SUMMARY
HIV-1 reverse transcriptase shares the key features of high fidelity polymerases, such as a closed architecture of the active site but displays a level of fidelity that is intermediate to that of high fidelity, replicative polymerases and low fidelity translesion synthesis (TLS) polymerases. The β3-β4 loop of the HIV-1 RT fingers subdomain makes transient contacts with the dNTP and template base. To investigate the role of active site architecture in HIV-1 RT fidelity, we truncated the β3-β4 loop, eliminating contact between Lys65 and the γ-phosphate of dNTP. The mutant, in a manner reminiscent of TLS polymerases, was only able to incorporate a nucleotide that was capable of base-pairing with the template nucleotide, but not a nucleotide shape-analog incapable of Watson-Crick hydrogen bonding. Unexpectedly, however, the deletion mutant differed from the TLS polymerases in that it displayed an increased fidelity. The increased fidelity was associated with reduced dNTP binding affinity as measured using the dead end complex formation. In an effort to delineate the specific amino acid residue in the deleted segment responsible for this phenotype, we examined the K65 residue. Two substitution mutants, K65R and K65A were studied. The K65A mutant behaved similarly to the deletion mutant displaying dependence on Watson-Crick hydrogen bonding, increased fidelity and reduced dNTP-binding, while the K65R was more akin to wild type enzyme. These results underscore the key role of the K65 residue in the phenotype observed in the deletion mutant. Based on the well-known electrostatic interaction between K65 and the γ-phosphate moiety of incoming dNTP substrate in the ternary complex structure of HIV-1 RT, we conclude that non-discriminatory interactions between β3-β4 loop and the dNTP in wild type HIV-1 RT help lower dNTP selectivity. Our results show that the fidelity of dNTP insertion is influenced by protein interactions with the triphosphate moiety.
Keywords: RT Fidelity, K65R, DNA replication, dNTP selection, steric effects
INTRODUCTION
HIV-1 reverse transcriptase (RT) is the viral polymerase responsible for both RNA-dependent and DNA-dependent DNA synthesis during viral replication. Error-prone synthesis by HIV-1 RT is thought to play a key role in viral pathogenesis and drug resistance.1,2 Previously, the fidelity of HIV-1 RT was generally considered one of the lowest.3 However, a number of polymerases with much lower fidelity have been identified among a panoply of cellular DNA polymerases described in recent years. In this context, HIV RT would now appear to be of an intermediate fidelity. In vitro studies have shown that even the proofreading-defective versions of replicative DNA polymerases, such as T7 DNA polymerase and Klenow fragment of DNA polymerase I display very high intrinsic polymerase fidelities (10−5 to 10−6). At the other end of the spectrum are specialized DNA repair enzymes, such as the B family member Polζ and the Y family members Polη, Polτ and Polκ, which lack proofreading and polymerize DNA with a much lower fidelity (10−1 to 10−3).4 The error rates of HIV-1 RT are intermediate to these extremes (10−4 to 10−5).5,6
Structural principles governing DNA polymerase fidelity are increasingly being better understood. There is evidence suggesting that fidelity is achieved by steric, or geometric, selection,7–10 in that polymerases with less solvent accessible active sites display higher fidelity.11 Replicative DNA polymerases also achieve higher fidelity via induced fit, where binding of the correct substrate induces a conformational change facilitating accurate dNTP insertion.12,13 Replicative DNA polymerases such as T7 DNA polymerase and Klenow use an induced fit mechanism, while many low fidelity polymerases have an open architecture exhibiting minimal change in conformation upon dNTP-binding.14,15 The role of Watson-Crick base-pairing in fidelity does not appear to be universal. For example, in replicative polymerases, the role of base pairing is limited, while low fidelity polymerases such as Y-family polymerases15 and primase,16,17 are dependent on hydrogen bonding. Their fidelity is thus often commensurate with differences in free energy between the correctly vs. incorrectly paired dNTPs.
The role of hydrogen bonding in polymerase fidelity has been studied using a non-polar thymine shape analog, difluorotoluene, which is incapable of forming hydrogen-bonds. The analog can be used either as a templating base or incorporated into a growing primer DNA with an efficiency and accuracy close to that of the natural nucleotide by many polymerases including Klenow,18 T7 DNA polymerase, HIV-1 RT and Thermus aquaticus DNA polymerase.19 Nevertheless, low fidelity polymerases of the Pol Y family, which have very wide, solvent accessible nucleotide binding pockets, accept non-hydrogen-bonding analogs poorly, suggesting that they require the formation of Watson-Crick hydrogen bonds for nucleotide incorporation.15,20
The polymerase active site is widely conserved from retroviral RTs to replicative human DNA polymerases.21,22 In HIV-1 RT, the template-primer duplex is bound in a cleft made up of fingers, palm and thumb subdomains (Fig 1A). The fingers subdomain, and specifically residues in the β3-β4 loop, modulates many aspects of RT activity, including fidelity,23,24 processivity,25,26 pyrophosphorolysis27 and strand displacement synthesis28. The β3-β4 loop is also a hotspot for nucleoside analog resistance mutations.29–32 The active site consists of primer 3′-OH, the primer terminal base, templating base and a triad of catalytically crucial aspartic acid residues (Asp110, Asp185 and Asp186). Residues in the fingers subdomain also contribute to the active site (Fig. 1). Upon binding of a dNTP at the active site, the fingers subdomain closes in on the nucleotide, and the Lys65 and Lys66 residues from the β3-β4 hairpin loop interact with, respectively, the incoming nucleotide and the primer terminus.33 The side chain of Arg72 (along with that of Gln151 in Palm subdomain) packs against the base of the incoming dNTP. The triphosphate moiety is coordinated by side chains Lys65 and Arg72, and two Mg2+ ions (Fig. 1C). Thus, the dNTP is sandwiched between surfaces for stacking (the primer 3′ terminal base and the Arg72 side chain) and hydrogen bonding with the template base, as well as contacts residues in the fingers and palm subdomains. In addition, residues in the β4 strand and loop (Lys65, Arg72 and Leu74) form part of a steric restraint, and the degree of conformational flexibility of amino acids within the nucleotide binding pocket,34 may further limit the potential size of the nascent base-pair. These contacts together contribute significantly to the discrimination of correct from incorrect dNTPs.35
Figure 1.
