Abstract
Background/Objective:
An assessment of neurological improvement after surgical intervention in the setting of traumatic thoracic spinal cord injury (SCI).
Methods:
A retrospective evaluation of a nonconsecutive cohort of patients with a thoracic SCI from T2 to T11. The analysis included a total of 12 eligible patients. The neurologic and functional outcomes were recorded from the acute hospital admission to the most recent follow-up. Data included patient age, level of injury, neurologic examination according to the Frankel grading system, the performance of surgery, and the mechanism of the time-related SCI decompression.
Results:
All patients had a complete thoracic SCI. The median interval from injury to surgery was 11 days (range, 1–36 days). Decompression, bone fusion, and instrumentation were the most common surgical procedures performed. The median length of follow-up was 18 months after surgery (range, 9–132 months). Motor functional improvement was seen in 1 patient (Frankel A to C).
Conclusion:
Surgical decompression and fusion imparts no apparent benefit in terms of neurologic improvement (spinal cord) in the setting of a complete traumatic thoracic SCI. To better define the role of surgical decompression and stabilization in the setting of a complete SCI, randomized, controlled, prospective studies are necessary.
Keywords: Spinal cord injuries, complete, Surgical decompression, Surgery, thoracic spine, Spinal fusion, thoracic
INTRODUCTION
Spinal trauma complicated by injury to the spinal cord is a devastating event on a personal and family level, as well as a tremendous financial burden to society because of its attendant morbidity, expense, and prolonged treatment requirements (1). Spinal injury occurs most frequently in young men with an average age of 35 years. The most frequent etiologies of injury are motor vehicle crashes and falls, followed by violence, sports-related injuries, and work-related accidents (2–4). Approximately 40% of patients with spinal cord injury (SCI) present with complete SCI, 40% with incomplete injury, and 20% with either no cord or only root lesions (5).
There has been a great deal of discussion as to which treatment course is most helpful in ensuring maximum neurologic improvement after an SCI (5–33). Spinal decompression in the setting of a traumatic thoracic SCI is controversial. To date, the role of decompression in patients with incomplete SCI is supported only by class 3 and limited class 2 evidence, but there is no definite evidence to support the role of decompression in complete SCI. The objective of this study was to examine the benefit of neural decompression in the setting of a complete thoracic SCI after trauma.
METHODS
Patients evaluated in this historical cohort study were all admitted to a regional level I trauma center in southeastern Iran from October 1994 through March 2005. The inclusion criteria were (a) complete neurological deficit attributable to a traumatic thoracic SCI (T2–T11); (b) at least a 6-month follow-up (9); and (c) SCI caused by an acute nonpenetrating traumatic event with radiographically documented spinal cord compression caused by cord encroachment by anterior vertebral body elements, disk material, or posterior vertebral elements as a result of fracture subluxation or dislocation.
The exclusion criteria were (a) Frankel grade B through E; (b) spinal cord abnormalities caused by other disease processes (eg, multiple sclerosis or pre-existing myelopathy as a result of severe spondylosis without trauma); and (c) severe cardiovascular shock. Of 108 patients who were evaluated during this period, 96 were excluded from the study because of the following reasons: neurologic examination consistent with a Frankel grade of B to E, inadequate documentation of pre-operative neurologic examination, complete SCI but no compression on myelography, lost to follow-up (<6-month follow-up), penetrating injury caused by gun shot or knife injury, or isolated root or cauda equina injury. This left 12 eligible patients with complete thoracic (T2–T11) SCI.
During the prehospital and acute care phase, the following data were collected for all patients: age, sex, associated injuries, mechanism of injury, and admitting and follow-up Frankel grade. The time intervals from injury to arrival at the Khatam-ol-anbia Emergency Department and to surgical decompression were also collected. Information was obtained from medical records, radiologic studies, and patient interviews. The type of surgical procedure was also recorded.
