 1990. Computer control of a wheelchair dynamometer; pp. 59–60. Proceedings of the 13th Annual RESNA Conference. [Google Scholar]
- DiGiovine CP, Cooper RA, Boninger ML. Dynamic calibration of a wheelchair dynamometer. J Rehabil Res Dev. 2001;38(1):41–55. [PubMed] [Google Scholar]
- Cooper RA, DiGiovine CP, Boninger ML, Shimada SD, Koontz AM, Baldwin MA. Filter frequency selection for manual wheelchair biomechanics. J Rehabil Res Dev. 2002;39(3):323–336. [PubMed] [Google Scholar]
- Cooper RA, Robertson RN, VanSickle DP, Boninger ML. Methods for determining three-dimensional wheelchair pushrim forces and moments: a technical note. J Rehabil Res Dev. 1997;34(2):162–170. [PubMed] [Google Scholar]
- Cooper RA, VanSickle DP, Robertson RN, Boninger ML, Ensminger GJ. A method for analyzing center of pressure during manual wheelchair propulsion. IEEE Trans Biomed Eng. 1995;3:289–298. [Google Scholar]
- Cooper RA, Robertson RN, VanSickle DP, Boninger ML, Shimada SD. Projection of the point of force application onto a palmar plane of the hand during wheelchair propulsion. IEEE Trans Biomed Eng. 1996;4:133–142. doi: 10.1109/86.536768. [DOI] [PubMed] [Google Scholar]
- Boninger ML, Souza AL, Cooper RA, Fitzgerald SG, Koontz AM, Fay BT. Propulsion patterns and pushrim biomechanics in manual wheelchair propulsion. Arch Phys Med Rehabil. 2002;83:718–723. doi: 10.1053/apmr.2002.32455. [DOI] [PubMed] [Google Scholar]
- Brubaker CE. Wheelchair prescription: an analysis of factors that affect mobility and performance. J Rehabil Res Dev. 1986;23(4):19–26. [PubMed] [Google Scholar]
- Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11:343–358. doi: 10.1002/ajim.4700110310. [DOI] [PubMed] [Google Scholar]
- Irlenbusch U, Gansen HK. Muscle biopsy investigations on neuromuscular insufficiency of the rotator cuff: a contribution to the functional impingement of the shoulder joint. J Shoulder Elbow Surg. 2003;12(5):422–426. doi: 10.1016/s1058-2746(03)00036-3. [DOI] [PubMed] [Google Scholar]
- David G, Magarey ME, Jones MA, et al. EMG and strength correlates of selected shoulder muscles during rotations of the glenohumeral joint. Clin Biomech. 2000;15:95–102. doi: 10.1016/s0268-0033(99)00052-2. [DOI] [PubMed] [Google Scholar]
- Rodgers MM, McQuade KJ, Rasch EK, Keyser RE, Finley MA. Upper-limb fatigue-related joint power shifts in experiences wheelchair users and nonwheelchair users. J Rehabil Res Dev. 2003;40(1):27–37. doi: 10.1682/jrrd.2003.01.0027. [DOI] [PubMed] [Google Scholar]
- Yang YS, Koontz AM, Boninger ML, Cooper RA. Orlando, FL: 2004. Influence of gripping moments on mechanical efficiency of wheelchair propulsion. Proceedings 28th Annual RESNA Conference. www.resna.org/ProfResoucesPublications/Proceedings/2004/Papers/StudentScientific/Winners/Gripping.php. Accessed October 26, 2005. [Google Scholar]