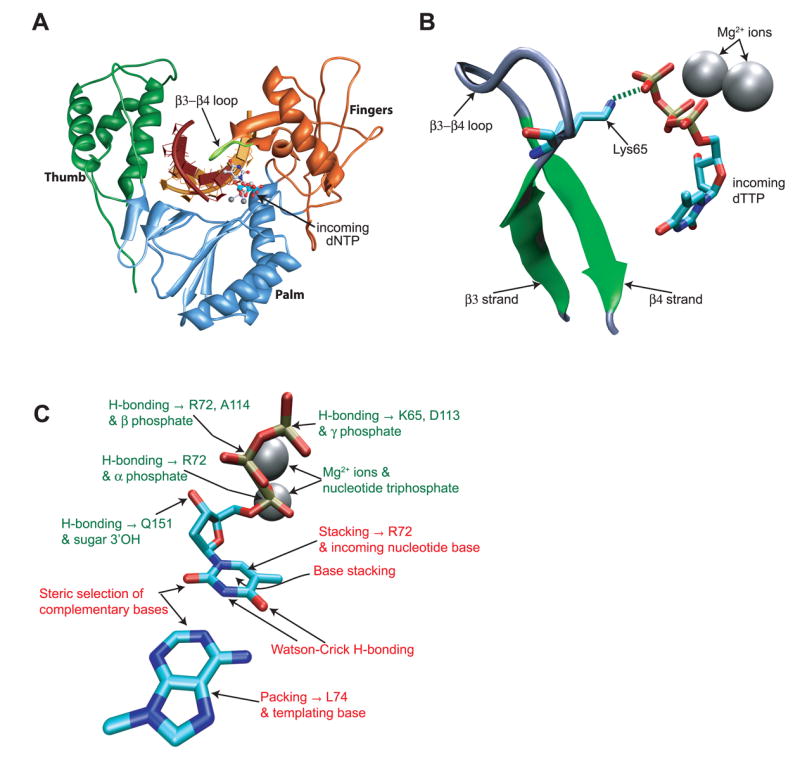
Interactions between HIV-1 RT and the newly formed basepair. A. Position of the β3-β4 loop within the fingers subdomain of HIV-RT. The primer and template strands are indicated in dark and light brown respectively. Fingers, Palm and Thumb subdomains of reverse transcriptase are colored orange, blue and green respectively. Atomic coordinates were from 1rtd.318 B. Interaction between the K65 residue in fingers β3-β4 loop and the γ-phosphate of the incoming dNTP. The hydrogen bond between residue K65 and the γphosphate of the incoming nucleotide is shown as a dashed green line. C. Diagrammatic representation of the informational (indicated in red) and non-informational (indicated in green) interactions affecting the incoming nucleotide:template pair in the HIV RT active site. Coordinates used are from the published HIV-1 RT ternary structure 1rtd,33 and the figures were created using VMD57 and Chimera.58,59
HIV-1 RT exhibits all the properties of a DNA polymerase with intrinsic high fidelity: an induced fit mechanism of nucleotide selection36,37 and the absence of a requirement for hydrogen bonding, which suggests the possibility of a steric mechanism of selection.19 Yet, HIV-1 RT exhibits an intermediate fidelity with error rates being in the range of 10−4 to 10−5,5,6 a frequency that is 10 to 100 times higher than T7 or Klenow. Therefore, we set out to understand the structural basis of this unusual behavior of HIV-1 RT. It has been suggested that rational, structure-based mutations could be made within the active site of DNA polymerases to alter solvent accessibility of the binding pocket.35 We hypothesized that a mutation that decreased active site tightness would decrease RT fidelity. We have tested this notion in HIV-1 RT by deleting five amino acid residues (65KKDST69) from the β3-β4 loop to create a truncation mutant, Δβ3-4L (Fig. 2a). The choice of this segment was based on a key contact with the dNTP–between residue K65 and the γ-phosphate of incoming dNTP. The recombinant purified Δβ3-4L mutant, in a manner reminiscent of low fidelity polymerases, exhibited an acquired dependence on hydrogen bonding between the incoming dNTP and template base. Interestingly, however, the truncation mutant also acquired an increased fidelity of dNTP insertion. Studies on substitution mutations at K65 residue suggested that the lower fidelity of wild type RT is likely due to an electrostatic interaction with the triphosphate moiety of the incoming dNTP. This non-discriminating interaction appears to considerably reduce the influence of other interactions that are more selective, thus favoring incorrect dNTP insertions. Our results provide biochemical evidence that the β3-β4 loop contains key determinants of dNTP selection.
Figure 2.

A. The sequence of HIV-1 RT, and the residues deleted in the Δβ3-4L mutant are shown. The residues comprising the β3 and β4 strands are shown in inverse, and the position of lysine 65 is indicated. B. Template-primers used in the running start and standing start primer extension assays. The position of the F or T residue in the template strand is indicated F/T. C. Structure of the thymine shape analog, difluorotoluene.18
RESULTS
A deletion in the β3-β4 hairpin loop leads to a dependence on template base-dNTP hydrogen-bonding
Structural evidence suggests that upon dNTP binding at the active site of HIV-1 RT, the fingers subdomain moves towards the active site, where the fingers β3-β4 hairpin loop makes contact with the nascent base pair, and sterically restrains the active site (Fig. 1B). In order to examine the role of this contact in fidelity, we engineered a deletion of residues 65 to 69 that form a part of the β3-β4 hairpin loop to create the mutant Δβ3-4L (Fig. 2A). The deletion was created in p66 and the mutant p66 reconstituted with the wild type p51 subunit. Initial characterization of the purified mutant heterodimer revealed that the Δβ3-4L mutant displayed increased Km and reduced kcat (Table 1 and Fig. 5). However, template-primer-RT dissociation kinetics of the Δβ3-4L mutant was identical to that of wild-type HIV-1 RT, suggesting that the gross folding of Δβ3-4L mutant is unaffected (Fig. 3).
Table 1.
Steady-state kinetic analysis of dAMP incorporation by wild-type HIV RT and the Δβ3-4L mutant, templated by either thymidine or difluorotoluene.
| Wild-type | Δβ3-4L | |||
|---|---|---|---|---|
| dATP:Ta | dATP:Fb | dATP:T | dATP:F | |
| 0.02 ± 0.004 | 20.3 ± 7.1 | 0.2 ±0.06 | 420 ±170 | |
| 2.3 ± 0.15 | 7.2 ± 1.3 | 1.5 ±0.13 | 0.1 ± 0.02 | |
| kcat/Km | 115 | 0.35 | 7.5 | 2.4×10−4 |
| f ins | 3.0×10−3 | 3.2×10−5 | ||
The incoming dNTP is specified first, followed by the template nucleotide.
Template nucleotide F, a non-polar thymidine analog.
Figure 5.

Quantitative analysis of incorporation of dATP opposite template F or template T by wild type and Δβ3-4L enzymes. Single-nucleotide extension assays were performed with a range of dATP substrate concentrations and an enzyme concentration of 5 nM (wild-type) or 10 nM (Δβ3-4L), and the proportion of extended primer determined by phophoimager analysis. The results were fitted to Michaelis-Menten curves using GraphPad Prism. The points are from at least two independent experiments. For clarity, separate graphs are displayed for the wild type and Δβ3-4L results.
Figure 3.

Binding of the wild-type and Δβ3-4L mutant enzyme to an RNA-DNA template-primer. Binding reactions were analyzed on native polyacrylamide gels, which were quantitated, and the percentage of unbound template-primer was plotted against the protein concentration. Dashed lines show the 95% confidence interval.
