Abstract
Background
Joint angular velocity (the rate of flexion and extension of a joint) is related to the dynamics of muscle activation and force generation during walking. Therefore, the goal of this research was to examine the joint angular velocity in normal and spastic gait and changes resulting from muscle-tendon lengthening (recession and tenotomy) in patients who have spastic cerebral palsy.
Methods
The gait patterns of forty patients who had been diagnosed with spastic cerebral palsy (mean age, 8.3 years; range, 3.7 to 14.8 years) and of seventy-three age-matched, normally developing subjects were evaluated with three-dimensional motion analysis and electromyography. The patients who had cerebral palsy were evaluated before muscle-tendon lengthening and nine months after treatment.
Results
The gait patterns of the patients who had cerebral palsy were characterized by increased flexion of the knee in the stance phase, premature plantar flexion of the ankle, and reduced joint angular velocities compared with the patterns of the normally developing subjects. Even though muscle-tendon lengthening altered sagittal joint angles in gait, the joint angular velocities were generally unchanged at the hip and knee. Only the ankle demonstrated modified angular velocities, including reduced dorsiflexion velocity at foot-strike and improved dorsiflexion velocity through midstance, after treatment. Electromyographic changes included reduced amplitude of the gastrocnemius-soleus during the loading phase and decreased knee coactivity (the ratio of quadriceps and hamstring activation) at toe-off. Principal component analyses showed that, compared with joint-angle data, joint angular velocity was better able to discriminate between the gait patterns of the normal and cerebral palsy groups.
Conclusions
This study showed that muscle-tendon lengthening corrects biomechanical alignment as reflected by changes in sagittal joint angles. However, joint angular velocity and electromyographic data suggest that the underlying neural input remains largely unchanged at the hip and knee. Conversely, electromyographic changes and changes in velocity in the ankle indicate that the activation pattern of the gastrocnemius-soleus complex in response to stretch was altered by recession of the complex.
By definition, spastic muscles demonstrate velocity-dependent hypertonic reflexes14. It follows that patients who have spasticity will demonstrate reduced joint angular velocity (the rate of joint flexion and extension in gait). Evidence in support of this argument includes the demonstration of reduced joint angular velocity in spastic muscles during isolated joint motion6,8,12,15,21. Similarly, joint angular velocities are reduced during free walking in patients who have spasticity due to spinal cord injury13. It is important to measure joint angular velocity because of its relationship to gait dynamics, including momentum, transfer of energy between limb segments, and the velocity of forward progression10,18,19,22,26.
Muscle-tendon lengthening (including recession and tenotomy) plays a prominent role in alleviating static deformity due to spasticity3. Research has verified that muscle-tendon lengthening alters joint angle profiles in spastic gait9,11,25. It has also been demonstrated that velocity of forward progression and stride length are increased after treatment2, although these findings are not unanimous9,25. However, it is unknown whether muscle-tendon lengthening can influence joint angular velocity in patients who have spastic cerebral palsy.
Arguments can be made to support or refute the claim that muscle-tendon lengthening modifies joint angular velocity. Joints move under the influence of muscle forces — specifically, the balance of the agonist and antagonist muscles that span the joint. Lengthening of the muscle-tendon unit alters this muscle balance as well as the stretch reflex that is mediated through the muscle spindle. Thus, muscle-tendon lengthening may alter joint angular velocity by unmasking agonist muscle force23. Conversely, one may predict that joint angular velocity will not be altered because muscle-tendon lengthening does not have an influence on the central nervous system's control of walking.
The goal of our research was to assess the influence of muscle-tendon lengthening on gait dynamics by analyzing joint angular velocities and electromyographic data. On the basis of what is known about spasticity and its origin in the central nervous system, we hypothesized that muscle-tendon lengthening would have a limited effect on joint angular velocity during gait.
Materials and Methods
Participants included forty patients diagnosed with spastic diplegia who had been managed, between February 1994 and October 1996, with muscle-tendon lengthening (recession and tenotomy) to treat contractures of the lower extremities and to improve walking ability, at the University of Virginia Children's Medical Center. Seventy-three age-matched, normally developing children were also evaluated. Evaluation protocols were set up prospectively. Exclusion criteria included moderate-to-severe mental retardation, extra-pyramidal motor involvement (characterized by athetosis, ataxia, or ballismus), and concurrent medical conditions that had necessitated an operation or hospitalization within six months before the study. Patients who had torsional deformities that were severe enough to warrant osteotomies were also excluded from the analysis.
The mean age at the time of treatment was 8.3 years (range, 3.7 to 14.8 years). Muscle-tendon units that had a broad aponeurotic expansion at the muscle-tendon junction were lengthened with transection (recession) of this aponeurosis, followed by manual stretching until the desired joint position was achieved. Typically, a one to three-centimeter gap in the aponeurosis resulted while the continuity of the muscle was maintained. Muscle-tendon structures that were conducive to aponeurotic lengthening included the semimembranosus, biceps femoris, gastrocnemius-soleus, and iliopsoas. Muscle-tendon release was performed at the muscle origin for the adductor longus and gracilis and distally for the semitendinosus (tenotomy). Distal hamstring lengthening was carried out in twenty-nine patients; eighteen of the twenty-nine patients were managed with medial and lateral lengthenings, and the other eleven were managed with medial lengthening only. Thirty patients were managed with recession of the gastrocnemius-soleus. Sixteen patients were managed with proximal gracilis and adductor longus releases. In fourteen patients, only a single muscle-tendon group was lengthened bilaterally (six patients had distal hamstring lengthenings only and eight had gastrocnemius-soleus lengthenings only). Other procedures included transfer of the rectus femoris to the hamstrings (six patients), recession of the iliopsoas (three patients), transfer of the posterior tibial tendon (two patients), subtalar stapling (four patients), and osteotomy of the first metatarsal (one patient). One of us (M. F. A.) performed all of the operations.
The operations were performed when restricted joint motion on physical examination was associated with kinematic abnormalities on gait analysis and was not amenable to bracing. For example, the adductor longus was released when passive abduction was less than 30 degrees with the hips extended and dynamic scissoring of the lower limb occurred during walking, hamstring lengthening was carried out when full passive extension of the knee was not possible and persistent knee flexion in single-limb stance was noted, and recession of the gastrocnemius-soleus was done when passive dorsiflexion of the ankle (measured with the subtalar joint in neutral and the knee extended) did not reach the neutral position and equinus in early single-limb stance was noted. For the hamstring muscles, we started lengthening on the medial side in an attempt to increase extension of the knee, abduction of the hip, and external rotation of the limb. The biceps femoris was recessed if full passive extension of the knee was not achieved following operations on the medial side.
