Abstract
Estrogen-related receptor α (ERRα) modulates estrogen receptor (ER)-mediated activity and is participating in the energy homeostasis by regulation of downstream target genes. The ERRα gene itself is proposed to be regulated by peroxisome proliferator-activated receptor γ coactivator (PGC-1α) through an autoregulatory loop under physiological stimulation. We have previously shown that the close family member ERRγ is a positive regulator of ERRα gene expression. ERRα and ERRγ are coexpressed in metabolically active tissues such as heart, kidney and muscle, yet the physiological role of ERRγ and its relationship with ERRα in gene regulation are currently unknown. The present study examined the interplay of ERRγ and ERRα in regulation of ERRα gene expression. Using real-time PCR analyses we found that ERRγ, like the ERRα and PGC-1α is induced in mouse liver during fasting. Overexpression of ERRγ in the HEC-1B cells robustly stimulated the multi-hormone response element (MHRE) of the ERRα gene promoter and this activity was repressed by increasing expression of ERRα. The two ERRs bind MHRE simultaneously in electrophoretic mobility shift assay (EMSA) and they were detected as multimeric complexes in cells by coimmunoprecipitation. Although ERRα and ERRγ share high sequence identity, they differ in biochemical and molecular characteristics as examined by trypsin digestion, reporter activation, and coactivator interaction and utilization. Using chromatin immunoprecipitation (ChIP) assay, we showed that ectopic expression of both ERRα and ERRγ modifies chromatin structure at the MHRE region while ectopic expression of PGC-1α in HEC-1B cells promotes ERRγ but not ERRα occupancy at the MHRE region of the ERRα gene promoter and enhances the recruitment of coactivator SRC1. These data suggested that ERRα and ERRγ regulate ERRα gene expression with different molecular mechanisms.
Introduction
The nuclear receptor superfamily consists of transcription factors that depend on ligands for their activation and a larger group of transcription factors with unidentified ligands or no ligand requirement (reviewed in (Mangelsdorf et al., 1995)). This latter group of nuclear orphan receptors (reviewed in (Giguere, 1999)) has diverse biological roles in tissue development and maintenance of homeostasis. Estrogen-related receptors (ERRs) belong to the NR3B orphan nuclear receptor subgroup, which consists of three members α, β and γ (Committee, 1999). ERRα and ERRβ were cloned based on sequence identity to the estrogen receptor α (ERα) DNA binding domain (Giguere et al., 1988) whereas the ERRγ was identified by a yeast two-hybrid screen (Hong et al., 1999). As with other nuclear receptors, ERRs are organized into modular domains with a less characterized N-terminal domain, a highly conserved DNA binding (DBD) domain, and a potential ligand-binding (LBD) domain that houses the activation function (AF2) domain. It is controversial whether a ligand is needed for ERR activation function (Kamei et al., 2003; Vanacker et al., 1999; Xie et al., 1999; Zhang and Teng, 2000). Recent crystallography studies suggest that the ERR functions as a constitutive activator and the classical nuclear receptor ligand is not required for its function (Greschik et al., 2002; Kallen et al., 2004). Nonetheless, several potential ligands that either stimulate or repress activity of the ERRs have been reported (Coward et al., 2001; Suetsugi et al., 2003; Tremblay et al., 2001; Willy et al., 2004; Yang and Chen, 1999; Zuercher et al., 2005).
ERRα has been found to enhance the ERα-mediated response of the human lactoferrin gene promoter via binding to an ERRE site. This site, TCAAGGTCA, is located 18 bp upstream from the well-characterized estrogen response element (ERE) (Yang et al., 1996). In contrast, ERRα and ERα function as a competitive repressor in transactivation activity on the synthetic EREs and natural promoter (Giguere, 2002; Johnston et al., 1997; Xie et al., 1999; Zhang and Teng, 2000; Zhang et al., 2006). The relationship of ERRα and ERα in the estrogen signaling pathway, therefore, is significantly influenced by the enhancer element organization of the target gene and the availability of cofactors in a given cellular environment.