Deletion of the β3-β4 loop removes active site contacts (including interactions between Lys65 and γ-phosphate and that between Lys66 and the penultimate primer nucleotide) between the protein, and the primer terminus and the newly formed base pair at the active site. Translesion (TLS) DNA polymerases, which likewise contain relatively few nucleotide-protein contacts, are apparently dependent on hydrogen bonding between the dNTP and template in order for polymerization to proceed. In order to determine whether this was the case for the Δβ3-4L mutant RT, a DNA oligonucleotide that contained either a thymidine or a difluorotoluene residue at a specific position was used as a template (Fig. 2B and 2C) and primer extension was carried out. Difluorotoluene is a shape homolog of thymidine that is incapable of forming hydrogen bonds.18 Previous reports have shown that both Klenow (KF) and wild-type HIV-1 RT can efficiently utilize difluorotoluene as a templating nucleotide.19 We found that the Δβ3-4L mutant was much less efficient than either KF or wild-type RT at using the F analog as a template (Fig. 4A). In order to ensure that the decreased efficiency was specific to difluorotoluene and independent of other factors, a control oligonucleotide template with a T at the same position (shown in Figure 2B) was also employed. All three enzymes displayed similar ability to insert dATP at that position and to extend it further. In the above experiment, the F base was several nucleotides ahead of the primer terminus (running start). In order to test the influence of the position of the F base with respect to primer terminus, two different template-primer pairs were used (Fig. 2B), in which the analog was either the first (standing start) or the fourth (running start) templating nucleotide. Titration of incoming nucleotide (dATP) in a standing-start primer extension reaction revealed that while the wild type could efficiently use F as a template even when dATP concentration was as low as 2.5 μM, the Δβ3-4L mutant exhibited very little extension even with dATP concentrations as high as 1.25 mM (Fig. 4C). A similar effect was observed when the template nucleotide was deoxyadenosine and the incoming nucleotide was non-hydrogen bonding F (deoxyribose-fluorotoluene triphosphate, dFTP). In this case, slightly higher concentrations of dFTP had to be employed for incorporation by wild type (250 μM), but very little extension was observed by the Δβ3-4L mutant even with a dFTP concentration of 2 mM (Fig. 4C). Quantitative analysis by single nucleotide insertion reactions confirmed that, while the catalytic efficiency of the Δβ3-4L mutant was reduced compared to the wild type, use of difluorotoluene as template nucleotide virtually abolished activity (Fig. 5 and Table I). In the case of the wild-type enzyme, while the Km was increased with the F analog template compared to T template, the F analog template also leads to a markedly increased kcat. The reason for this increase in kcat is not known, but we speculate that the electronegative nature of the analog increases the dissociation rate following nucleotide insertion. The Δβ3-4L mutant had a marked increase in Km and decrease in kcat when using the template analog. The analog lead to a 330-fold reduction in the efficiency of nucleotide insertion by the wild-type enzyme; the reduction in efficiency was 31, 000-fold for the Δβ3-4L mutant. These results showed that the fingers β3-β4 loop truncation increases reliance on hydrogen-bonding for correct dNTP insertion by HIV-1 RT, in a manner reminiscent of low fidelity polymerases.
Figure 4.

Ability of wild type and Δβ3-4L mutant of HIV-1 RT to utilize the thymidine analog difluorotoluene (F). A. Incorporation of adenosine opposite either template thymidine or F base by Klenow, wild type and Δβ3-4L mutant HIV-1 RT enzymes. Reactions contained the running-start template-primer (2 nM) and either dATP (100 μM), allowing extension up to and including the modified nucleotide, or dATP, dCTP and dGTP (100 μM each), allowing extension to proceed to the end of the template. Reactions contained 20 nM enzyme, and were incubated for 1.5, 5 or 15 minutes. The position of the F analog in the modified template is labeled F/T. B. A basic residue is required at position 65 for HIV reverse transcriptase to utilize the F template nucleotide under either running or standing-start conditions. Reactions were incubated for 10 minutes, and contained 100 nM of the respective template-primer, and either 5nM enzyme (wild-type and K65R) or 10 nM enzyme (Δβ3-4L and K65A mutants). Lanes contained running or standing start primers, indicated “R” and “S” respectively. The templates contained either a thymine (template “T”) or difluorotoluene analog (template “F”). C. Utilization of F as either the template nucleotide or incoming nucleotide is impaired in the Δβ3-4L mutant. Reactions contained 100 nM template-primer, 5 nM wild type HIV-1 RT or 10 nM Δβ3-4L mutant, and were incubated for 5 minutes. Reactions contained either dATP or dFTP at the concentrations indicated. The unextended primer is labeled p, and the extension product p+1. D. The K65R mutant utilizes a template F, but K65A does not. Reactions contained standing-start primer and F containing template and dATP at the concentrations indicated, and 5 nM each enzyme.
Alanine substitution at a single residue (K65) recapitulates the dependence on hydrogen-bonding
Since the deletion mutant is missing five residues (65 to 69), it was unclear whether the acquired dependence on template base-incoming dNTP hydrogen bonding is due to an overall conformational change or due to loss of a specific contact. The residues deleted in the Δβ3-4L mutant include K65, which is known to interact with the incoming nucleotide via interaction with the γ phosphate. Therefore, we chose to first examine the result of substituting this residue alone, either via a conservative change (K65R) or by a substitution in which the positive charge is removed (K65A). Interestingly, the K65R mutant behaved similar to wild type RT in that it was able to utilize the difluorotoluene template for insertion of dATP. In contrast, the K65A mutant was analogous to the Δβ3-4L mutant in that it was incapable of utilizing difluorotoluene template in either the running or standing start assays (Fig 4B).
The relative ability of K65A and K65R mutants to use the thymidine analog deoxydifluorotoluene as a template nucleotide is shown in Fig. 4D. The results indicate that while the K65A mutant behaved similar to the Δβ3-4L deletion in its inability to insert dATP opposite template F, the K65R mutant was more similar to the wild-type enzyme. These results are interesting in that they reveal the importance of K65 and its interaction with the γ-phosphate in stabilization of the incoming dNTP in the absence of hydrogen bonding. They also suggest that the charge at Lys65 is important, revealing the significance of charge interactions at this site.
The Δβ3-4L mutant displays an unexpected increase in the fidelity of dNTP insertion
TLS DNA polymerases display low fidelity - their ability to distinguish correct nucleotide from incorrect is relatively low and they are generally unable to accept dNTPs in the absence of hydrogen bonding. Since the Δβ3-4L mutant was demonstrably similar to the TLS polymerases in being unable to insert dNTPs in the absence of hydrogen bonding between nucleotide and the template base, we surmised that the fidelity of this mutant would now be lower than wild-type RT and similar to TLS DNA polymerases. To test this hypothesis, fidelity of wild-type and Δβ3-4L mutant RTs were measured using the ‘standing start’ template-primer described in Fig. 2B, but with no modified analogs in the template. The polymerases were assayed for their ability to extend this primer by a single nucleotide. All four nucleotides were tested individually for their ability to be incorporated opposite a template thymidine. The results of our experiments indicated that wild-type RT incorporated the incorrect nucleotides (C, G or T) relatively efficiently opposite the template thymidine (Fig. 6). In contrast, even at nucleotide concentrations as high as 1.25 mM, the Δβ3-4L mutant was nearly incapable of incorporating deoxycytidine or deoxyguanosine (Fig. 6). The reaction with these incorrect nucleotides was so inefficient that catalytic constants could not be accurately calculated for the Δβ3-4L mutant. Interestingly, the incorrect incorporation of thymidine by the Δβ3-4L mutant was not reduced to the same extent (Fig. 6).
Figure 6.