The gait patterns of the forty children diagnosed with spastic cerebral palsy and of the seventy-three age-matched, normally developing children were evaluated with standard three-dimensional motion analysis and electromyography. The normally developing subjects included siblings of the patients and other children from the university community. In total, 154 limbs were evaluated in the study of the children with spastic cerebral palsy (eighty limbs were examined preoperatively, eighty were examined postoperatively, and six were later excluded because the video motion data on them were lost) and 146 limbs were evaluated in the study of the normally developing children. The study was approved by the university's Human Investigations Committee, and informed consent was obtained from the patients' guardians before each measurement session.
The children who had cerebral palsy were evaluated with three-dimensional video motion analysis before orthopaedic treatment and nine months after treatment. The period between assessments was limited to one year to minimize the influence of growth and maturation on gait and motor function. Preoperative tests were performed at a mean of 2.3 months (range, zero to six months) before treatment. Twenty-one of the patients were found, on serial evaluations done every three months, to have had recovery by a mean of six months after muscle-tendon lengthening2. Postoperative evaluations were performed at a mean of nine months (range, 7.7 to eleven months) after treatment. Two-year follow-up data were collected and subsequently reported for twenty-nine of the patients2. In the additional fifteen months, no functional or kinematic changes occurred that could not be explained by growth (increase in limb length).
Analyses were performed with a VICON six-camera kinematic system (Oxford Metrics, Oxford, England) at a sampling rate of sixty hertz. Limb segments were represented by fifteen reflective markers on the lower extremities and the pelvis. Electromyographic data (Noraxon, Scottsdale, Arizona) were collected from the children who had a spastic gait with use of bipolar surface electrodes over the rectus femoris, biceps femoris, gastrocnemius, and tibialis anterior muscles.
All of the subjects walked barefoot along a ten-meter carpeted walkway at freely selected speeds. A minimum of three representative trials were processed and averaged with use of VICON Clinical Manager software (version 1.21; Oxford Metrics). Temporal-distance factors (velocity of forward progression, stride length, and cadence) and total joint excursions in the sagittal plane were calculated at the hip, knee, and ankle for each of the subjects. Joint angles were calculated in order to determine changes in magnitude and timing during the gait cycle. An experienced physical therapist (D. L. D.) measured passive motion of the joint.
Joint angular velocities were calculated with numerical differentiation of the time-based angle data. Joint excursion is related to the mathematical integral of joint angular velocity and stride duration. Hence, joint angular velocity data were normalized to the stride duration (the angular velocity in terms of degrees per percent of the gait cycle) to remove cadence artifact, thereby considering strictly the relation between excursion and joint angular velocity. Peak joint angular velocities were determined for flexion and extension for each subject. Angle and joint angular velocity at foot-strike were calculated from the mean value at foot-strike ± 3 percent of the gait cycle. Similar analyses were performed for toe-off data. Mid-stance data represented the mean values from 20 to 40 percent of the gait cycle while the patient was in single-limb stance.
Electromyographic data were recorded from the gastrocnemius, tibialis anterior, hamstrings, and quadriceps muscle groups for all of the patients who had cerebral palsy and from seventeen limbs of the normally developing children. Data on muscle activities were collected with bipolar surface electrodes and were normalized to the mean activity level observed throughout the gait cycle28. This method permits comparison of the dynamic range and relative changes in amplitude between muscles and between the patients' preoperative and postoperative conditions, but it is limited in its ability to compare mean or absolute levels. Muscle coactivation about the knee (quadriceps versus hamstrings) and ankle (gastrocnemius versus tibialis anterior) was evaluated to assess the balance of muscle activity before and after treatment. Coactivity was quantified by scaling the most active muscle about each joint by the relative difference in total activity:
In this relation, EMGAg represents the normalized myoelectric activity in the primary agonist muscle and EMGAntag represents that in the antagonist. At each point in time, the agonist was assumed to be the more active of the pair as proposed by Falconer and Winter7. Multiplying the scaling factor by the agonist avoids mis-interpretation of coactivity when both muscles are near resting levels. The coactivity relation describes antagonistic activity as a percentage of total myoelectric energy about the joint of interest relative to the agonist behavior. The relation was derived from and was similar to the coactivity measures developed by Olney20 and by Falconer and Winter7.
Statistical analyses were performed to identify differences between the motions in the normally developing subjects and the preoperative motions in the patients who had cerebral palsy, between the data on the normally developing subjects and the postoperative data on the patients who had cerebral palsy, and between the preoperative data and the postoperative results for the patients who had cerebral palsy. Data representing the joint angle, joint angular velocity, electromyographic findings, and coactivity at foot contact, mid-stance, toe-off, peak flexion, and peak extension were examined with paired comparison tests. The level of significance was considered to be p < 0.05. Principal component analyses were performed to evaluate the joint angle and joint angular velocity profiles as a whole. This technique allows quantification of the shape and the timing of the data reporting joint angle and joint angular velocity profiles (Figs. 1 and 2). An excellent description and application of principal component analyses may be found in the paper by Wootten et al.27. Weighting coefficients based on principal component analyses were evaluated with forward stepwise linear regression to analyze the ability to categorize subjects into the spastic cerebral palsy and normally developing groups. Intersubject variance at each point in time was documented within each group. The mean variance provided an estimate of the intersubject variability.
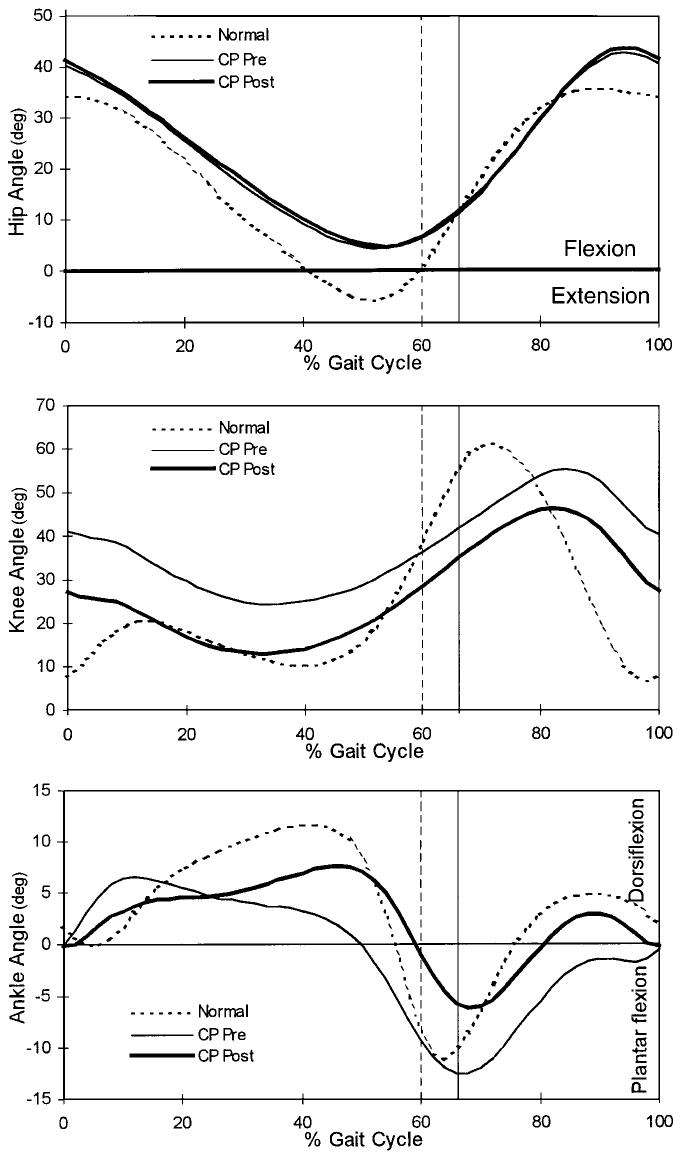
Fig. 1.