Neurologic Evaluation
Motor and sensory examinations were performed at admission, before surgery, immediately after surgery, and at the most recent follow-up. Neurologic function was measured by 3 parameters; neurologic recovery was recorded as any improvement in (a) motor or sensory function; (b) Frankel grading system (10); or (c) motor index score. Patients were assigned an initial motor index score that included manual muscle test scores of all key muscles, sensory examination (prick and touch), sacral and deep tendon reflexes, and muscle tone evaluation. Sensory level was recorded as the most caudal dermatomal level of bilateral intact sensation. Neurologic examinations were documented on admission, daily during the acute hospitalization, and at all follow-up outpatient encounters.
Treatment
Standard spinal immobilization and resuscitation were implemented by emergency medical personnel. All patients were prescribed intravenous methylprednisolone (30 mg/kg intravenous [IV] bolus over 15 minutes followed 45 minutes later by a 5.4 mg/kg/h intravenous infusion over 23 hours) if they arrived at the emergency room within 8 hours of the accident (34). All patients underwent preoperative myelography, computerized tomography (CT) imaging, and/or magnetic resonance imaging (MRI). Patients with CT-myelography or MRI-documented spinal cord compression (from vertebral burst fractures or fracture dislocations, persistent misalignment, epidural hematoma, or intracanalicular bone fragments) underwent surgical decompression and spinal column stabilization. The standard chosen procedure was a posterior transpedicular or extracavitary decompression and instrumented fusion. Adequacy of decompression was determined by a comparison of preoperative and postoperative CT and MRI scans (35).
Data Collection
Neurologic follow-up examinations were performed at in-hospital and outpatient follow-up visits by the primary author. In cases of the deceased, the last documented neurologic examination was used. Data collected were analyzed for age, sex, number of days before surgery in the acute hospital setting, number of days to most recent follow-up, motor score on admission to the acute hospital, both preoperative and postoperative, and motor score at most recent follow-up.
Outcome Assessment
A patient was considered to have an excellent result if they became ambulatory in the household or community or had marked improvement in ambulatory status. A good outcome was recorded if there was recovery of 1 or more motor-root levels in the lower extremities or partial recovery of multiple levels. A fair result was recorded if the individual had less recovery, but at least partial improvement of 1 or 2 motor-root levels, and a poor result was no improvement.
RESULTS
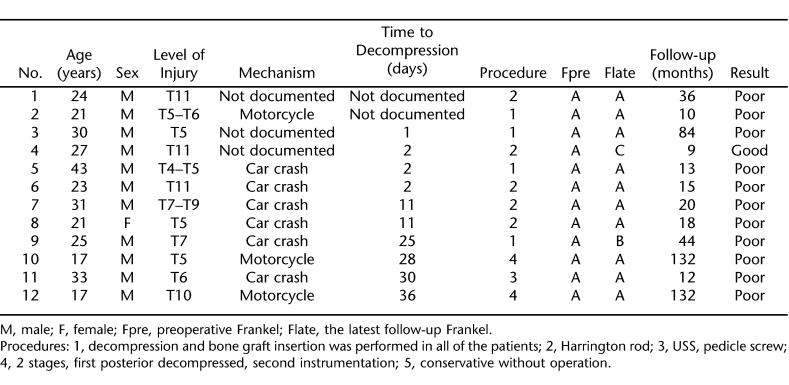