Recently, ERRα was reported to be upregulated by estrogen in the uterus and heart (Liu et al., 2003), by fasting in liver (Ichida et al., 2002) and by cold stress in brown fat and skeletal muscle (Schreiber et al., 2003). The increased ERRα expression during fasting and cold exposure is correlated with the induction of a coactivator, peroxisome-proliferation-activated receptor-γ (PPARγ) coactivator-1α (PGC-1α), which is a master regulator in executing the energy metabolism programs (reviewed in (Knutti and Kralli, 2001; Puigserver and Spiegelman, 2003)). ERRα was identified as a key partner for PGC-1α in regulation of genes involved in the mitochondria oxidative phosphorylation (Huss et al., 2002; Laganiere et al., 2004; Mootha et al., 2004; Schreiber et al., 2003). Interestingly, ERRα itself is PGC-1α inducible (Schreiber et al., 2003). Inhibition of ERRα expression or function compromises the ability of PGC-1α to stimulate genes in mitochondria biogenesis (Huss et al., 2004; Schreiber et al., 2004).
The ERRα gene lacks the typical TATA and CAAT boxes, but has multiple consensus Sp1 binding elements in the GC-rich promoter (Shi et al., 1997). Previously, our laboratory has shown that ERRα expression is upregulated in the mouse uterus by estrogen (Shigeta et al., 1997) and recently we identified a multiple hormone response element (MHRE), a 57 bp region in human and a 34 bp region in mouse, that plays a significant role in estrogen-stimulated activity (Liu et al., 2003). The MHRE is a pleiotropic response element for other nuclear receptors and also serves as the binding site for ERRα and ERRγ (Laganiere et al., 2004; Liu et al., 2005; Mootha et al., 2004). It has been proposed that, in response to physiological cues, PGC-1α is induced and partners with ERRα to form an autoregulatory loop in the stimulation of ERRα gene expression. This event stimulates the expression of downstream target genes that are involved in energy production (Laganiere et al., 2004; Mootha et al., 2004). Nonetheless, the mechanism of ERRα gene induction by PGC-1α remains to be elucidated. ERRγ is coexpressed with ERRα in metabolically active tissues such as kidney, skeletal muscle and cardiac muscle, where it binds to the MHRE and is a stronger activator than the ERRα in self-stimulating the ERRα gene promoter (Liu et al., 2005). In particular, PGC-1α interacts with ERRγ and coactivates ERRγ’s transactivation function on the βPDGF (SIS) element (Hentschke et al., 2002) and the ERRα MHRE (Liu et al., 2005). These findings indicate that ERRγ may have a major role in regulation of ERRα gene expression and in energy homeostasis.
In the present study, we demonstrate that ERRγ expression is increased in liver of a fasting animal. We investigated the relationship of ERRα and ERRγ in the regulation of ERRα gene expression and showed that ERRs have different molecular characteristics. Our results suggest that ERRα and ERRγ may have different yet complimenting roles in modulating the ERRα gene expression during the PGC-1α induction.