Gait profiles showing the mean hip, knee, and ankle angles for the normally developing subjects (dotted line), the patients with spastic cerebral palsy (CP) before muscle-tendon lengthening (thin line), and the patients with spastic cerebral palsy after muscle-tendon lengthening (heavy line). The vertical lines represent the time of toe-off for the normally developing subjects (dashed line) and for the patients who had spastic cerebral palsy (solid line).
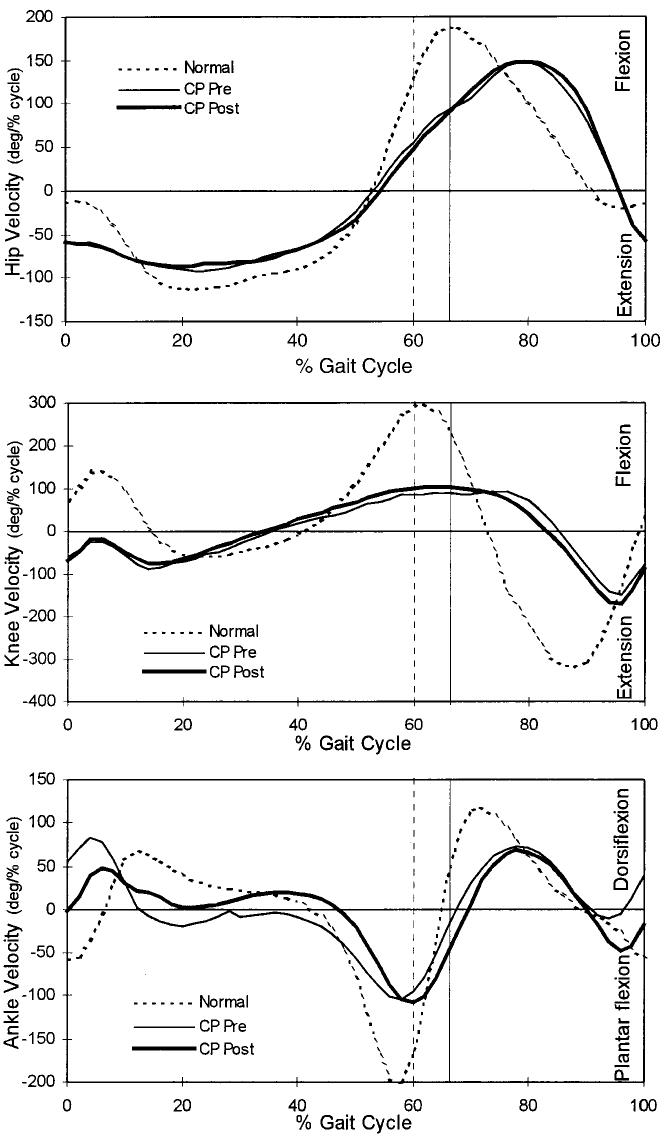
Fig. 2.
Gait profiles showing the mean hip, knee, and ankle angular velocities of the normally developing subjects (dotted line), the patients with spastic cerebral palsy (CP) before muscle-tendon lengthening (thin line), and the patients with spastic cerebral palsy after muscle-tendon lengthening (heavy line). The vertical lines represent the time of toe-off for the normally developing subjects (dashed line) and for the patients with spastic cerebral palsy (solid line).
Results
Nine months after the operation, the mean stride length increased by ten centimeters and the mean velocity of forward progression increased by ten centimeters per second2.
Joint Angle
The patients who had spastic cerebral palsy demonstrated hip excursion values similar to those of the normally developing subjects, but the entire angle profile was shifted toward flexion (Fig. 1). With the numbers available for study, we could not detect any significant differences between preoperative and postoperative hip angles.
Knee angles during gait decreased as a result of muscle-tendon lengthening (Fig. 1). Before treatment, the patients who had cerebral palsy demonstrated crouched gait characterized by increased knee flexion throughout the stance phase compared with that of the normally developing subjects. After treatment, mean knee flexion and mid-stance flexion were significantly reduced (improved knee extension) (p < 0.05), resulting in knee angles within the range of the angles of the normally developing subjects (Table I). The knee angles at foot contact and toe-off were also more extended but remained significantly different from normal (p < 0.05). Peak knee flexion (swing) was reduced and occurred earlier in the cycle after the operation (Table III). Muscle-tendon lengthening failed to substantially modify total knee excursions (Table I). Instead, the treatment shifted the entire knee angle curve toward extension (Fig. 1).
TABLE I.
Joint Angles in Normal Subjects and Before and After Muscle-Tendon Lengthening in Patients with Cerebral Palsy
| Angle (degrees) |
||||
|---|---|---|---|---|
| Effect Size* | Normal Subjects | Patients with Cerebral Palsy† |
||
| Preop. | Postop. | |||
| Hip | ||||
| Excursion | 3.8 | 42.4 | 41.4 | 41.9 |
| Mean angle | 4.0 | 18.1 | 22.4 | 22.8 |
| Maximum flexion | 5.6 | 36.4 | 44.1 | 44.9 |
| Maximum extension | 5.9 | 6.0 | −2.7 | −3.0 |
| Flexion angle | ||||
| At foot contact | 4.5 | 34.0 | 38.5 | 39.1 |
| At mid-stance | 5.3 | 4.6 | 11.5 | 12.5 |
| At toe-off | 4.9 | 1.1 | 10.2 | 10.9 |
| Knee | ||||
| Excursion | 5.2 | 56.6 | 36.4 | 38.8 |
| Mean angle | 4.6 | 24.9 | 37.3 | 26.9‡ |
| Maximum flexion | 5.4 | 61.7 | 58.2 | 49.4‡ |
| Minimum flexion | 5.0 | 5.1 | 21.8 | 10.7‡ |
| Flexion angle | ||||
| At foot contact | 5.3 | 11.3 | 40.3 | 26.3‡ |
| At mid-stance | 5.3 | 10.9 | 24.3 | 13.0‡ |
| At toe-off | 4.9 | 40.1 | 40.4 | 35.0‡ |
| Ankle | ||||
| Excursion | 4.1 | 24.7 | 28.5 | 22.1‡ |
| Mean angle | 4.3 | 3.4 | −1.3 | 2.0 |
| Maximum dorsiflexion | 4.5 | 12.8 | 11.3 | 11.8 |
| Maximum plantar flexion | 5.9 | 11.8 | 17.2 | 10.3‡ |
| Dorsiflexion angle | ||||
| At foot contact | 5.1 | 1.4 | 0.1 | −0.2 |
| At mid-stance | 4.3 | 11.1 | 2.8 | 6.3 |
| Plantar flexion angle at toe-off | 6.2 | 9.3 | 14.0 | 6.0‡ |
Effect size = the difference required for significance at α < 0.05 with a statistical power of 0.9.