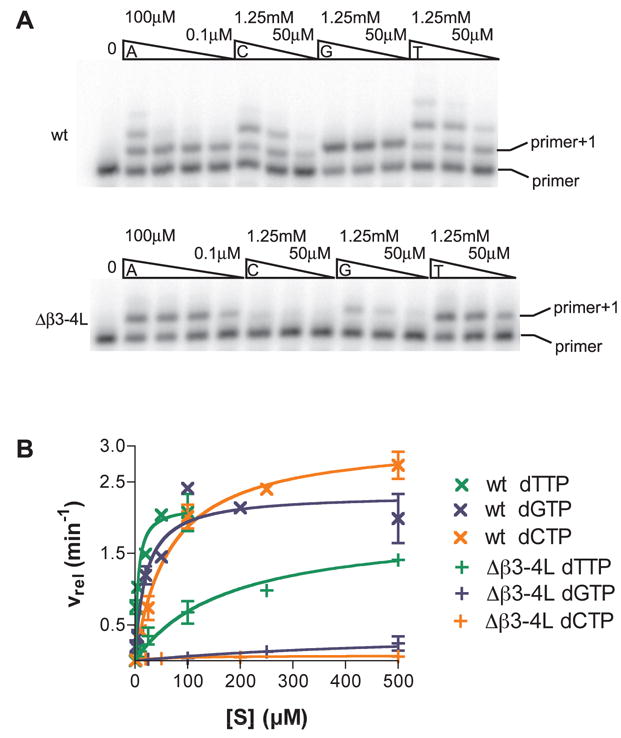
Quantitative analysis of the incorporation of a non-complementary nucleotide opposite template thymidine by Δβ3-4L and wild type enzymes. Single-nucleotide extension assays performed with wild type (5 nM) and Δβ3-4L mutant (10 nM) RTs. A. Reactions contained template-primer (100 nM) and either the next complementary nucleotide (dATP) 0.1, 1, 10 and 100μM or incorrect nucleotides (dCTP, dGTP or dTTP) at 50, 250 or 1250μM respectively. The unextended primer and the expected extension product (primer+1) are labeled. B. Single-nucleotide extension assays were performed as above, and the proportion of extended primer determined by phophoimager analysis. The results were fitted to a Michaelis-Menten curve using GraphPad Prism. The points are from at least two independent experiments. Wild-type fins were calculated to be 4.3×10−4, 8.4×10−4 and 2.9×10−3 for misincorporation of dCTP, dGTP and dTTP opposite a template thymine; Δβ3-4L fins for the same misincorporations were calculated as 1.6×10−4, 9.4×10−5 and 1.4×10−3.
The increased dNTP insertion fidelity of Δβ3-4L mutant is paralleled by that of K65A mutant
In order to determine whether the K65A mutation also influences the fidelity of HIV-1 RT, we examined the ability of K65A and K65R mutant RTs to insert correct and incorrect dNTPs using the same template-primer as described above for the Δβ3-4L mutant. Interestingly, while K65A displayed a striking similarity to Δβ3-4L in its increased ability to discriminate against all three mispairs involving a template T, the K65R mutant exhibited a degree of fidelity that was slightly higher than wild-type HIV-1 RT, but lower than the Δβ3-4L mutant (compare Fig. 6, panel ‘wt’ and Fig. 7A, panel ‘K65R’: insertion of dCTP opposite template T). This increase in fidelity for K65R was confirmed by measuring insertion of correct and incorrect dNTPs opposite a different template base (Adenine) (Fig. 7B). The increased fidelity of K65R mutant is consistent with our previous report that it displays an overall reduction in forward mutation rate.23
Figure 7.

A. Single-nucleotide extension assays performed with K65R and K65A mutant enzymes (5 nM each). Reactions contained template-primer (100 nM) and either the next complementary nucleotide (dATP) at 10, 100 or 1000 μM or incorrect nucleotides (dCTP, dGTP or dTTP) at 5, 50 or 500 μM respectively. The unextended primer and the expected extension product (primer+1) are labeled. B. Single nucleotide extension assays performed with the K65R mutant and wild-type reverse transcriptase (both at 5nM). Reactions contained template-primer in which the next complimentary nucleotide was dTTP, which was included at 0.1, 1, 10 or 100 μM. Incorrect nucleotides (dATP, dCTP or dGTP) were included at concentrations of 0.05, 0.25, 1 or 2 mM. The template-primer pairs used in each case are shown at the bottom of the panels.
Increased fidelity of Δβ3-4L and K65A mutants is associated with a decreased affinity for dNTP
In order to measure the influence of the K65 residue on nucleotide binding, we performed a dead end complex assay. This assay measures the formation of a stable complex between reverse transcriptase bound to primer-template in which the primer has a dideoxy-blocked 3′ terminus, and the incoming nucleotide.37 The incoming nucleotide was titrated in a range between 0.5 μM and 1 mM, and the resulting stable complex was separated from the template-primer on a native PAGE gel (Fig. 8). The gels were quantified, and nucleotide binding constants derived by curve-fitting data from two independent experiments. The results followed the same trend as the other experiments discussed here; the wild-type and K65R nucleotide binding constants were similar (Kd,app 9.2±1.2 μM and 12.3±1.1 μM respectively). Consistent with a role for a positive charge at position 65 in nucleotide binding, the Kd,app for both the K65A and Δβ3-4L was greater than 1 mM.
Figure 8.

Nucleotide binding by wild-type, Δβ3-4L, K65R and K65A HIV reverse transcriptase, analysed by dead-end complex formation. Ternary complexes consisting of reverse transcriptase, template annealed with 5′ end-labelled, 3′-blocked primer and the next complementary nucleotide, dATP (in a concentration range between 0.5 μM and 1 mM) were formed, and challenged with an unlabeled nucleic acid competitor. The dead end complex was separated from unbound primer-template by native PAGE.
The major difference between K65A and K65R substitutions is the charge and the length of the side chain of the amino acid residue. Therefore, we surmise that the wild-type enzyme owes its intermediate fidelity to a non-sequence specific stabilizing interaction between the K65 side chain and the negative charge on the γ-phosphate of dNTP. It is likely that when this non-specific interaction is eliminated, as in Δβ3-4L or K65A mutants, the enzyme now relies more on other interactions within the newly formed base pair (hydrogen bonding) and with other RT residues such as L74, R72 and Q151 thus increasing the accuracy of insertion.
DISCUSSION
In this report, we have presented results delineating the role of an HIV-1 RT-dNTP contact that is not discriminatory and that contributes to decreased fidelity of a DNA polymerase that otherwise behaves similarly to high fidelity polymerases. A small deletion in the fingers β3-β4 loop of HIV-1 RT results in an apparent, acquired dependence on hydrogen bonding. The unexpected increase in fidelity of this deletion mutant helped uncover a non-discriminating, stabilizing interaction between β3-β4 loop of wild-type RT and the triphosphate moiety of dNTP. The absence of this interaction in this mutant highlighted the role of other interactions that are discriminatory.