Materials and Methods
Animal fasting, real time-PCR and Western blotting
Mature female CD-1 mice at 36-days of age (Charles River Laboratories, Wilmington, Mass) were housed in the National Institute of Environmental Health Sciences (NIEHS) animal laboratories (at 72° F, 40–60% humidity, and 12L:12D photoperiod) and provided with unlimited water and food (NIH31 chow). The animals were handled and the experiments conducted according to the approved method by the Animal Care and Use Committee of NIEHS. For fasting, food was removed at 6PM and the animals were killed at either 6AM (12 h) or 6PM (24 h) the next day. The control mice (fed ad libitum) were housed in the same facility and killed at 6PM together with the 24h fasting mice. Each experimental group consisted of four mice whose livers were immediately frozen on dry ice after collection and processed for RNA or nuclear protein preparation individually. Total RNA was extracted with Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) and the nuclear protein was prepared by standard methods. TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) were used to measure the ERRγ (assay ID Mm00516267_m1) and PGC-1α (assay ID Mm00447183_m1) mRNA levels whereas SYBR green assays were used for ERRα and β-actin according to the method previously described (Liu et al., 2003). Each sample was quantified against its β-actin transcript contents, and then normalized with the control group. The results are presented as fold of stimulation ± S.D. Nuclear protein extracts from three individual mice of control group and 24h fasting group were cleaned and concentrated with PAGEprep Protein Clean-up and Enrichment Kit (Pierce, Rockford, IL). Protein concentration was determined by the bicinchoninic acid (BCA) method (Pierce Chemical C., Madison, WI), and a total of 30 μg protein was separated by 4–12% Bis-Tris NuPAGE gel. After electroporesis, the proteins were electrotransferred to polyvinylidene difluoride membrane (NOVEX, San Diego, CA). Western blotting was consequentially carried out by specific antibody to ERRα (rabbit polyclonal antibody to the ERRα peptide at the C-terminal region (P3–80) as previously described (Shigeta et al., 1997)), ERRγ (a gift from U. Borgmeyer, University of Hamburg, Germany), PGC-1α (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and ERα (TE111.5D11, NeoMarker, Fremont, CA). The dilution of each antibody used in Western was indicated in the individual figure. ECL detection system ( Amersham Biosciences, Piscataway, NJ) was used and the x-ray films were exposure for 1–5 minutes depending on the intensity of the band. Blots were tripped according to the instruction of NOVEX and reprobed with other antibodies.
Plasmids and primers
All of the plasmids used in the study are described in Table I, and the primers are presented in Table II. The gift plasmids were obtained from the following sources: pSG-ERRα from S Chen (Backman Research Institute, City of Hope, Duarte, CA); pflag-ERRα from T Finkel (NHLB, NIH, Bethesda, MD); the mutation constructs ERRα N, ERRα C, ERRα P-boxm (ERRα p-box) from JE Mertz (University of Wisconsin Medical School, Madison, WI); pcDNA3-ERRγ, pcDNA3-Myc-ERRγ, GST-ERRγ, GST-ERRγ ΔAF2 from U. Borgmeyer (University Hamburg, Germany); ERα (HEGO) from P. Chambon (College de France, Cedex, France); ERβ from D. McDonald (Duke, Durham, NC); RORα from V. Giguere (McGill University, Montreal, Quebec, Canada); pCR3.1-hSRC-1a from B.W. O’Malley (Baylor College of Medicine, Houston, TX); pSG5-mGRIP1 from M.R. Stallcup (USC, Los Angeles, CA); pSG5-hACTR from RM Evans (Salk Institute, La Jolla, CA); pCDNA3-hPGC-1α from A. Kralli (Scripps Research Institute, La Jolla, CA). References for the plasmids are included in Table I.
Table I.
Plasmids
| Reporters | Description | Reference |
| AAB-TATA-Luc | hERRα MHRE | (Liu et al., 2003) |
| 3x ERE-TATA-Luc | synthetic triple ERE | (Zhang & Teng, 2000) |
| 1x ERE-TATA-Luc | vit A2 ERE | (Zhang & Teng, 2000) |
| NRRE-TATA-Luc | MCAD, NRRE | (Disch et al., 1996) |
| Expression constructs | ||
| Plasmid | Description | Reference |
| pcDNA3.1-ERRα-myc-his | with myc and his tag | (Zhang and Teng, 2000) |
| pSG-ERRα | hERRα | (Yang et al., 1998) |
| pFlag-ERRα | with flag tag | (Ichida et al., 2002) |
| pGEX-4T-1 | GST tag expression vector | Amersham |
| GST-ERRα | GST tag | (Yang et al., 1996) |
| pcDNA3.1-ERRγ | hERRγ | (Hentschke et al., 2002) |
| pcDNA3.1-myc-ERRγ | myc tag | (Hentschke et al., 2002) |
| GST-ERRγ | GST tag | (Hentschke et al., 2002) |
| GST-ERRγ ΔAF2 | delete AF2 | (Hentschke et al., 2002) |
| pSG-HEGO | (Migliaccio et al., 1991) | |
| pcDNA3.1-ERβ | (Hall and McDonnell, 1999) | |
| RORα | (Giguere et al., 1994) | |
| Mutant constructs | ||
| Construct | Mutation | Reference |
| ERRα-N | N-terminal 1–174 | (Kraus et al., 2002) |
| ERRα-C | C-terminal 76–422 | (Kraus et al., 2002) |
| ERRα - pboxmut | E97G/A98S/A101V | (Kraus et al., 2002) |
| GST-ERRα ΔAF2 | hERRα (1–406, delete 16aa) | current study |
| Coactivator expression constructs | ||
| Construct | Description | Reference |
| PCR3.1-hSRC-1a | human | (Onate et al., 1995) |
| pcDNA3.1-hPGC-1α | human | (Knutti et al., 2000) |
Table II.