Boldface type indicates that the data were significantly different from the value for normally developing subjects (p < 0.05).
These postoperative data were significantly different from the preoperative values (p < 0.05).
TABLE III.
Time of Peak Angle (Percent of Cycle)
| Effect Size* | Normal Subjects | Patients with Cerebral Palsy† |
||
|---|---|---|---|---|
| Preop. | Postop. | |||
| Hip | ||||
| Flexion angle | 1.9 | 93.4 | 95.5 | 95.3 |
| Extension angle | 2.2 | 51.6 | 53.2 | 54.2 |
| Knee | ||||
| Flexion angle | 2.6 | 71.6 | 85.6 | 82.1‡ |
| Extension angle | 3.8 | 41.2/97.9 | 33.7 | 30.0‡ |
| Ankle | ||||
| Dorsiflexion angle | 5.1 | 39.1 | 27.0 | 36.3‡ |
| Plantar flexion angle | 1.6 | 63.6 | 66.2 | 66.5 |
Effect size = the difference required for significance at α < 0.05 with a statistical power of 0.9.
Boldface type indicates that the data were significantly different from the value for normally developing subjects (p < 0.05).
These postoperative data were significantly different from the preoperative values (p < 0.05). The results suggest that muscle-tendon lengthening can modify the timing of peak angles.
Preoperatively, the patients who had cerebral palsy demonstrated premature peak dorsiflexion and reduced mid-stance dorsiflexion of the ankle compared with the normally developing subjects (Fig. 1 and Table I). Treatment delayed the time of peak dorsiflexion from 27.0 to 36.3 percent of the gait cycle (Table III) but did not affect the magnitude of dorsiflexion. Total ankle excursion was reduced postoperatively because the maximum plantar flexion angle was reduced (Table I).
Joint Angular Velocity
Although significant differences (p < 0.05) in joint angular velocities were found between the normally developing children and the patients who had spastic diplegia, no significant changes in hip or knee velocity following muscle-tendon lengthening were detected, with the numbers available in this study (Tables II and IV).
TABLE II.
Joint Angular Velocities in Normal Subjects and Before and After Muscle-Tendon Lengthening in Patients with Cerebral Palsy
| Velocity (degrees/percent of cycle) |
||||
|---|---|---|---|---|
| Effect Size* | Normal Subjects | Patients with Cerebral Palsy† |
||
| Preop. | Postop. | |||
| Hip | ||||
| Maximum flexion velocity | 19 | 192.1 | 181.9 | 184.3 |
| Maximum extension velocity | 15 | 118.7 | 128.1 | 128.5 |
| Extension velocity | ||||
| At foot contact | 21 | 11.5 | 50.9 | 61.6 |
| At mid-stance | 13 | 96.7 | 77.1 | 70.0 |
| Flexion velocity at toe-off | 20 | 138.0 | 91.9 | 98.5 |
| Knee | ||||
| Maximum flexion velocity | 28 | 308.0 | 179.8 | 183.8 |
| Maximum extension velocity | 81 | 360.2 | 200.8 | 209.4 |
| Flexion velocity at foot contact | 31 | 87.5 | −52.6 | −58.5 |
| Extension velocity at mid-stance | 16 | 34.5 | 7.8 | 0.8 |
| Flexion velocity at toe-off | 29 | 295.9 | 112.2 | 121.6 |
| Ankle | ||||
| Maximum dorsiflexion velocity | 39 | 135.8 | 119.6 | 113.0 |
| Maximum plantar flexion velocity | 43 | 200.9 | 166.2 | 151.1 |
| Plantar flexion velocity at foot contact | 34 | 12.9 | −58.5 | −0.1‡ |
| Dorsiflexion velocity at mid-stance | 15 | 5.1 | −11.3 | 11.9‡ |
| Plantar flexion velocity at toe-off | 39 | 104.5 | 42.1 | 51.1 |
Effect size = the difference required for significance at α < 0.05 with a statistical power of 0.9.
Boldface type indicates that the data were significantly different from the value for normally developing subjects (p < 0.05).
These postoperative data were significantly different from the preoperative values (p < 0.05). The results suggest that muscle-tendon lengthening has no effect on knee or hip velocities but can modify ankle dynamics.
TABLE IV.
Time of Peak Velocity (Percent of Cycle)
| Effect Size* | Normal Subjects | Patients with Cerebral Palsy† |
||
|---|---|---|---|---|
| Preop. | Postop. | |||
| Hip | ||||
| Flexion velocity | 2.6 | 66.8 | 78.3 | 78.5 |
| Extension velocity | 5.3 | 24.4 | 18.9 | 17.2 |
| Knee | ||||
| Flexion velocity | 5.6 | 61.5 | 61.9 | 62.3 |
| Extension velocity | 3.5 | 87.4 | 2.2 | 99.3 |
| Ankle | ||||
| Dorsiflexion velocity (swing/stance) | 3.4/3.3 | 71.1/11.4 | 79.4/6.5 | 78.4/9.9 |
| Plantar flexion velocity | 1.7 | 57.0 | 59.7 | 60.5 |
Effect size = the difference required for significance at α < 0.05 with a statistical power of 0.9.
Boldface type indicates that the data were significantly different from the value for normally developing subjects (p < 0.05). The results suggest that muscle-tendon lengthening has no effect on knee or hip velocity timing.
Hip extension velocities at foot contact were greater than normal in the patients who had cerebral palsy (Table II). However, hip extension velocities during mid-stance and hip flexion velocities at toe-off were less than normal (Table II), with peak flexion velocity occurring later than normal during the swing phase (Table IV). The operation had no effect on the timing or magnitude of hip angular velocities.
