Abstract
Neurites projecting to their target tissues during embryogenesis are subject to many perturbations that could influence their rate of growth. For example, environmental influences such as supply of neurotrophic factor or electrical activity profoundly influence the rate of neuronal protein synthesis. Because accumulation of protein is necessary for outgrowth to proceed normally, a perturbation in protein synthesis could cause a net change in the rate of accumulation of proteins with the result that neurite outgrowth rate increases or decreases. That neurite outgrowth does not normally seem to be subject to such perturbations suggests involvement of a homeostatic system controlling the rate of outgrowth. Consistent with this hypothesis, we show here that the rate of growth of neurites of sympathetic neurons is highly resistant to decreased rates of protein synthesis. Chronic suppression of protein synthesis by sixty percent had no significant effect on neurite outgrowth over a two day period while complete suppression halted it almost immediately. By the third day of exposure, sixty percent suppression slowed outgrowth. Sustained suppression of protein synthesis rate by thirty-three percent had no effect on rate of outgrowth even after seven days. We show that the ability of the growing neurites to resist protein synthesis suppression appears to be caused, at least in part, by a parallel decrease in the rate of protein degradation. The result of this coupling between degradation and synthesis is that proteins can continue to accumulate even when protein synthesis rate decreases, allowing normal rates of neurite outgrowth.
Keywords: protein synthesis, protein degradation, sympathetic neuron, neurite, protein turnover
Introduction
The rate of degradation of long-lived proteins in sympathetic neurons exposed to their required neurotrophic factor, nerve growth factor (NGF), is regulated by the rate of protein synthesis [6]. Sustained inhibition of synthesis by a specific amount causes a nearly equivalent sustained reduction in the rate of degradation of long-lived proteins in these cells, indicating that the degradation of these proteins is coupled to protein synthesis in a nearly 1:1 ratio. Protein degradation/synthesis coupling appears to function to protect these neurons from protein loss during periods of decreased synthesis. The net result of such coupling is that protein synthesis can decrease without a loss of total cellular protein due to ongoing protein turnover.
We previously demonstrated a role for protein degradation/synthesis coupling in controlling the size of the somas of sympathetic neurons experiencing prolonged periods of reduced rates of protein synthesis [6]. A major cause of cellular atrophy in all cells is loss of total cellular protein [13]. Therefore, sustained suppression of protein synthesis would cause a net loss of cellular protein and a decrease in cell size if degradation were not also suppressed. We found that this does not happen. Complete suppression of protein synthesis for many days in NGF-maintained rat sympathetic neurons in cell culture does not cause atrophy. The primary reason appears to be that coupling between degradation and synthesis prevents net loss of protein. Removal of NGF uncouples degradation from protein synthesis and allows it to proceed at the normal rate. Uncoupling of the two processes leads to loss of total cellular protein concurrent with profound somatic atrophy preceding apoptotic death [3, 14, 18]. Thus, NGF-mediated coupling of the rate of protein degradation to that of protein synthesis appears to be an important means by which neurotrophic factors maintain homeostatic control of neuronal size in sympathetic neurons. Such an effect of coupling of protein degradation to protein synthesis has now been described in other cell types and may well be a universal mechanism for controlling cell size homeostasis [2].