Primers
| For Northern blotting | ||
| hERRα | ||
| Direction | Exon | Sequence |
| forward | exon 1 | 5′-ACA AGC AGC CGG CGG CGC CGC CGA GTG A3′ |
| reverse | exon 2 | 5′AT GTC GAC TCC TCC TCT TCC TTG3′ |
| forward | exon 6 | 5′AGA TGT CAG TAC TGC AGA GCG T3′ |
| reverse | Exon 7 | 5′GCT TCA TAC TCC AGC AGG3′ |
| hERRγ | ||
| forward | exon 1 | 5′ATG TCA AAC AAA GAT CGA CAC3′ |
| reverse | exon 2 | 5′ GCC CAC TAC CTC CCA GGA TA3′ |
| hPGC-1α | ||
| forward | exon 2 | 5′TTT GCC CAG ATC TTC CTG AAC3′ |
| reverse | exon 3 | 5′ATG AGG CCA ATC CGT CTT C3′ |
| hβ-Actin | ||
| forward | exon 5 | 5′GAC AGG ATG CAG AAG GAG ATC AC3′ |
| reverse | exon 6 | 5′GCT GAT CCA CAT CTG CTG GAA3′ |
| For real time PCR | ||
| mERRα | ||
| forward | exon 6 | 5′TTC GGC GAC TGC AAG CTC3′ |
| reverse | exon 7 | 5′CAC AGC CTC AGC ATC TTC AAT G3′ |
| mβ-Actin | ||
| forward | exon 5 | 5′GAC AGG ATG CAG AAG GAG ATT AC3′ |
| reverse | exon 6 | 5′GCT GAT CCA CAT CTG CTG GAA3′ |
| mERRγ | ||
| Applied Biosystems assay ID Mm00516267_m1 | ||
| mPGC-1α | ||
| Applied Biosystems assay ID Mm00447183_m1 | ||
| For EMSA | ||
| 1x ERE | ||
| top strand | 5′CCC GAA GCT TCT AGG TCA CAG TGA C3′ | |
| bottom strand | 5′GC TCG AGG TCA CTG TGA CCT AGA AG3′ | |
| For ChIP assay | ||
| MHRE region | ||
| forward | 5′GTC AGT GCA GGA CAG CCC GCG AG3′ | |
| reverse | 5′GAT AGG GCC CGG ACG GAG AAA GC3′ | |
| 4 kb upstream | ||
| forward | 5′GCA TAG CGA CAC TCG GGA CCT3′ | |
| reverse | 5′GCG GGT CTT GCT AAT GTT G3′ | |
Northern blot analysis
The FirstChioce Northern Human Blot I (Ambion, Austin, TX) containing 2 μg of poly(A) RNA from 10 different human tissues was used. The human ERRα and ERRγ, PGC-1α and β-actin cDNA were labeled with [α-32P]dATP by StripAble PCR Probe Synthesis and Removal Kit (Ambion). Human ERRα probe was made to detect ERRα exon 1–2, 345 bp length and exon 6–7, 322bp; ERRγ exon 1–2, 216 bp; PGC-1α exon 2–3, 240 bp; β-actin exon 5–6, 144 bp. The specific activities of the probes were about 3 x 105 cpm/μl and a total of 1 x 107 cpm of each probe was used in the NorthernMax (Ambion) hybridization solution. The human tissue blot was independently hybridized with 4 different radiolabeled probes and therefore under went three stripping procedures. Each probe was hybridized for 16 h. Exposure times were 48 h for the ERRγ and PGC-1α probes, 24 h for the ERRα probe, and 2 h for the βactin probe.