Knee angular velocities in the patients who had spastic cerebral palsy were significantly reduced (p < 0.05) compared with those in the normally developing subjects (Fig. 2). Maximum flexion and extension velocities of the knee in the patients who had cerebral palsy (preoperatively and postoperatively) were approximately 58 percent of the values for the normally developing subjects (Table II). The operation failed to substantially modify knee angular velocities.
Only the ankle demonstrated changes in joint angular velocity following muscle-tendon lengthening; these changes occurred at foot contact through mid-stance. The normally developing subjects demonstrated ankle plantar flexion velocity at heel contact (that is, heel-strike was quickly followed by toe-strike) (Fig. 1). Conversely, preoperatively the patients who had cerebral palsy demonstrated dorsiflexion velocity at contact (a negative value in plantar flexion [Table II]) and premature peak dorsiflexion velocity during the stance phase compared with the normally developing subjects (Fig. 2 and Table IV). Muscle-tendon lengthening reduced this abnormal dorsiflexion motion so that ankle velocity approached zero at foot-strike. Similarly, the abnormal plantar flexion velocity demonstrated preoperatively through mid-stance was corrected to a dorsiflexion velocity that was closer to normal. Ankle plantar flexion velocity at toe-off was preserved following treatment despite a priori suspicions that it might have been reduced because of concomitant reduction in the capacity to generate muscle force as a result of the muscle-tendon lengthening
Analysis of Kinematic Patterns
Principal component analyses were used to mathematically compare group differences on the basis of the fundamental shapes of the angle and joint angular velocity profiles of the hip, knee, and ankle joints. With this technique, motion data were categorized into one of three groups: the normally developing subjects; the patients who had cerebral palsy, before operative treatment; and the patients who had cerebral palsy, after operative treatment. Mathematically predicted categories were compared with actual group assignments. On comparison of angle data of the normally developing subjects and the patients who had cerebral palsy (preoperative and postoperative groups combined), 140 of the 146 normal limbs were categorized with principal component analyses as normal and sixty-five of the 154 limbs of the patients with cerebral palsy (preoperative and postoperative) were mathematically categorized into a cerebral palsy group. With use of joint angular velocity data, 97 percent of the limbs were correctly categorized; all 146 normal limbs were categorized as normal, and 146 of the 154 limbs of the patients with cerebral palsy were categorized into a cerebral palsy group. However, principal component analyses failed to discriminate between the preoperative and postoperative gait patterns of the patients who had cerebral palsy. Angle data correctly categorized only thirty-eight of the seventy-seven limbs that had not been treated into the preoperative cerebral palsy group and fourteen of the seventy-six limbs that had been treated into the postoperative cerebral palsy group. Data on joint angular velocity correctly categorized fifty-six of the seventy-seven limbs that had not been treated and forty-four of the seventy-seven limbs that had been treated. This limited ability to accurately discriminate between the preoperative and postoperative groups with use of the data on joint angular velocity underscores the fact that muscle-tendon lengthening has a limited capacity to modify joint angular velocity. Nonetheless, the results illustrate that, compared with joint angle parameters, joint angular velocity profiles may discriminate more effectively between normally developing subjects and patients who have cerebral palsy.
Electromyography
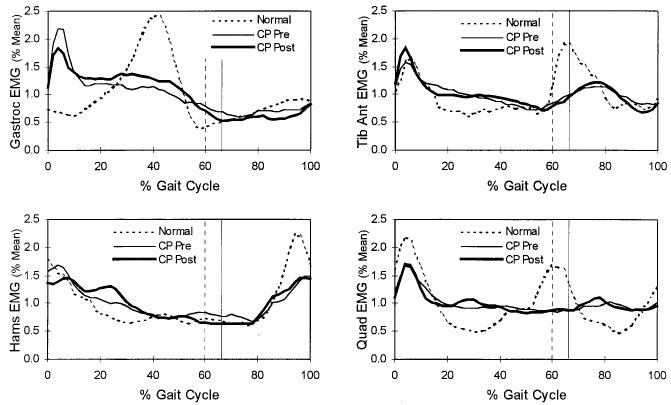
In general, the muscle activation pattern on electromyography changed little after the operations (Table V). The only change in muscle activity associated with the muscle-tendon lengthening was a relative increase in mid-stance plantar flexion electromyographic activity of the gastrocnemius-soleus muscle group (Fig. 3). Post priori repeated-measures analysis of variance of the gastrocnemius-soleus activity during weight acceptance demonstrated a significant reduction (p < 0.05) in electromyographic amplitude. These changes were primarily evident in the patients who had had an operation on the gastrocnemius-soleus muscle.
TABLE V.
Electromyographic Data for Normal Subjects and Before and After Muscle-Tendon Lengthening in Patients with Cerebral Palsy
| Muscle Activity (percent of mean) |
||||
|---|---|---|---|---|
| Effect Size* | Normal Subjects | Patients with Cerebral Palsy† |
||
| Preop. | Postop. | |||
| Gastrocnemius | ||||
| Range | 0.35 | 3.00 | 2.14 | 1.89 |
| Mean | — | 1 | 1 | 1 |
| Maximum | 0.33 | 3.17 | 2.54 | 2.24 |
| Minimum | 0.06 | 0.17 | 0.39 | 0.36 |
| At foot contact | 0.13 | 0.79 | 1.38 | 1.25 |
| At mid-stance | 0.17 | 1.75 | 1.14 | 1.31‡ |
| At toe-off | 0.19 | 0.48 | 0.63 | 0.55 |
| Tibialis anterior | ||||
| Range | 0.28 | 2.06 | 1.41 | 1.62 |
| Mean | — | 1 | 1 | 1 |
| Maximum | 0.26 | 2.37 | 1.93 | 2.05 |
| Minimum | 0.06 | 0.30 | 0.52 | 0.43‡ |
| At foot contact | 0.20 | 1.56 | 1.16 | 1.26 |
| At mid-stance | 0.09 | 0.69 | 0.97 | 0.97 |
| At toe-off | 0.18 | 1.13 | 0.97 | 0.98 |
| Hamstrings | ||||
| Range | 0.27 | 2.77 | 1.47 | 1.71 |
| Mean | — | 1 | 1 | 1 |
| Maximum | 0.25 | 2.93 | 1.95 | 2.08 |
| Minimum | 0.05 | 0.16 | 0.49 | 0.37‡ |
| At foot contact | 0.35 | 1.73 | 1.57 | 1.39 |
| At mid-stance | 0.12 | 0.73 | 0.92 | 1.06 |
| At toe-off | 0.19 | 0.72 | 0.78 | 0.58 |
| Quadriceps | ||||
| Range | 0.31 | 2.62 | 1.50 | 1.66 |
| Mean | — | 1 | 1 | 1 |
| Maximum | 0.28 | 2.86 | 2.02 | 2.09 |
| Minimum | 0.06 | 0.24 | 0.51 | 0.43‡ |
| At foot contact | 0.21 | 1.66 | 1.25 | 1.22 |
| At mid-stance | 0.10 | 0.58 | 0.93 | 0.99 |
| At toe-off | 0.24 | 1.62 | 0.87 | 0.97 |
Effect size = the difference required for significance at α < 0.05 with a statistical power of 0.9.