It has been reported that complete block of protein synthesis in sympathetic neurons rapidly halts neurite outgrowth [5]. The effects of reducing synthesis by smaller amounts on outgrowth have not yet been explored. While investigating the effects of protein synthesis suppression on protein degradation and on somatic cell size in rat sympathetic neurons in cell culture, we noticed that neurite outgrowth appeared to be resistant to partial suppression of protein synthesis. To explore this phenomenon in more detail, we utilized the protein synthesis inhibitor cycloheximide (CHX) [17] to clamp the rate of protein synthesis in these cells at various levels for prolonged periods. First, we established the effects of prolonged exposure to several concentrations of CHX on the rate of protein synthesis in these cells. Timed-pregnant Sprague Dawley rats were obtained from Harlan (Indianapolis, IN). The superior cervical ganglia were dissected from embryos on the 21st day of gestation. Neurons were dissociated from the ganglia and plated on a collagen substrate in 24-well tissue culture dishes (Costar, Cambridge, MA) as described [7, 11]. Culture medium (Life Technologies, Gaithersburg, MD) consisted of Eagle’s minimum essential medium with Earle’s salts w/o L-glutamine (Invitrogen, Carlsbad, CA). This medium was supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 20 μM fluorodeoxyuridine, 20 μM uridine, 1.4 mM L-glutamine, and 50 ng/ml 2.5S NGF, all from Sigma (St. Louis, MO). Cultures were maintained in this medium for a week before experiments. They were then incubated for 68 hours at 35° C in an incubator having a 5 % CO2 and 95 % air atmosphere in culture medium containing NGF and various concentrations of CHX (Sigma). For the last 4 hours of the experiment, cultures were exposed to a metabolic labeling medium consisting of 10 μCi/ml TRAN 35S-label (70% L-methionine, 15% L-cysteine; ICN, Irvine, CA) and containing the same concentrations of CHX that the cells had been exposed to for the previous 68 hours. Figure 1A shows the dose-dependent effect of CHX on suppression of protein synthesis over this period. Our previous work demonstrated that the dose-response curve for suppression of protein synthesis by CHX is identical after four or seventy-two hours of exposure [6]. Therefore, we could use these CHX concentrations to clamp rate of protein synthesis in sympathetic neurons at a desired level for prolonged periods.
Fig. 1. Effect of CHX on rates of protein synthesis and neurite outgrowth.
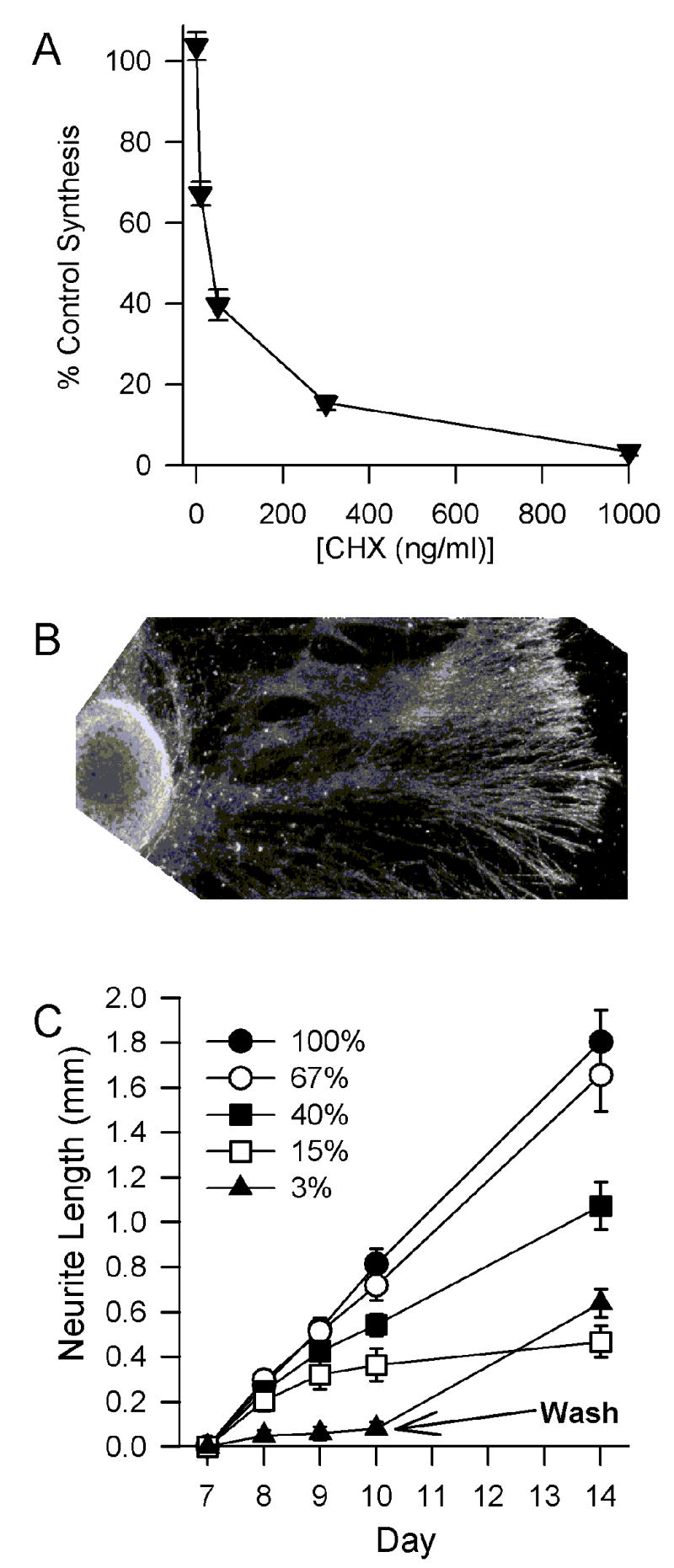
(a) Rates of protein synthesis in dissociated cultures of sympathetic neurons exposed to different concentrations of CHX for 72h. N = 26–30 cultures from 4 separate platings for each concentration of CHX tested.