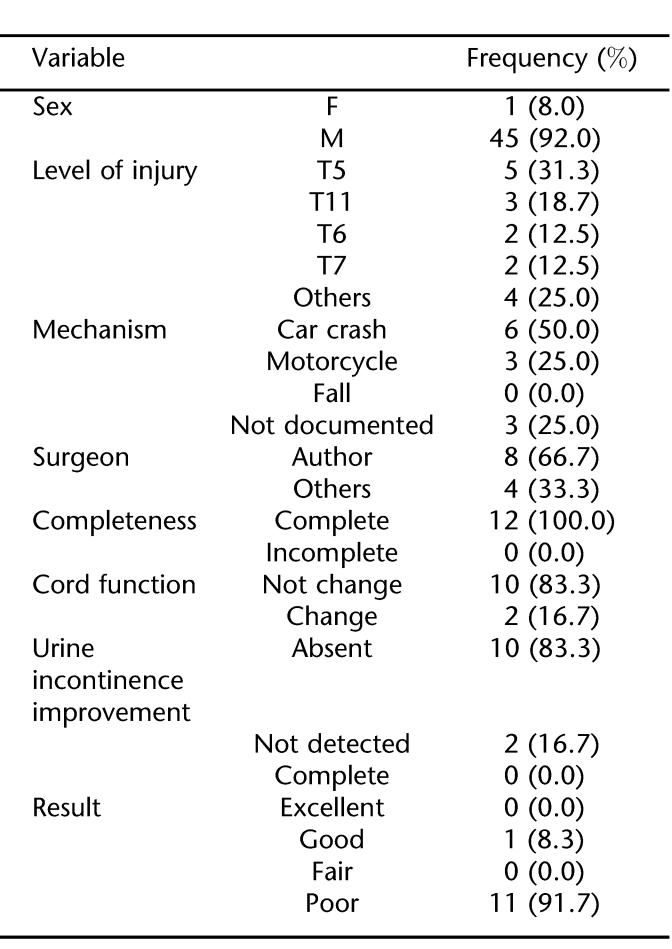
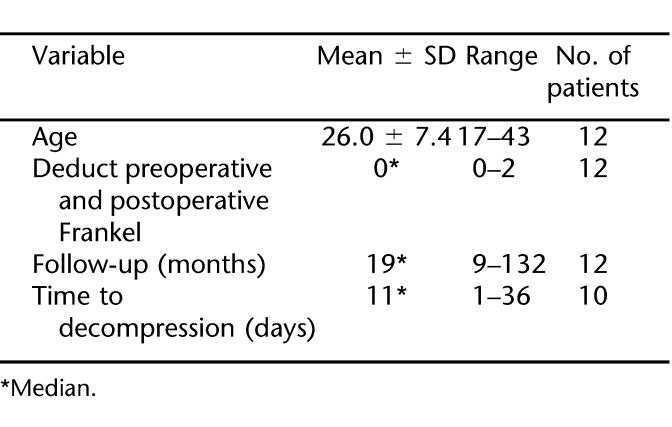
There were 12 eligible patients with neurologic injury levels from T2 to T11 (Table 1). Before treatment, all patients (100.0%) had a functionally complete neurological deficit below the level of injury. Mean patient age was 26.7 ± 7.5 years; 92% were men. The most common level of injury was T5, and the most frequent mechanism of injury was motor vehicle crashes. The median time interval from injury to surgery was 11 days and ranged from 1 to 36 days. The length of follow-up ranged from 9 months to 12 years, with a median time period of 18 months (Tables 2 and 3). The primary indications for surgery were documented spinal cord compression in the setting of a neurologic complete deficit and spinal instability. Sensori-motor functional improvement was seen in 2 patients; motor functional improvement was seen in only 1 patient (Frankel A–C).
Table 1.
Summary of Data From 12 Individuals With Complete Thoracic Spinal Cord Injury
Table 2.
Frequency Distribution of Some Variables in the Patients With Thoracic SCI
Table 3.
Mean, Median, and Range of Some Variables in the Patients With SCI
Eight complications were recorded in 5 patients, including continued cord compression from an inadequate decompression, deep wound infection, Klebsiella urinary tract infection, fever, bed sore, spasticity, and Amikacin-induced hearing loss. One patient with a complete T7 Frankel A SCI after a motor vehicle crash required 2 surgical procedures. An initial decompression and fusion procedure without instrumentation resulted in progressive kyphosis after cast removal. Twenty-two months after the initial index procedure, a revision posterior stabilization procedure was performed with instrumentation. At the 44-month follow-up, the patient was noted to have return of sensation in 1 lower extremity (Frankel B).
DISCUSSION
After spinal cord decompression, motor functional improvement was seen in only 1 patient (Frankel A–C), and sensory (without motor) improvement was seen in 1 other patient. Only 2 of 11 patients (18%) with initial adequate decompression experienced improvement of 1 grade on the Frankel or American Spinal Injury Association (ASIA) scales. One patient had an initial decompression that was later determined to be inadequate but was included in the study because of its fulfillment of the inclusion criteria of the study. Vale et al (36) observed that 33% of patients with a complete thoracic SCI improved at least 1 Frankel or ASIA grade. Why our results are different in terms of functional improvement is difficult to tell. It may be that the patients in the study of Vale et al were treated with aggressive blood pressure support, which may have improved the potential for neurologic improvement.