Boldface type indicates that the data were significantly different from the value for normally developing subjects (p < 0.05).
These postoperative data were significantly different from the preoperative values (p < 0.05). The results suggest that muscle-tendon lengthening has little effect on knee or hip muscle activities but can modify myoactivity of stance-phase ankle dynamics.
Fig. 3.
Electromyographic activities measured from the tibialis anterior, gastrocnemius, quadriceps femoris, and hamstring muscles, representing the mean behaviors of the normally developing subjects (dotted line), the patients with spastic cerebral palsy (CP) before muscle-tendon lengthening (thin line), and the patients with spastic cerebral palsy after muscle-tendon lengthening (heavy line). Only gastrocnemius-soleus activity during weight acceptance and mid-stance was significantly modified following the muscle-tendon lengthening. The vertical lines represent the time of toe-off for the normally developing subjects (dashed line) and for the patients with spastic cerebral palsy (solid line).
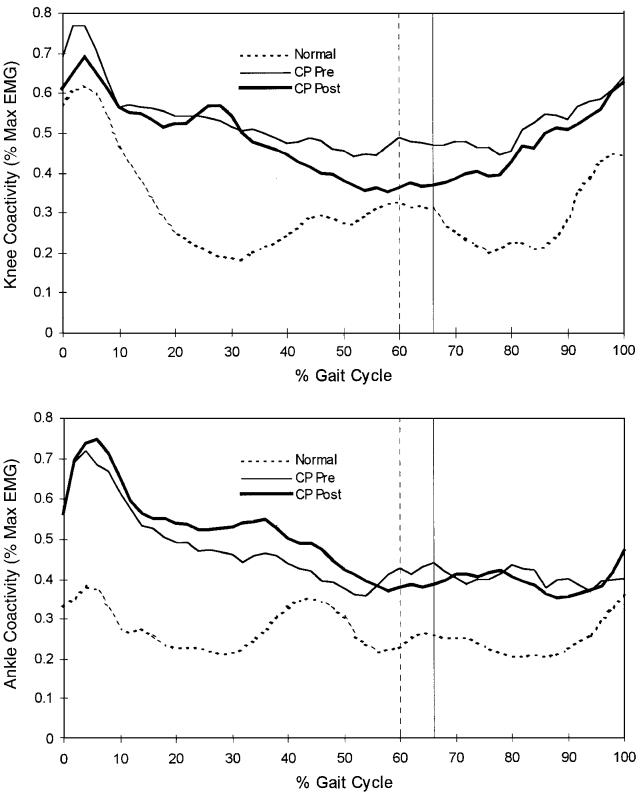
Muscle coactivity of agonist-antagonist pairs was evaluated to reflect the balance of activity before and after treatment. Hamstring versus quadriceps coactivity at toe-off was significantly reduced (p < 0.05) as a result of muscle-tendon lengthening (Fig. 4 and Table VI). Therefore, the data suggest a slight reduction in stiffness of the knee at toe-off, which may contribute to the earlier flexion peak in the swing phase despite the lack of change in knee velocity.
Fig. 4.
Muscle coactivity quantified about the knee (quadriceps femoris versus hamstrings) and ankle (gastrocnemius versus tibialis anterior). Coactivity represents the fraction of antagonistic activity comprising the total activity of the system, scaled relative to the agonist level. The agonist and antagonist were defined as the most active and the least active muscles, respectively. Coactivity in the patients with spastic cerebral palsy (CP) was significantly greater than that in normally developing subjects (dotted line) both before (thin line) and after (heavy line) muscle-tendon lengthening. The vertical lines represent the time of toe-off for the normally developing subjects (dashed line) and for the patients with spastic cerebral palsy (solid line).
TABLE VI.
Electromyographic Coactivity for Normal Subjects and Before and After Muscle-Tendon Lengthening in Patients with Cerebral Palsy
| Coactivity (percent of maximum) |
||||
|---|---|---|---|---|
| Effect Size* | Normal Subjects | Patients with Cerebral Palsy† |
||
| Preop. | Postop. | |||
| Knee | ||||
| Range | 0.05 | 0.77 | 0.61 | 0.64 |
| Mean | 0.05 | 0.31 | 0.53 | 0.48 |
| Maximum | 0.04 | 0.85 | 0.89 | 0.86 |
| Minimum | 0.04 | 0.08 | 0.28 | 0.21† |
| At foot contact | 0.09 | 0.54 | 0.69 | 0.63 |
| At mid-stance | 0.07 | 0.21 | 0.51 | 0.51 |
| At toe-off | 0.08 | 0.31 | 0.49 | 0.36† |
| Ankle | ||||
| Range | 0.06 | 0.42 | 0.60 | 0.62 |
| Mean | 0.05 | 0.26 | 0.45 | 0.48 |
| Maximum | 0.06 | 0.51 | 0.84 | 0.87 |
| Minimum | 0.04 | 0.09 | 0.25 | 0.25 |
| At foot contact | 0.06 | 0.35 | 0.55 | 0.57 |
| At mid-stance | 0.06 | 0.24 | 0.46 | 0.53 |
| At toe-off | 0.07 | 0.25 | 0.41 | 0.39 |
Effect size = the difference required for significance at α < 0.05 with a statistical power of 0.9.
Boldface type indicates that the data were significantly different from the value for normally developing subjects (p < 0.05).
These postoperative data were significantly different from the preoperative values (p < 0.05). Only knee coactivity at toe-off was modified by muscle-tendon lengthening.
Discussion
Joint angular velocities during walking, particularly at the knee, were reduced in the patients who had spastic diplegia compared with those of normally developing subjects. This is characteristic of spastic muscle behavior4,5,8 and is consistent with the findings of previous studies that demonstrated reduced joint velocity in spastic gait13. We observed that reduced joint angular velocities were associated with higher-than-normal levels of antagonistic coactivity in the patients who had cerebral palsy7. Consequently, reduced joint angular velocity may involve velocity-dependent hypertonicity in antagonistic or co-spastic muscles that generate deceleration forces, thereby limiting the magnitude of the joint angular velocity. The reduced joint angular velocities may also reflect the contracture of the fibrous components of the muscle-tendon unit, such as the muscle sheath and tendon. In other words, limited joint angular velocity may be the result of reduced muscle-tendon extensibility. However, knee and hip joint velocities were not changed by muscle-tendon lengthening even though passive range of motion improved2. These results imply that spastic neural mechanisms primarily reduce joint angular velocities, which in turn contribute to limited joint excursion in spastic gait. This helps to explain why patients who have spastic cerebral palsy rely mainly on increased cadence rather than on increased stride length to increase walking speed1.