(b) Dark-field photomicrograph of representative superior cervical ganglion explant used to measure neurite outgrowth.
(c) Effects of protein synthesis suppression on neurite outgrowth. Medium contained NGF and concentrations of CHX that clamped protein synthesis rate to the indicated percentages of control rate. On the third d after treatment with 1 μg/ml CHX, the CHX was washed out with control medium. Outgrowth then resumed at about the normal rate. Neurite length shown is increased length beyond that measured on the seventh day after initiation of cultures. Initial neurite length was measured from the edge of the ganglia. N = 8 – 11 ganglia from 2 separate platings for each concentration of CHX.
To determine the effects of protein synthesis suppression on neurite outgrowth, we utilized explants of superior cervical ganglia. Ganglia were dissected from embryonic-day-21 rats, placed on a collagen substrate in 24-well tissue culture dishes, and maintained as described for cultures of dissociated cells. All procedures were approved by the Animal Care and Use Committee of the University of Georgia. Explants were exposed to different concentrations of CHX in the presence of NGF and outgrowth was measured beginning on the seventh day after plating (Fig. 1B). Linear outgrowth in cells not exposed to CHX continued for at least a month after the beginning of the experiment (not shown). Neurite outgrowth was partially protected from protein synthesis inhibition (Fig. 1C). Control outgrowth from ganglia maintained in NGF-containing medium without CHX occurred at an average rate of 0.27 mm/d over a 7 day period. Sustained suppression of synthesis by about 30% with 10 ng/ml CHX had no significant effect on neurite outgrowth over a 7d period (0.24 mm/d; p > 0.1 by paired t test). Sustained suppression of synthesis by 80% with 50–300 ng/ml CHX did not significantly affect neurite outgrowth during the first 24 hours of exposure (growth rate was 0.2 ± 0.05 mm/d; p > 0.2). However, after 2 days of exposure neurite outgrowth had slowed. As previously reported [5], complete suppression of synthesis with 1μg/ml CHX blocked almost all outgrowth over a 3 day period (0.03 mm/day). Neurites remained the same length throughout this period and appeared to be unharmed by the suppression of synthesis. Washout of CHX from ganglia exposed to 1 μg/ml CHX for 3 days caused resumption of normal growth, rate indicating that cells remained viable and capable of rapid neurite extension after reversal of protein synthesis inhibition. As a control, we tested the effect of different CHX concentrations on neurite outgrowth from dissociated cultures of superior cervical ganglia. These cultures were plated on a thin strip of collagen to allow measurement of linear outgrowth [7]. No differences were noted in the effects of CHX on outgrowth of neurites from these neurons than from that of the ganglionic explants (not shown).