Although HIV-1 wild-type RT can utilize the thymine analog difluorotoluene, the β3-β4 loop deletion mutant could not (Fig. 4 and Table 1). In the wild-type enzyme, even though the template analog leads to an increase in both Km and kcat, the analog is utilized relatively efficiently. The inability of the Δβ3-4L mutant to insert the difluorotoluene analog suggests a greater dependence on Watson-Crick hydrogen bonding. Possible alternative explanations include the inability of the F analog to form minor-groove hydrogen bonds. However, using two nucleotide analogs, one of which is capable of minor groove interactions, it was demonstrated that HIV-1 RT does not require this sort of interaction for effective synthesis.38 Furthermore, the less drastic K65A substitution also led to an inability to insert a dATP opposite F template base (Fig. 4D) and there is no structural evidence that the lysine 65 residue is involved in minor groove interactions. Therefore, we propose that in the β3-β4 loop deletion mutant the absence of two interactions, the Watson-Crick base pairing and K65-γ-phosphate interaction, prevents proper positioning of nucleotide involving an A-F pair, so that nucleotidyl transfer cannot occur. Conversely, in the wild-type enzyme, the triphosphate group is stabilized by the β3-β4 loop, obviating the need for Watson-Crick hydrogen bonds for the correct positioning of dFTP opposite template A or dATP opposite template F. This emphasizes that in the absence of stabilizing interaction between RT and the triphosphate of the nucleotide, the importance of the Watson-Crick hydrogen bonding is greater.
The increase in fidelity displayed by the Δβ3-4L enzyme (Fig. 6) provided new insights into the determinants of fidelity in HIV-1 RT. The removal of residues that form part of the active site, as in the Δβ3-4L mutant, could be expected to cause less restriction on incorrect base pairing due to size constraints. However, fidelity actually increased compared to the wild-type. The increase in fidelity was the highest for insertion of G or C opposite T compared to insertion of T opposite T. A plausible explanation for the relative reduction in fidelity for a mismatched T-T base pair by the mutant enzyme is that an asymmetric ‘wobble’ base pair (in which hydrogen bonds are formed between O2-N3H and N3H-O4) is more easily accommodated in the active site of the deletion mutant. Such a base-pair retains hydrogen bonding, which we have shown is essential for nucleotide incorporation by the mutant, and causes minimal distortion of the phosphodiester backbone39,40.
The increased fidelity suggests that the influence of the β3-β4 loop via extra-stabilization at the non-informational part of the nucleotide has been lost. It is well known that the discriminatory interactions occur not near the sugar phosphate edges of the base pair, bur rather on the inner core of the basepair structure. Our results show that removal of a non-discriminatory stabilizing interaction between the β3-β4 loop and the γ-phosphate of dNTP increases fidelity, possibly via interactions with the discriminatory portions of the base-pair, which comprises the actual bases, their interaction via hydrogen bonds and their proper stacking with the preexisting base pair. In Fig. 1C, the potential interactions between the newly formed base-pair and both active site amino acids and the metal cofactors are shown.
Our experiments with the K65A mutant of HIV-1 RT confirm that the behavior of the Δβ3-4L mutant can be attributed mainly to the loss of K65, which makes contacts with the γ-phosphate of dNTP. The K65A mutant behaves exactly like the Δβ3-4L mutant, both in its apparent acquired dependence on hydrogen bonding as shown by its inability to insert dATP opposite F template base, and its increased fidelity especially for formation of dCTP:T, and dGTP:T mispairs and to a lesser extent than that of dTTP:T mispair.
A number of mutations including M184V,41,42 E89G43,44 and K65R23,34 have previously been reported to increase the fidelity of dNTP incorporation by HIV-1 RT. These mutations presumably alter the conformation of the dNTP-binding pocket to enhance discrimination of correct vs. incorrect dNTPs. Our experiments, using K65R as a representative of this class of RT variants with increased fidelity, show that the mechanisms by which K65R and the Δβ3-4L mutant increase discrimination are different. The Arg65 and Lys65 residues were both proposed to make bidentate electrostatic interactions with the nucleotide γ-phosphate, but an additional restraint was placed on the position of the Arg65 residue because of electrostatic interaction with Trp71.23 The differences in mechanism of increased fidelity between K65R and both the Δβ3-4L mutant and K65A is supported by the similarity in requirement for Watson-Crick base pairing in the latter two enzymes, which is not required by either wild type or K65R enzymes (Fig. 4).
It has been shown that interactions between RT and the γ-phosphate of the incoming nucleotide occur. Interactions were observed in the crystal structure of the ternary complex involving the γphosphate and the metal ion, and residues K65 and D113 of the protein.33 Interactions between the γ-phosphate of the incoming nucleotide and the polymerase active site have been shown to be crucial for stable nucleotide binding, and the work presented in this paper suggests that the charge on the K65 residue has a large affect on initial nucleotide binding (Fig. 8). The apparent Kd for nucleotide binding by either the Δβ3-4L or K65A mutants was at least two orders of magnitude higher than the wild-type Kd. In both human polymerase α and Klenow fragment, binding to nucleotide triphosphates occurred with higher affinity than binding to diphosphates or monophosphates.45,46 Interestingly, this difference in affinity was only observed for complementary nucleotide addition, suggesting that addition of the correct nucleotide induced a conformational change in the protein, and that the γ-phosphate plays a crucial role in this conformational change. Further evidence that the γ-phosphate could be involved in polymerase fidelity is provided in a report by Mulder et. al.47 They showed that modified nucleotides containing 1-aminonaphthalene-5-sulfonate (ANS) attached to their γ-phosphates were incorporated with higher fidelity by HIV RT, which suggested that in unmodified dNTPs the γphosphate plays a critical role in polymerase fidelity. In this paper, we show that interactions between the enzyme and the γ-phosphate of the incoming nucleotide reduce the dNTP selectivity.
Wilson and colleagues have argued that polymerase fidelity is largely governed by the efficiency with which the correct nucleotide is inserted.48,49 According to this hypothesis, incorrect nucleotide insertion is not related to the polymerase fidelity; instead, the catalytic efficiency with which the correct nucleotide is inserted determines fidelity, and is higher in high fidelity polymerases. This was demonstrated by plotting the catalytic efficiency with which a nucleotide is inserted against fidelity for a number of different enzymes, and a linear relationship between the two was demonstrated for the correct nucleotide, but not for incorrect nucleotide insertion.48 Interestingly, HIV-1 RT is an outlier of this linear relationship; the enzyme has a lower fidelity than would be expected from its enzymatic efficiency. Our data suggests that this is, at least in part, due to the β3-β4 loop, which increases the enzyme efficiency. However, the interactions between the β3-β4 loop and the incoming dNTP are not selective, and do not increase the fidelity of the reverse transcriptase. In the absence of the non-selective β3-β4 loop interactions with the incoming nucleotide other, more selective interactions play a more significant role; these could include base stacking and steric effects and, as we have demonstrated, the increased importance of Watson-Crick hydrogen bonding.
MATERIALS AND METHODS
Reagents for polymerase experiments
Primers (Invitrogen and IDTDNA) were gel purified through denaturing 10% polyacrylamide gels. Nucleotide triphosphates were purchased from CLP (Mercury Ultra-Pure nucleotides). Klenow Fragment (exo−) was purchased from NEB.