An exception to reduced joint angular velocity in the patients who had cerebral palsy was demonstrated at the hip during foot contact, when extension velocity was greater than normal. This increased hip angular velocity at foot contact is believed to be attributable to a compensatory mechanism in response to a power loss due to abnormal motions of the knee and ankle. This proposed mechanism is supported by previous analyses1 showing that patients who have spastic diplegia achieve increased walking speed by means of increased hip excursions.
Joint angular velocity successfully discriminated between the kinematic patterns of the cerebral palsy group and the motion patterns of the normal controls. Previous analyses comparing the gait patterns of patients who have cerebral palsy with those of normal subjects have emphasized joint angle data24. Sutherland et al.24 used so-called prediction regions based on angle data to correctly classify 75 percent (fifty-seven) of seventy-six limbs, as compared with our accuracy of 68 percent (205 of 300 limbs) with use of angle data. However, with joint angular velocity data, normal and spastic profiles could be correctly classified 97 percent of the time (292 of 300 limbs). One reason for the improved accuracy was that joint angular velocity demonstrated less intersubject variability (standard deviation/peak velocity = 13 percent) than did joint angle data (standard deviation/peak angle = 31 percent). Another consideration is that angle bias errors (that is, shifting the entire joint angle kinematic curve up or down) due to marker placement, calibration procedures, and assumptions made with the motion-analysis models are not present in joint angular velocity data. Because velocity represents the relative change in angle, it is unaffected by bias errors. Thus, joint angular velocity provides a unique motion signature: abnormally reduced values that are consistently observed in nearly all of the patients who have spasticity.
Knee dynamics (specifically, joint angular velocities) were markedly different between the normal and cerebral palsy groups. Near the end of the swing phase, the normally developing subjects demonstrated positive flexion velocities, indicating that the knee had completed extension and was beginning to flex just before heel contact. Deceleration activity in the knee flexors through the late-swing phase leads to this reversal in knee velocity and contributes to load-damping and hip extension in the early stance phase. Hamstring versus quadriceps coactivity was increased in the patients who had spastic cerebral palsy. We believe that this caused a delayed reversal of knee extension velocity during the swing phase. As a result, the knee traveled toward extension at foot contact, imposing abnormal reaction loads on the knee and increasing energy expenditure.
Reduced knee flexion during the swing phase in spastic gait was attributed in part to abnormally reduced knee and hip angular velocity at toe-off17-19. Meancknee velocity at toe-off in the patients who had cerebral palsy was less than 40 percent of normal. Hip flexion velocity at toe-off was less than 70 percent of normal. Analyses confirmed that the peak knee flexion angle during the swing phase was correlated with the knee flexion velocity at toe-off (r = 0.48). Thus, reduced joint angular velocity limited knee excursion because joint motion was too slow to achieve a normal range of motion within the time-span of the gait cycle.
Muscle-tendon lengthening effectively modified the mean knee joint angle, but excursions remained unchanged because knee velocities were unchanged. Excursion is related to the mathematical integral of joint angular velocity (the area under the velocity curve). If joint angular velocity does not change, neither can excursion. After the operation, the patients continued to demonstrate reduced knee velocity and achieved foot-strike while the knee was moving toward extension. However, it should be emphasized that techniques for muscle-tendon lengthening are varied. In fact, excessive lengthening of the hamstrings could shift the knee into severe extension, thereby reducing excursion9 and disturbing angular velocity of the knee during the swing phase. Transfer of the rectus femoris to the hamstrings may influence angular velocities of the knee, but this procedure was performed in only six patients, so its effect on angular velocity of the knee could not be analyzed separately.
Muscle-tendon lengthening reduced ankle dorsiflexion velocity during the loading phase. The normally developing subjects achieved heel-strike first, generating ankle plantar flexion velocity until forefoot contact. Children who have spastic cerebral palsy often achieve floor contact with the forefoot, so that the heel is driven toward the ground (dorsiflexion velocity). Maki et al.16 concluded that ankle dorsiflexion velocity during foot contact exceeded the spastic threshold velocity in the plantar flexion muscles, producing a reflex activation and generating internal plantar flexion moments, which resulted in toe-walking during mid-stance. Our data clearly demonstrate the abnormal dorsiflexion angular velocity during loading, the characteristic gastrocnemius-soleus spastic reflex activity that follows, and the abrupt plantar flexion velocity in the early stance phase in the patients who had cerebral palsy. After muscle-tendon lengthening, reduced dorsiflexion velocity during loading was accompanied by reduced gastrocnemius-soleus spastic stretch activity in this period. Peak electromyographic activity during weight acceptance was reduced to 40 percent of the mean relative to preoperative levels. These factors, when combined with the added length in the muscle-tendon units, allowed for more sustained dorsiflexion through the stance phase.
Muscle-tendon lengthening changed the angular velocity of the ankle during terminal swing. The operation allowed the ankle to assume a dorsiflexed posture in the mid-swing phase, providing improved foot clearance (Fig. 1). This postoperative improvement in the dorsiflexion angle allowed the foot to drop toward a neutral posture in the late-swing phase in a motion similar to that of the normally developing group (that is, plantar flexion velocity in the late-swing phase).
Many patients with spastic cerebral palsy had several muscle-tendon units lengthened; therefore, we cannot form strong conclusions about specific muscle-tendon lengthening procedures. However, the results provide insight into the mechanisms of gait in spastic cerebral palsy and the influence of muscle-tendon lengthening in improving gait. The gait patterns of the patients who had spastic cerebral palsy were characterized by reduced joint angular velocities compared with the patterns of the normally developing subjects. The joint angular velocity data were more accurate at categorizing the subjects into the appropriate clinical groups than were the angle data, suggesting a more intrinsic relationship between joint angular velocity and spastic behavior than can be derived from time-dependent joint angle measures. Muscle-tendon lengthening, as described, successfully modified the joint angle profiles associated with gait but had little effect on the dynamic components (angular velocity of the joints). Therefore, the capacity to change gait dynamics, including joint angular velocity, momentum, and energy transfer between limb segments, seems to be limited with muscle-tendon lengthening. The notable exception was modified ankle velocities at foot contact through the stance phase and concomitant reduced spastic activity in the gastrocnemius-soleus muscles. The findings at the ankle suggest that peripheral feedback seems to be influenced by the operation. Nonetheless, benefits derived from alleviating static contractures and modifying joint angles during gait included greater stride lengths and improved velocity of forward progression2.
Footnotes
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. Funds were received in total or partial support of the research or clinical study presented in this article. The funding sources were the Orthopaedic Research and Education Fund, the University of Virginia Children's Medical Center Grant-in-Aid, and the National Institutes of Health (Grant R29HD36516-01).