These data suggest that, in addition to contributing to somatic size homeostasis, coupling of protein degradation to protein synthesis in sympathetic neurons may aid in maintaining neuronal growth homeostasis in the face of altered rates of protein synthesis. To correlate the effects of protein synthesis suppression on neurite outgrowth with the relationship between protein synthesis and protein degradation, we determined the effects of the different concentrations of CHX used in Figure 1 on protein degradation. On the seventh day after plating, cultures were metabolically labeled by incubation in medium containing 10 μCi/ml TRAN 35S-label (70% L-methionine, 15% L-cysteine) as described [4, 6]. Neurons were exposed to labeling medium for 24h. This treatment resulted in a greater percentage of the labeled pool of proteins being long-lived because of the continuous turnover of short-lived proteins during the labeling period. The cells were then washed with standard culture medium and incubated for 6 hours before the initial time-point was taken to allow time for incorporation of labeled cytosolic amino acids that had not been incorporated at the time of washout. Cultures were lysed with a buffer containing 0.5% N-lauryl sarcosine, 1 mM EDTA, and 10 mM Tris HCl, pH 7.5. Protein was precipitated with a solution of ice-cold 10% trichloroacetic acid (TCA) and retained by filtration through a 0.45-μm nitrocellulose filter. Radioactivity was measured by liquid scintillation counting. Loss of radiolabel was normalized to TCA-precipitable counts measured at the beginning of the experiment [1, 5, 12, 16]. Figure 2A shows that the concentrations of CHX used in Figure 1 had profound effects on protein degradation. Almost complete suppression of degradation of long-lived proteins occurred in cultures maintained in medium containing 1 μg/ml CHX. Lower concentrations of CHX caused less suppression. Figure 2B shows the effect of CHX on protein degradation as a function of the same concentrations on protein synthesis. The relationship is linear.
Fig. 2. Dose-dependent suppression of degradation of long-lived proteins by CHX.

(a) CHX effect on protein degradation. n = 18–23 cultures.
(b) Rate of degradation of long-lived proteins was a linear function of protein synthesis rate (R = 0.99). Suppression of protein synthesis rate for 72h caused an almost equivalent percentage reduction in protein degradation rate over the same period. Degradation suppression data is from Fig 2A and protein synthesis data from Fig. 1A.
(c) Suppression of protein synthesis by CHX decreased polyubiquitination of proteins in NGF-supported rat sympathetic neurons. Representative immunoblots demonstrating a decrease in polyubiquitination of proteins in NGF-maintained cultures that had been exposed to CHX (1 μg/ml) for 24h. Immunoblotting was done by enhanced chemiluminescence essentially as described [19]. The anti-ubiquitin antibody was from StressGen (Ann Arbor, MI). The secondary antibody and chemoluminescence kit were from Amersham (Piscataway, NJ). Blots are representative of three similar experiments.
The linear outgrowth of the neurites of NGF-maintained sympathetic neurons in cell culture is accompanied by a linear increase in total cellular protein [7]. Somas only increase slightly in size during the period in which outgrowth was measured. Therefore, it appears that most newly-produced protein in these cells is directed to neurites. The rapid slowing of neurite outgrowth only at the highest concentration of CHX (~97% protein synthesis suppression) may, in part, indicate a need for proteins with half-lives that are not significantly affected by suppression of protein synthesis. With complete suppression, such proteins may rapidly decrease in concentration to levels that are incompatible with continued neurite outgrowth. Additionally, continued outgrowth requires new protein [7]. The almost complete suppression of synthesis in these cells would prevent new cytoskeletal proteins necessary for neurite extension from being produced.
The mechanism that couples protein degradation to protein synthesis in sympathetic neurons and other cells is unknown. The principal means of degradation of most proteins in all cells is the ubiquitin-proteasome system [9, 10]. The proteasome is a large proteolytic complex that degrades proteins that have been tagged with polyubiquitin [8]. Therefore, the most likely site at which protein synthesis suppression can have an effect on protein degradation is via this system. Consistent with this hypothesis, we found that CHX suppresses polyubiquitination of many proteins in NGF-maintained sympathetic neurons (Fig. 2C). Such a global decrease in polyubiquitination could cause a decrease in degradation of these proteins. It appears that only the degradation of long-lived proteins such as cytoskeletal elements is coupled to protein synthesis rate [6]. This suggests the possibility that a protein or proteins necessary for ubiquitination of these proteins may be short-lived and decrease in concentration when protein synthesis decreases. The result of this decrease would be a decline in polyubiquitination of long-lived proteins and, subsequently, a decrease in their degradation.