Protein purification
All wild type and mutant HIV-1 RT enzymes employed were in heterodimeric form and derived from HIV-1HXB2. All mutations except K65A were in the p66 subunit alone and the two subunits were expressed separately using pRT or pRT6H51 plasmids. RT expression constructs pRT and p6HRT51 were from Dr. Stuart Le Grice (Drug Resistance Program, NCI, Frederick). Expression and purification of the wild type, Δβ3-4L and K65R mutants were largely based on the method described by Le Grice and Gruninger-Leitch50 and for most of the enzymes studied here, it is similar to that described previously.28,50 To purify wild-type RTHXB2 and its Δβ3-4L mutant, RT subunits were over-expressed in E. coli strain DH5_ F’IQ containing the RT p66 or p51 expression constructs and a plasmid expressing rare tRNAs CodonPlus-RIL (Stratagene). Purification was carried out using an Äkta Explorer (Amersham Biosciences) at room temperature. In order to eliminate nucleic acids, the lysates were treated with 1% (w/v) streptomycin sulfate before loading onto chromatography columns. The RT heterodimers were purified via a hexahistidine tag on the p51 subunit. Following initial purification with a Ni2+-Sepharose column, the preparation was desalted and subjected to ion exchange chromatography on Mono-S. The enzyme specific activities were measured using a standard poly(rA)-oligo(dT) assay.51 All preparations were found to be free of nucleases. The purified K65A was expressed from a pET28a vector27 and the purified protein contained the mutation in both subunits.
Electrophoretic mobility shift assay
RNA template (the template and primer sequence is as used in Meyer et al.52) was synthesized by high yield in vitro transcription (Ampliscribe T7, Epicentre) from a partially double-stranded T7 promoter with a single-stranded oligonucleotide template.53 Template-primer was prepared by annealing an excess of RNA template W50 with a 32P end-labeled primer L33. Binding reactions contained RT in a concentration range of 0.16 nM to 1 μM and 0.1 nM template primer in a reaction mix containing 50 mM Tris-HCl, pH 8.0, 80 mM KCl, 6 mM MgCl2, 1 mM DTT, 0.1 mg/ml acetylated BSA and 5% glycerol. The reactions were incubated on ice for 10 minutes, and loaded on 10% native acrylamide gels run in 50 mM Tris-bicine, pH 8.3. The gels were run at a constant current of 10 mA at 4ºC for 1 hour, dried and exposed to phosphoimager screen. Binding was quantified by measuring the proportion of the template-primer that was unbound at each protein concentration, and the data was fitted to a sigmoidal dose-response curve using GraphPad Prism.
Single nucleotide insertion assays
Experiments that investigated a thymine or difluorotoluene template base utilized a 5′ 32P end-labeled primer (running-start: 5′ACGCCAGGGTTTTCCCAGTCACGACGTTGT3′ or standing-start 5′ACGCCAGGGTTTTCCCAGTCACGACGTTGTAAA3′) was annealed to the template oligonucleotide (5′CCCGXTTTACAACGTCGTGACTGGGAAAACCCTGGCGT), in which X represents either deoxythymidine or deoxydifluorotoluene. In experiments examining an adenine template base, the template sequence described above was used, with the 5′ 32P end-labeled primer 5′ACGCCAGGGTTTTCCCAGTCACGACGTTG3′. The enzyme and template primer were incubated together in a reaction mix containing 50 mM Tris-HCl, pH 8.0, 80 mM KCl, 6 mM MgCl2, 1 mM DTT, 0.1 mg/ml acetylated BSA and either one or three dNTPs. The concentrations of dNTP, enzyme and template primer are specified in the figure legends. The reaction products were separated on a denaturing 12% polyacrylamide sequencing gel, and exposed to a phosphoimager screen. For the quantitative single nucleotide insertion assays, less than 20% of the starting primer was extended, the extension time was 10 minutes or less, and there was at least a 10-fold excess of primer-template over enzyme. The proportion of primer that had been extended was quantitated, and fitted to a Michaelis-Menten function using GraphPad Prism. The frequency of misinsertion was calculated as fins = (Vmax/Km)incorrect dNTP/(Vmax/Km)correct dNTP.54
Nucleotide binding assay
An oligonucleotide with the same sequence as the standing-start primer described above, but truncated by a single nucleotide at the 3′ terminus, was blocked at the 3′-end with ddATP, using a procedure similar to that described previously.55,56 The primer (15μM) was incubated for 30 minutes at 37ºC in a reaction containing 1xTdT buffer, 1mM ddATP, 200 μg/ml BSA and 20 units TdT (USB). The blocked primer was phenol/chloroform extracted, ethanol precipitated and purified by size exclusion chromatography (P-30 Micro Bio-Spin column, Bio-Rad).
The procedure for dead end complex formation assays was based on Tong, et al.37 The blocked primer was 32P 5′ end-labeled and annealed to a 2 fold molar excess of template primer (CGCAGTATCCCGTTTTACAACGTCGTGACCTGGGAAAACCCTGGCGT). The binding reaction, containing 0.1 nM template-primer, 20 nM reverse transcriptase, 50 mM Tris, pH 8.0, 50 mM KCl, 6 mM MgCl2 and 1 mM DTT, was incubated at 37ºC for 5 minutes. The next complementary nucleotide (dATP) was then added to the reaction at a concentration ranging from 0.5 μM to 2 mM, and incubated for 10 minutes at 37ºC. A half volume of competitive trap mixture was added, comprising 12 μg/ml poly rA and 3 μg/ml oligo(dT) in 10 mM Tris, pH 8.0, 100 mM KCl, 30% glycerol and 50 μg/ml bromophenol blue, and the reactions were incubated for a further 5 minutes at 37ºC, and then placed on ice. The reaction products were separated by 6% native PAGE in 0.5× TBE. Gels were dried and exposed to a phosphoimager screen.
Footnotes
The authors would like to acknowledge Dr. Wei Yang (NIDDK, NIH) for invaluable suggestions, Ganjam Kalpana and Ian Willis for critically reading the manuscript and Albert Einstein Comprehensive Cancer Center’s DNA core facility for the use of DNA sequencing services. This work was supported in part by the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health (NIH AI-51519). The research described in this report was supported by a Public Health Service research grant to V.P. (AI030861), and an NIH research grant to E.T.K. (GM072705).