References
- 1.Abel MF, Damiano DL. Strategies for increasing walking speed in diplegic cerebral palsy. J. Pediat. Orthop. 1996;16:753–758. doi: 10.1097/00004694-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Abel MF, Damiano DL, Pannunzio M, Bush J. Muscle-tendon surgery in diplegic cerebral palsy: functional and mechanical changes. J. Pediat. Orthop. 1999;19:366–375. [PubMed] [Google Scholar]
- 3.Bleck EE. Goals, treatment and management. In: Bleck EE, editor. Orthopaedic Management in Cerebral Palsy. MacKeith Press; Philadelphia: 1987. pp. 190–212. [Google Scholar]
- 4.Brown RA, Lawson DA, Leslie GC, Part NJ. Observations on the applicability of the Wartenberg pendulum test to healthy, elderly subjects. J. Neurol., Neurosurg. and Psychiat. 1988;51:1171–1177. doi: 10.1136/jnnp.51.9.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown RA, Lawson DA, Leslie GC, MacArthur A, MacLennan WJ, McMurdo ME, Mutch WJ, Part NJ. Does the Wartenberg pendulum test differentiate quantitatively between spasticity and rigidity? A study in elderly stroke and Parkinsonian patients. J. Neurol., Neurosurg. and Psychiat. 1988;51:1178–1186. doi: 10.1136/jnnp.51.9.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcos DM, Gottlieb GL, Penn RD, Myklebust BM, Agarwal GC. Movement deficits caused by hyperexcitable stretch reflexes in spastic humans. Brain. 1986;109:1043–1058. doi: 10.1093/brain/109.5.1043. [DOI] [PubMed] [Google Scholar]
- 7.Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyog. and Clin. Neurophysiol. 1985;25:135–149. [PubMed] [Google Scholar]
- 8.Fellows SJ, Kaus C, Thilmann AF. Voluntary movement at the elbow in spastic hemiparesis. Ann. Neurol. 1994;36:397–407. doi: 10.1002/ana.410360311. [DOI] [PubMed] [Google Scholar]
- 9.Gage JR, Fabian D, Hicks R, Tashman S. Pre- and postoperative gait analysis in patients with spastic diplegia: a preliminary report. J. Pediat. Orthop. 1984;4:715–725. doi: 10.1097/01241398-198411000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Gage JR, Perry J, Hicks RR, Koop S, Werntz JR. Rectus femoris transfer to improve knee function of children with cerebral palsy. Devel. Med. and Child. Neurol. 1987;29:159–166. doi: 10.1111/j.1469-8749.1987.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 11.Gage JR, DeLuca PA, Renshaw TS. Instructional Course Lectures, American Academy of Orthopaedic Surgeons. Vol. 45. American Academy of Orthopaedic Surgeons; Rosemont, Illinois: 1996. Gait analysis: principles and applications with emphasis on its use in cerebral palsy; pp. 491–507. [PubMed] [Google Scholar]
- 12.Knutsson E, Martensson A. Dynamic motor capacity in spastic paresis and its relation to prime mover dysfunction, spastic reflexes and antagonist co-activation. Scandinavian J. Rehab. Med. 1980;12:93–106. [PubMed] [Google Scholar]
- 13.Krawetz P, Nance P. Gait analysis of spinal cord injured subjects: effects of injury level and spasticity. Arch. Phys. Med. and Rehab. 1996;77:635–638. doi: 10.1016/s0003-9993(96)90000-3. [DOI] [PubMed] [Google Scholar]
- 14.Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30:1303–1313. doi: 10.1212/wnl.30.12.1303. [DOI] [PubMed] [Google Scholar]
- 15.Lin JP, Brown JK, Brotherstone R. Assessment of spasticity in hemiplegic cerebral palsy. II: Distal lower-limb reflex excitability and function. Devel. Med. and Child Neurol. 1994;36:290–303. doi: 10.1111/j.1469-8749.1994.tb11849.x. [DOI] [PubMed] [Google Scholar]
- 16.Maki BE, Rosen MJ, Simon SR. Modification of spastic gait through mechanical damping. J. Biomech. 1985;18:431–443. doi: 10.1016/0021-9290(85)90278-7. [DOI] [PubMed] [Google Scholar]
- 17.Mena D, Mansour JM, Simon SR. Analysis and synthesis of human swing leg motion during gait and its clinical applications. J. Biomech. 1981;14:823–832. doi: 10.1016/0021-9290(81)90010-5. [DOI] [PubMed] [Google Scholar]
- 18.Mochon S, McMahon TA. Ballistic walking. J. Biomech. 1980;13:49–57. doi: 10.1016/0021-9290(80)90007-x. [DOI] [PubMed] [Google Scholar]
- 19.Mochon S, McMahon TA. Ballistic walking: an improved model. Math. Biosci. 1980;52:241–260. [Google Scholar]
- 20.Olney SJ. Quantitative evaluation of cocontraction of knee and ankle muscles in normal walking. In: Winter DA, Norman RW, Wells RP, Hayes KC, Potla AE, editors. Biomechanics IX-A. Human Kinetics; Champaign, Illinois: 1985. pp. 431–435. [Google Scholar]
- 21.Phillips CA, Repperger DW, Chelette TL. The acceleration-velocity relationship: identification of normal and spastic upper extremity movement. Comput. Biol. and Med. 1997;27:309–328. doi: 10.1016/s0010-4825(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 22.Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. J. Biomech. 1996;29:723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 23.Reimers J. Static and dynamic problems in spastic cerebral palsy. J. Bone and Joint Surg. 1973;55-B(4):822–827. [PubMed] [Google Scholar]
- 24.Sutherland DH, Kaufman KR, Campbell K, Ambrosini D, Wyatt M. Clinical use of prediction regions for motion analysis. Devel. Med. and Child Neurol. 1996;38:773–781. doi: 10.1111/j.1469-8749.1996.tb15111.x. [DOI] [PubMed] [Google Scholar]
- 25.Thometz J, Simon S, Rosenthal R. The effect on gait of lengthening of the medial hamstrings in cerebral palsy. J. Bone and Joint Surg. 1989 March;71-A:345–353. [PubMed] [Google Scholar]
- 26.Winter DA. Biomechanics and Motor Control of Human Movement. Wiley Interscience Publication; New York: 1990. [Google Scholar]
- 27.Wootten ME, Kadaba MP, Cochran GVB. Dynamic electromyography. I. Numerical representation using principal component analysis. J. Orthop. Res. 1990;8:247–258. doi: 10.1002/jor.1100080214. [DOI] [PubMed] [Google Scholar]
- 28.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch. Phys. Med. and Rehab. 1984;65:517–521. [PubMed] [Google Scholar]