Our study showed that, in cases of complete thoracic SCI, there was no correlation between spinal cord decompression and motor improvement. In several studies, no patients with a complete neurological deficit improved after an anterior spinal decompression and fusion (13–15) or nonoperative management (16,37,38). In the opinions of these authors, emergent spinal decompression has no indication in the setting of a complete thoracic traumatic SCI.
In general, patients with a partial neurological deficit often show improved lower extremity motor and/or bladder function with either nonoperative or operative intervention (10–12,15–20,37–39).
Grootboom and Govender (14) treated the majority of their patients with injuries to the upper thoracic spine from T2 to T9 nonoperatively. All patients with a partial neurological deficit improved over time.
The review of the relevant clinical literature shows that most studies comparing decompressive surgery with conservative management fail to show any advantage of surgery in terms of neurologic improvement in the setting of a complete SCI (21–25). In the series of Tator et al (23), Bedbrook (40), and Wilmot and Hall (26), operative treatment did not seem to be superior to nonoperative treatment. Boerger et al (27) reported a meta-analysis on the value of surgical decompression in affecting neurological outcome in patients with thoracolumbar fractures. Their results showed that surgery did not offer a significant advantage compared with conservative treatment with respect to neurological outcome. Waters et al (28) showed motor recovery did not significantly differ between patients categorized in various surgical subgroups or between those having surgery and those treated nonoperatively. Geisler et al (29) concluded that the sparseness of prospective data on the treatment of traumatic SCI at 28 centers in North America suggested that treatment guidelines have limited empirical support and should be made cautiously. Bohlman and Freehafer (30) has reported that greater neurologic recovery occurs if surgical decompression is performed within 2 years after the injury.
The efficacy of decompression after SCI in enhancing neurological recovery in animal models has been widely shown (9,41–50). There are 8 prospective nonrandomized case series (class 2 evidence) (23,25,31–33,51–53) and several retrospective case series with historical controls (class 3 evidence) that have addressed the role of spinal cord decompression. None has shown an advantage to surgery in the setting of a complete SCI.
Spinal fusion with instrumentation is useful for purposes of mobilization, prevention of deformity with late pain, and less reliance on the need for cumbersome braces in the patient population with paraplegia.
Anterior thoracolumbar decompression is a useful surgical strategy in cases of trauma, infection, or tumor that causes compression of the neural tissues, resulting in an incomplete neurologic deficit (54).
In our patient population, victims of motorcycle crashes experienced more severe injury in terms of extremity trauma than those involved in motor vehicle crashes. The typical mechanism of injury after a motorcycle accident was a flexion injury to the thoracic spine (55).
Our study did not show that surgical decompression was effective in terms of neurologic improvement in the setting of complete thoracic SCI. A problem with our study is the small number of cases, which decreases the power of our study and prevents us from employing any meaningful statistical analysis. Another potential problem is that the majority of surgeries were delayed, although again, the timing of surgical decompression has not been shown in clinical trials to affect neurologic improvement. A true understanding of the role of surgical intervention in the setting of traumatic thoracic SCI can only be determined through a randomized controlled clinical trial.
CONCLUSIONS
Surgical decompression and fusion did not result in spinal cord recovery after complete SCI in the thoracic spine. Clearly, to better define the role of surgery in the management of acute SCI, randomized, controlled prospective trials are required.
Acknowledgments
I thank Dr Alexander R. Vaccaro for careful review of the manuscript.
Footnotes
This study was partially supported by Grant 644 from the Zahedan University of Medical Sciences.