References
- 1.Rezende LF, Prasad VR. Nucleoside-analog resistance mutations in HIV-1 reverse transcriptase and their influence on polymerase fidelity and viral mutation rates. Int J Biochem Cell Biol. 2004;36:1716–1734. doi: 10.1016/j.biocel.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Menéndez-Arias L. Molecular basis of fidelity of DNA synthesis and nucleotide specificity of retroviral reverse transcriptases. Prog Nucleic Acid Res Mol Biol. 2002;71:91–147. doi: 10.1016/s0079-6603(02)71042-8. [DOI] [PubMed] [Google Scholar]
- 3.Preston BD, Poiesz BJ, Loeb LA. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 5.Bebenek K, Abbotts J, Roberts JD, Wilson SH, Kunkel TA. Specificity and mechanism of error-prone replication by human immunodeficiency virus-1 reverse transcriptase. J Biol Chem. 1989;264:16948–16956. [PubMed] [Google Scholar]
- 6.Rezende LF, Drosopoulos WC, Prasad VR. The influence of 3TC resistance mutation M184I on the fidelity and error specificity of human immunodeficiency virus type 1 reverse transcriptase. Nucleic Acids Res. 1998;26:3066–3072. doi: 10.1093/nar/26.12.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry AA, Olsen AG, Matsuda S, Yu C, Geierstanger BH, Romesberg FE. Efforts to expand the genetic alphabet: identification of a replicable unnatural DNA self-pair. J Am Chem Soc. 2004;126:6923–6931. doi: 10.1021/ja049961u. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda S, Henry AA, Romesberg FE. Optimization of unnatural base pair packing for polymerase recognition. J Am Chem Soc. 2006;128:6369–6375. doi: 10.1021/ja057575m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kincaid K, Beckman J, Zivkovic A, Halcomb RL, Engels JW, Kuchta RD. Exploration of factors driving incorporation of unnatural dNTPS into DNA by Klenow fragment (DNA polymerase I) and DNA polymerase alpha. Nucleic Acids Res. 2005;33:2620–2628. doi: 10.1093/nar/gki563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Lee I, Berdis AJ. The use of nonnatural nucleotides to probe the contributions of shape complementarity and pi-electron surface area during DNA polymerization. Biochemistry. 2005;44:13101–13110. doi: 10.1021/bi050585f. [DOI] [PubMed] [Google Scholar]
- 11.Vaisman A, Ling H, Woodgate R, Yang W. Fidelity of Dpo4: effect of metal ions, nucleotide selection and pyrophosphorolysis. EMBO J. 2005;24:2957–2967. doi: 10.1038/sj.emboj.7600786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong I, Patel SS, Johnson KA. An induced-fit kinetic mechanism for DNA replication fidelity: direct measurement by single-turnover kinetics. Biochemistry. 1991;30:526–537. doi: 10.1021/bi00216a030. [DOI] [PubMed] [Google Scholar]
- 13.Sawaya MR, Prasad R, Wilson SH, Kraut J, Pelletier H. Crystal structures of human DNA polymerase beta complexed with gapped and nicked DNA: evidence for an induced fit mechanism. Biochemistry. 1997;36:11205–11215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- 14.Ling H, Boudsocq F, Woodgate R, Yang W. Crystal structure of a Y-family DNA polymerase in action: a mechanism for error-prone and lesion-bypass replication. Cell. 2001;107:91–102. doi: 10.1016/s0092-8674(01)00515-3. [DOI] [PubMed] [Google Scholar]
- 15.Washington MT, Helquist SA, Kool ET, Prakash L, Prakash S. Requirement of Watson-Crick hydrogen bonding for DNA synthesis by yeast DNA polymerase eta. Mol Cell Biol. 2003;23:5107–5112. doi: 10.1128/MCB.23.14.5107-5112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore CL, Zivkovic A, Engels JW, Kuchta RD. Human DNA primase uses Watson-Crick hydrogen bonds to distinguish between correct and incorrect nucleoside triphosphates. Biochemistry. 2004;43:12367–12374. doi: 10.1021/bi0490791. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Aguilar KA, Moore CL, Kuchta RD. Herpes simplex virus 1 primase employs Watson-Crick hydrogen bonding to identify cognate nucleoside triphosphates. Biochemistry. 2005;44:15585–15593. doi: 10.1021/bi0513711. [DOI] [PubMed] [Google Scholar]
- 18.Moran S, Ren RX, Kool ET. A thymidine triphosphate shape analog lacking Watson-Crick pairing ability is replicated with high sequence selectivity. Proc Natl Acad Sci USA. 1997;94:10506–10511. doi: 10.1073/pnas.94.20.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales JC, Kool ET. Varied Molecular Interactions at the Active Sites of Several DNA Polymerases: Nonpolar Nucleoside Isosteres as Probes. J Am Chem Soc. 2000;122:1001–1007. doi: 10.1021/ja993464+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfle WT, Washington MT, Kool ET, Spratt TE, Helquist SA, Prakash L, Prakash S. Evidence for a Watson-Crick hydrogen bonding requirement in DNA synthesis by human DNA polymerase kappa. Mol Cell Biol. 2005;25:7137–7143. doi: 10.1128/MCB.25.16.7137-7143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doublié S, Sawaya MR, Ellenberger T. An open and closed case for all polymerases. Struct Fold Des. 1999;7:R31–R35. doi: 10.1016/S0969-2126(99)80017-3. [DOI] [PubMed] [Google Scholar]
- 22.Steitz TA. A mechanism for all polymerases. Nature. 1998;391:231–232. doi: 10.1038/34542. [DOI] [PubMed] [Google Scholar]
- 23.Shah FS, Curr KA, Hamburgh ME, Parniak M, Mitsuya H, Arnez JG, Prasad VR. Differential influence of nucleoside analog-resistance mutations K65R and L74V on the overall mutation rate and error specificity of human immunodeficiency virus type 1 reverse transcriptase. J Biol Chem. 2000;275:27037–27044. doi: 10.1074/jbc.M002881200. [DOI] [PubMed] [Google Scholar]
- 24.Kim B, Ayran JC, Sagar SG, Adman ET, Fuller SM, Tran NH, Horrigan J. New human immunodeficiency virus, type 1 reverse transcriptase (HIV-1 RT) mutants with increased fidelity of DNA synthesis. Accuracy, template binding, and processivity. J Biol Chem. 1999;274:27666–27673. doi: 10.1074/jbc.274.39.27666. [DOI] [PubMed] [Google Scholar]
- 25.Kew Y, Olsen LR, Japour AJ, Prasad VR. Insertions into the beta3-beta4 hairpin loop of HIV-1 reverse transcriptase reveal a role for fingers subdomain in processive polymerization. J Biol Chem. 1998;273:7529–7537. doi: 10.1074/jbc.273.13.7529. [DOI] [PubMed] [Google Scholar]
- 26.Arion D, Borkow G, Gu Z, Wainberg MA, Parniak MA. The K65R mutation confers increased DNA polymerase processivity to HIV-1 reverse transcriptase. J Biol Chem. 1996;271:19860–19864. doi: 10.1074/jbc.271.33.19860. [DOI] [PubMed] [Google Scholar]
- 27.Sluis-Cremer N, Arion D, Kaushik N, Lim H, Parniak MA. Mutational analysis of Lys65 of HIV-1 reverse transcriptase. Biochem J. 2000;348(Pt 1):77–82. [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher TS, Darden T, Prasad VR. Substitutions at Phe61 in the beta3-beta4 hairpin of HIV-1 reverse transcriptase reveal a role for the Fingers subdomain in strand displacement DNA synthesis. J Mol Biol. 2003;325:443–459. doi: 10.1016/s0022-2836(02)01225-1. [DOI] [PubMed] [Google Scholar]
- 29.Deval J, Navarro JM, Selmi B, Courcambeck J, Boretto J, Halfon P, Garrido-Urbani S, Sire J, Canard B. A loss of viral replicative capacity correlates with altered DNA polymerization kinetics by the human immunodeficiency virus reverse transcriptase bearing the K65R and L74V dideoxynucleoside resistance substitutions. J Biol Chem. 2004;279:25489–25496. doi: 10.1074/jbc.M313534200. [DOI] [PubMed] [Google Scholar]