In conclusion, we have shown that the rate of outgrowth of the neurites of sympathetic neurons is highly resistant to suppression of protein synthesis. This resistance appears to be mediated by a concurrent decrease in the rate of degradation of long-lived proteins with the result that accumulation of proteins necessary for outgrowth proceeds at a normal pace. The decrease in degradation may be caused by a decrease in protein polyubiquitination.
Acknowledgments
This work was supported by grant from the National Institutes of Health (NS37110).
Footnotes
Classification Items: Theme A (10.000): Development, Regeneration, and Repair Topic (10.210): Process outgrowth, growth cones, and sprouting
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ballard FJ. Regulation of intracellular protein breakdown with special reference to cultured cells. In: Glauman H, Ballard FJ, editors. Lysosomes: Their Role in Protein Breakdown. Academic Press; New York: 1987. pp. 285–318. [Google Scholar]
- 2.Conlon I, Raff M. Differences in the way a mammalian cell and yeast cells coordinate cell growth and cell-cycle progression. J Biol. 2003;2:1475–4924. doi: 10.1186/1475-4924-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deckwerth TL, Johnson EM., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123:1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle D, Tweto J. Measurement of protein turnover in animal cells. Meth Cell Biol. 1975;10:235–260. doi: 10.1016/s0091-679x(08)60740-2. [DOI] [PubMed] [Google Scholar]
- 5.Estridge M, Bunge R. Compositional analysis of growing axons from rat sympathetic neurons. J Cell Biol. 1978;79:138–155. doi: 10.1083/jcb.79.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin JL, Johnson EM., Jr Control of neuronal size homeostasis by trophic factor-mediated coupling of protein degradation to protein synthesis. J Cell Biol. 1998;142:1313–1324. doi: 10.1083/jcb.142.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklin JL, Sanz-Rodriguez C, Juhasz A, Deckwerth TL, Johnson EM., Jr Chronic depolarization prevents programmed death of sympathetic neurons in vitro but does not support growth: Requirement for Ca2+ influx but not Trk activation. J Neurosci. 1995;15:643–664. doi: 10.1523/JNEUROSCI.15-01-00643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg AL, Rock KL. Proteolysis, proteasomes and antigen presentation. Nature. 1992;357:375–379. doi: 10.1038/357375a0. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg AL, St John AC. Intracellular protein degradation in mammalian and bacterial cells: part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MI, Argiro V. Techniques in the tissue culture of rat sympathetic neurons. Meth Enzymol. 1983;103:334–347. doi: 10.1016/s0076-6879(83)03022-0. [DOI] [PubMed] [Google Scholar]
- 12.Kirkland RA, Franklin JL. Evidence for redox regulation of cytochrome c release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition. J Neurosci. 2001;21:949–1963. doi: 10.1523/JNEUROSCI.21-06-01949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macknight ADC. Principles of cell volume regulation. Renal Physiol Biochem. 1988;11:114–141. doi: 10.1159/000173158. [DOI] [PubMed] [Google Scholar]
- 14.Martin DP, Schmidt RE, DiStefano PS, Lowry OH, Carter JG, Johnson EM., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988;106:829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poole B, Wibo M. Protein degradation in cultured cells. J Biol Chem. 1973;248:6221–6226. [PubMed] [Google Scholar]
- 16.Rock KLC, Gramm L, Rothstein K, Clark R, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 17.Wettstein FOH, Noll S, Penman S. Effect of cycloheximide on ribosomal aggregates engaged in protein synthesis in vitro. Biochim Biophys Acta. 1964;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- 18.Wyllie AH, Kerr JFR, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 19.Kirkland RA, Windelborn JA, Kasprzak JM, Franklin JL. A Bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J Neurosci. 2002;22:6480–6490. doi: 10.1523/JNEUROSCI.22-15-06480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