REFERENCES
- Kiwerski J, Weiss M. Neurological improvement in traumatic injuries of cervical spinal cord. Paraplegia. 1981;19:31–37. doi: 10.1038/sc.1981.9. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Sekhon LHS, Tator C. The role and timing of decompression in acute spinal cord injury: what do we know? What should we do? Spine. 2001;26:S101–S110. doi: 10.1097/00007632-200112151-00017. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH. An evidence-based review of surgical decompression for acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg (Spine) 1999;91:1–11. doi: 10.3171/spi.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Franti CE, Riggins RS, Richards D, Borhani NO. Incidence of traumatic spinal cord lesions. J Chronic Dis. 1975;28:471–492. doi: 10.1016/0021-9681(75)90057-0. [DOI] [PubMed] [Google Scholar]
- Rizzolo SJ, Vaccaro AR, Cotler JM. Cervical spine trauma. Spine. 1994;19:2288–2298. doi: 10.1097/00007632-199410150-00007. [DOI] [PubMed] [Google Scholar]
- Donovan WH, Cifu DX, Schotte DE. Neurological and skeletal outcomes in 113 patients with closed injuries to the cervical spinal cord. Paraplegia. 1992;30:533–542. doi: 10.1038/sc.1992.111. [DOI] [PubMed] [Google Scholar]
- Heiden JS, Weiss MH, Rosenberg AW. Management of cervical spinal cord trauma in southern California. J Neurosurg. 1975;43:732–736. doi: 10.3171/jns.1975.43.6.0732. [DOI] [PubMed] [Google Scholar]
- Marshall LF, Garfin S. Incidence and causes of neurological deterioration following spinal cord injury. In: Peipmeier JM, editor. The Outcome Following Traumatic Spinal Cord Injury. Mount Kisco, NY: Futura Publishing; 1992. pp. 13–30. [Google Scholar]
- Rosa GLA, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503–512. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- Frankel H, Hancock D, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Part 1. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- Young B, Brooks WH, Tibbs PA. Anterior decompression and fusion for thoracolumbar fractures with neurological deficits. Acta Neurochir (Wien) 1981;57:287–298. doi: 10.1007/BF01664845. [DOI] [PubMed] [Google Scholar]
- Benzel EC, Larson SJ. Functional recovery after decompressive operation for thoracic and lumbar spine fractures. Neurosurgery. 1986;19:772–778. doi: 10.1227/00006123-198611000-00009. [DOI] [PubMed] [Google Scholar]
- Petitjean ME, Mousselard H, Pointillart V, Lassie P, Senegas J, Dabadie P. Thoracic spinal trauma and associated injuries: should early spinal decompression be considered? J Trauma. 1995;39:368–372. doi: 10.1097/00005373-199508000-00030. [DOI] [PubMed] [Google Scholar]
- Grootboom MJ, Govender S. Acute injuries of the upper dorsal spine. Injury. 1993;24:389–392. doi: 10.1016/0020-1383(93)90103-d. [DOI] [PubMed] [Google Scholar]
- Bostman OM, Myllynen PJ, Riska EB. Unstable fractures of the thoracic and lumbar spine: the audit of an 8-year series with early reduction using Harrington instrumentation. Injury. 1987;18:190–195. doi: 10.1016/0020-1383(87)90135-5. [DOI] [PubMed] [Google Scholar]
- Riska EB, Myllynen P, Bostman O. Anterolateral decompression for neural involvement in thoracolumbar fractures. A review of 78 cases. J Bone Joint Surg Br. 1987;69:704–708. doi: 10.1302/0301-620X.69B5.3680328. [DOI] [PubMed] [Google Scholar]
- Transfeldt EE, White D, Bradford DS, Roche B. Delayed anterior decompression in patients with spinal cord and cauda equina injuries of the thoracolumbar spine. Spine. 1990;15:953–957. doi: 10.1097/00007632-199009000-00021. [DOI] [PubMed] [Google Scholar]