- 30.Gu Z, Gao Q, Fang H, Salomon H, Parniak MA, Goldberg E, Cameron J, Wainberg MA. Identification of a mutation at codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1994;38:275–281. doi: 10.1128/aac.38.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller MD. K65R, TAMs and tenofovir. AIDS Rev. 2004;6:22–33. [PubMed] [Google Scholar]
- 32.Tantillo C, Ding J, Jacobo-Molina A, Nanni RG, Boyer PL, Hughes SH, Pauwels R, Andries K, Janssen PA, Arnold E. Locations of anti-AIDS drug binding sites and resistance mutations in the three-dimensional structure of HIV-1 reverse transcriptase. Implications for mechanisms of drug inhibition and resistance. J Mol Biol. 1994;243:369–387. doi: 10.1006/jmbi.1994.1665. [DOI] [PubMed] [Google Scholar]
- 33.Huang H, Chopra R, Verdine GL, Harrison SC. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 34.Harris D, Kaushik N, Pandey PK, Yadav PN, Pandey VN. Functional analysis of amino acid residues constituting the dNTP binding pocket of HIV-1 reverse transcriptase. J Biol Chem. 1998;273:33624–33634. doi: 10.1074/jbc.273.50.33624. [DOI] [PubMed] [Google Scholar]
- 35.Kool ET. Active site tightness and substrate fit in DNA replication. Annu Rev Biochem. 2002;71:191–219. doi: 10.1146/annurev.biochem.71.110601.135453. [DOI] [PubMed] [Google Scholar]
- 36.Rittinger K, Divita G, Goody RS. Human immunodeficiency virus reverse transcriptase substrate-induced conformational changes and the mechanism of inhibition by nonnucleoside inhibitors. Proc Natl Acad Sci USA. 1995;92:8046–8049. doi: 10.1073/pnas.92.17.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong W, Lu CD, Sharma SK, Matsuura S, So AG, Scott WA. Nucleotide-induced stable complex formation by HIV-1 reverse transcriptase. Biochemistry. 1997;36:5749–5757. doi: 10.1021/bi962410z. [DOI] [PubMed] [Google Scholar]
- 38.Morales JC, Kool ET. Functional hydrogen-bonding map of the minor groove binding tracks of six DNA polymerases. Biochemistry. 2000;39:12979–12988. doi: 10.1021/bi001578o. [DOI] [PubMed] [Google Scholar]
- 39.Gantchev TG, Cecchini S, Hunting DJ. Dynamic conformational states of DNA containing T.T or BrdU.T mispaired bases: wobble H-bond pairing versus cross-strand interatomic contacts. J Mol Model. 2005;11:141–159. doi: 10.1007/s00894-005-0238-9. [DOI] [PubMed] [Google Scholar]
- 40.Sherer EC, Cramer CJ. Structural and dynamic variations in DNA hexamers containing T-T and F-F single and tandem internal mispairs. Theor Chem Acc. 2004;111:311–327. [Google Scholar]
- 41.Pandey VN, Kaushik N, Rege N, Sarafianos SG, Yadav PN, Modak MJ. Role of methionine 184 of human immunodeficiency virus type-1 reverse transcriptase in the polymerase function and fidelity of DNA synthesis. Biochemistry. 1996;35:2168–2179. doi: 10.1021/bi9516642. [DOI] [PubMed] [Google Scholar]
- 42.Wainberg MA, Drosopoulos WC, Salomon H, Hsu M, Borkow G, Parniak M, Gu Z, Song Q, Manne J, Islam S, Castriota G, Prasad VR. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science. 1996;271:1282–1285. doi: 10.1126/science.271.5253.1282. [DOI] [PubMed] [Google Scholar]
- 43.Drosopoulos WC, Prasad VR. Increased polymerase fidelity of E89G, a nucleoside analog-resistant variant of human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1996;70:4834–4838. doi: 10.1128/jvi.70.7.4834-4838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oude Essink BB, Back NK, Berkhout B. Increased polymerase fidelity of the 3TC-resistant variants of HIV-1 reverse transcriptase. Nucleic Acids Res. 1997;25:3212–3217. doi: 10.1093/nar/25.16.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doronin SV, Lavrik OI, Nevinsky GA, Podust VN. The efficiency of dNTP complex formation with human placenta DNA polymerase alpha as demonstrated by affinity modification. FEBS Lett. 1987;216:221–224. doi: 10.1016/0014-5793(87)80693-2. [DOI] [PubMed] [Google Scholar]
- 46.Doronin SV, Nevinsky GA, Malygina TO, Podust VN, Khomov VV, Lavrik OI. The efficiency of interaction of deoxyribonucleoside-5′-mono-, di- and triphosphates with the active centre of E. coli DNA polymerase I Klenow fragment. FEBS Lett. 1989;259:83–85. doi: 10.1016/0014-5793(89)81500-5. [DOI] [PubMed] [Google Scholar]
- 47.Mulder BA, Anaya S, Yu P, Lee KW, Nguyen A, Murphy J, Willson R, Briggs JM, Gao X, Hardin SH. Nucleotide modification at the gamma-phosphate leads to the improved fidelity of HIV-1 reverse transcriptase. Nucleic Acids Res. 2005;33:4865–4873. doi: 10.1093/nar/gki779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beard WA, Shock DD, Vande Berg BJ, Wilson SH. Efficiency of correct nucleotide insertion governs DNA polymerase fidelity. J Biol Chem. 2002;277:47393–47398. doi: 10.1074/jbc.M210036200. [DOI] [PubMed] [Google Scholar]
- 49.Beard WA, Wilson SH. Structural insights into the origins of DNA polymerase fidelity. Struct Fold Des. 2003;11:489–496. doi: 10.1016/s0969-2126(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 50.Le Grice SF, Grüninger-Leitch F. Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur J Biochem. 1990;187:307–314. doi: 10.1111/j.1432-1033.1990.tb15306.x. [DOI] [PubMed] [Google Scholar]
- 51.Kew Y, Qingbin S, Prasad VR. Subunit-selective mutagenesis of Glu-89 residue in human immunodeficiency virus reverse transcriptase. Contribution of p66 and p51 subunits to nucleoside analog sensitivity, divalent cation preference, and steady state kinetic properties. J Biol Chem. 1994;269:15331–15336. [PubMed] [Google Scholar]
- 52.Meyer PR, Matsuura SE, So AG, Scott WA. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc Natl Acad Sci USA. 1998;95:13471–13476. doi: 10.1073/pnas.95.23.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Milligan JF, Uhlenbeck OC. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- 54.Mendelman LV, Boosalis MS, Petruska J, Goodman MF. Nearest neighbor influences on DNA polymerase insertion fidelity. J Biol Chem. 1989;264:14415–14423. [PubMed] [Google Scholar]
- 55.Kaushik N, Rege N, Yadav PN, Sarafianos SG, Modak MJ, Pandey VN. Biochemical analysis of catalytically crucial aspartate mutants of human immunodeficiency virus type 1 reverse transcriptase. Biochemistry. 1996;35:11536–11546. doi: 10.1021/bi960364x. [DOI] [PubMed] [Google Scholar]
- 56.Mas A, Vázquez-Alvarez BM, Domingo E, Menéndez-Arias L. Multidrug-resistant HIV-1 reverse transcriptase: involvement of ribonucleotide-dependent phosphorolysis in cross-resistance to nucleoside analogue inhibitors. J Mol Biol. 2002;323:181–197. doi: 10.1016/s0022-2836(02)00911-7. [DOI] [PubMed] [Google Scholar]
- 57.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–8, 27–8. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 58.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 59.Sanner MF, Olson AJ, Spehner JC. Reduced surface: an efficient way to compute molecular surfaces. Biopolymers. 1996;38:305–320. doi: 10.1002/(SICI)1097-0282(199603)38:3%3C305::AID-BIP4%3E3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]