- Huang TJ, Chao EK, Chen YJ, Du YK, Chen JY, Hsu RW. Complete fracture-dislocation of the thoracic spine with spontaneous neurologic decompression: a case report. Changgeng Yi Xue Za Zhi. 1995;18:387–391. [PubMed] [Google Scholar]
- Krengel WF, III, Anderson PA, Henley MB. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine. 1993;18:2080–2087. doi: 10.1097/00007632-199310001-00027. [DOI] [PubMed] [Google Scholar]
- Sridhar K, Vasudevan MC, Ramamurthi B. Posttraumatic total dislocation of the upper thoracic spine. Surg Neurol. 2004;61:343–346. doi: 10.1016/S0090-3019(03)00489-0. [DOI] [PubMed] [Google Scholar]
- Levi L, Wolf A, Rigamonti D, Ragheb J, Mirvis S, Robinson WL. Anterior decompression in cervical spine trauma: does timing of surgery affect the outcome? Neurosurgery. 1991;29:216–222. [PubMed] [Google Scholar]
- Donovan WH, Kopaniky D, Stolzman E, Carter RE. The neurological and skeletal outcome in patients with closed cervical spinal cord injury. J Neurosurg. 1994;66:690–694. doi: 10.3171/jns.1987.66.5.0690. [DOI] [PubMed] [Google Scholar]
- Tator CH, Duncan EG, Edmonds VE, Lapzack LI, Andrews DF. Comparison of surgical and conservative management in 208 patients with acute spinal cord injury. Can J Neurol Sci. 1987;14:60–69. doi: 10.1017/s0317167100026858. [DOI] [PubMed] [Google Scholar]
- Wagner FC, Jr, Cherazi B. Early decompression and neurological outcome in acute cervical spinal cord injuries. J Neurosurg. 1982;56:699–705. doi: 10.3171/jns.1982.56.5.0699. [DOI] [PubMed] [Google Scholar]
- Waters RL, Adkins RH, Yakura JS, Sie I. Effect of surgery on motor recovery following traumatic spinal cord injury. Spinal Cord. 1996;34:188–192. doi: 10.1038/sc.1996.37. [DOI] [PubMed] [Google Scholar]
- Wilmot CB, Hall KM. Evaluation of the acute management of tetraplegia: conservative versus surgical treatment. Paraplegia. 1986;24:148–153. doi: 10.1038/sc.1986.19. [DOI] [PubMed] [Google Scholar]
- Boerger TO, Limb D, Dickson RA. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures? J Bone Joint Surg Br. 2000;82:629–635. doi: 10.1302/0301-620x.82b5.11321. [DOI] [PubMed] [Google Scholar]
- Waters RL, Adkins RH, Yakura JS, Sie I. Effect of surgery on motor recovery following traumatic spinal cord injury. Spinal Cord. 1996;34:188–192. doi: 10.1038/sc.1996.37. [DOI] [PubMed] [Google Scholar]
- Geisler FH, Coleman WP, Grieco G, Poonian D. Sygen Study Group. Recruitment and early treatment in a multicenter study of acute spinal cord injury. Spine. 2001;26(24 Suppl):58–67. doi: 10.1097/00007632-200112151-00013. [DOI] [PubMed] [Google Scholar]
- Bohlman HH, Freehafer A. Late anterior decompression of spinal cord injuries. J Bone Joint Surg Am. 1975;57:10–25. [Google Scholar]
- Ng WP, Fehlings MG, Cuddy B, et al. Surgical treatment for acute spinal cord injury study pilot # 2: evaluation of protocol for decompressive surgery within 8 hours of injury. Neurosurg Focus. 1999;6(1) doi: 10.3171/foc.1999.6.1.4. article 3. www.aans.org/education/journal/neurosurgical/jan99/6-1-3.asp. Accessed November 14, 2005. [DOI] [PubMed] [Google Scholar]
- Vaccaro AR, Daugherty RJ, Sheehan TP, et al. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine. 1997;22:239–246. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- Waters RL, Meyer PR, Adkins RH, Felton D. Emergency, acute, and surgical management of spine trauma. Arch Phys Med Rehabil. 1999;80:1383–1390. doi: 10.1016/s0003-9993(99)90248-4. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Shepard MJ, Collins WF, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- Quencer RM, Sheldon JJ, Post MJD, et al. Magnetic resonance imaging of the chronically injured cervical spinal cord. Am J Neuroradiol. 1986;7:457–464. [Google Scholar]
- Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- Mayer H, Schaaf D, Kudernatsch M. Use of internal fixator in injuries of the thoracic and lumbar spine. Chirurg. 1992;63:944–949. (German) [PubMed] [Google Scholar]
- Beisse R, Muckley T, Schmidt MH, Hauschild M, Buhren V. Surgical technique and results of endoscopic anterior spinal canal decompression. J Neurosurg Spine. 2005;2:128–136. doi: 10.3171/spi.2005.2.2.0128. [DOI] [PubMed] [Google Scholar]
- Lobosky JM, Hitchon PW, McDonnell DE. Transthoracic anterolateral decompression for thoracic spinal lesions. Neurosurgery. 1984;14:26–30. doi: 10.1227/00006123-198401000-00007. [DOI] [PubMed] [Google Scholar]
- Bedbrook GM. Spinal injuries with tetraplegia and paraplegia. J Bone Joint Surg Br. 1979;61:267–284. doi: 10.1302/0301-620X.61B3.225332. [DOI] [PubMed] [Google Scholar]
- Brodkey JS, Richards DE, Blasingame JP, Nulsen FE. Reversible spinal cord trauma in cats. Additive effects of direct pressure and ischemia. J Neurosurg. 1972;37:591–593. doi: 10.3171/jns.1972.37.5.0591. [DOI] [PubMed] [Google Scholar]
- Carlson GD, Minato Y, Okada A, et al. Early time-dependent decompression for spinal cord injury, vascular mechanisms of recovery. J Neurotrauma. 1997;14:951–962. doi: 10.1089/neu.1997.14.951. [DOI] [PubMed] [Google Scholar]
- Croft TJ, Brodkey JS, Nulsen FE. Reversible spinal cord trauma: a model for electrical monitoring of spinal cord function. J Neurosurg. 1972;36:402–406. doi: 10.3171/jns.1972.36.4.0402. [DOI] [PubMed] [Google Scholar]
- Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury: recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042–1049. doi: 10.2106/00004623-199507000-00010. [DOI] [PubMed] [Google Scholar]
- Dolan EJ, Tator CH, Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980;53:749–755. doi: 10.3171/jns.1980.53.6.0749. [DOI] [PubMed] [Google Scholar]
- Guha A, Tator CH, Endrenyi L, Piper I. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324–339. doi: 10.1038/sc.1987.61. [DOI] [PubMed] [Google Scholar]
- Kobrine AI, Evans DE, Rizzoli HV. Correlation of spinal cord blood flow and function in experimental compression. Surg Neurol. 1978;10:54–59. [PubMed] [Google Scholar]
- Kobrine AI, Evans DE, Rizzoli HV. Experimental balloon compression of the spinal cord: factors affecting disappearance and return of spinal evoked potential. J Neurosurg. 1979;51:841–845. doi: 10.3171/jns.1979.51.6.0841. [DOI] [PubMed] [Google Scholar]
- Nystrom B, Berglund JE. Spinal cord restitution following compression injuries in rats. Acta Neurol Scand. 1988;78:467–472. doi: 10.1111/j.1600-0404.1988.tb03689.x. [DOI] [PubMed] [Google Scholar]
- Sekhon LHS, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- Chen TY, Dickman CA, Eleraky M, Sonntag VKH. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine. 1998;22:2398–2403. doi: 10.1097/00007632-199811150-00007. [DOI] [PubMed] [Google Scholar]
- Duh MS, Shepard MJ, Wilberger JE, Bracken MB. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35:240–249. doi: 10.1227/00006123-199408000-00009. [DOI] [PubMed] [Google Scholar]
- Pointillart V, Petitjean ME, Wiart L, et al. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71–76. doi: 10.1038/sj.sc.3100962. [DOI] [PubMed] [Google Scholar]
- Bohlman HH, Kirkpatrick JS, Delamarter RB, Leventhal M. Anterior decompression for late pain and paralysis after fractures of the thoracolumbar spine. Clin Orthop Relat Res. 1994;300:24–29. [PubMed] [Google Scholar]
- Robertson A, Branfoot T, Barlow IF, Giannoudis PV. Spinal injury patterns resulting from car and motorcycle accidents. Spine. 2002;27:2825–2830. doi: 10.1097/00007632-200212150-00019. [DOI] [PubMed] [Google Scholar]