Cell culture, transient transfection and luciferase assay
HEC-1B (ATCC# HTB-113, endometrial) and HeLa (ATCC# CCL-2, cervix) cells were maintained in Minimum Essential Medium-Eagle (American Type Culture Collection, Manassas, VA) and supplemented with 100 units/ml penicillin, 10 μg/ml streptomycin, and 10% fetal bovine serum (Sigma, St. Louis, MO) at 37°C under 5% CO2. The transfections were carried out with Qiagen Effectene Transfection Reagent (Qiagen, Valencia, CA). Total DNA (201 ng) transfected in each experiment was kept constant with reporter constructs (100 ng per well), internal control pRL-CMV plasmid (1 ng per well, the CMV immediate early enhancer/promoter region were linked to the cDNA encoding Renilla luciferase; Promega, Madison, WI), nuclear receptor expression plasmids (50 ng per well or specified in individual experiments), coactivator expression plasmids (5 ng per well) and the empty expression vector pSG5 to make up the difference in plasmid concentration. Prior to transfections, cells were plated in 24-well plates and grown overnight in the medium containing 10% dextran-coated charcoal-stripped serum (Atlanta Biologicals, Norcross, GA). Twenty four h after transfection, the cells were collected and the firefly and Renilla luciferase activities measured with Dual-Luciferase Reporter Assay System (Promega) on the Fluoroskan Ascent FL instrument (Labsystems, Franklin, MA).
In vitro transcription and translation, and nuclear protein preparation
Receptors and coactivators (ERRα, ERRγ, ERα, ERβ, RORα, PGC-1α and SRC1) were transcribed and translated in vitro with either unlabeled or [35S]-labeled methionine (Amersham Biosciences, Piscataway, NJ) using the TNT Coupled Reticulocyte Lysate Systems (Promega, Madison, WI). For the dimerization experiments, ERRα and myc-ERRγ were co-transcribed/translated at either a 10:0, 10:1, 7:4, 4:7, 1:10, or 0:10 ratio, while ERRα and ERα were at a 10:0, 10:2, 8:4, 6:6, 4:8, 2:10 and 0:10 ratio. Nuclear proteins of the HEC-1B cells were prepared with the TransFactor Extraction Kit (Clontech, Palo Alto, CA) and used in the EMSA, GST pull-down and limited proteolysis (trypsin digestion) assay.
Electrophoretic mobility shift assay (EMSA)
Double-stranded MHRE elements (AAB, AB, A, m1, m2, m3, m4, sequence in Fig. 3) were cut out from the SV40-CAT reporters by NheI and XhoI, gel purified and used in EMSA (Liu et al., 2003). The oligonucleotides (1xERE) were synthesized by Sigma Genosys (The Woodlands, TX) and the probe was labeled by fill-in with 32P-dGTP, dNTP mixture and DNA polymerase I (Klenow large fragment). The unlabeled oligonucleotides encoding the MHRE: wild type (AAB for human; AB for mouse; A for truncated) and mutant (m1, m2 and m3 are single site mutation of the AB element; m4 is multiple sites mutation of the AB element) elements were used as the competitors at 1:50 molar ratio. Antibodies used in supershifted experiments are: rabbit polyclonal antibody to the ERRα peptide at the C-terminal region (P3–80), purified with protein-A column as described previously (Shigeta et al., 1997), human lactoferrin (LF) and pre-immune serum (PS) were prepared in our laboratory (Teng et al., 1986), and ERRγ (a gift from Dr. U. Borgmeyer, University of Hamburg, Germany). The antibody (1 μl/reaction) was incubated with the in vitro translated or nuclear proteins on ice for 30 min before EMSA. The EMSA has been previously described (Shigeta et al., 1997; Yang et al., 1996).
Figure 3.
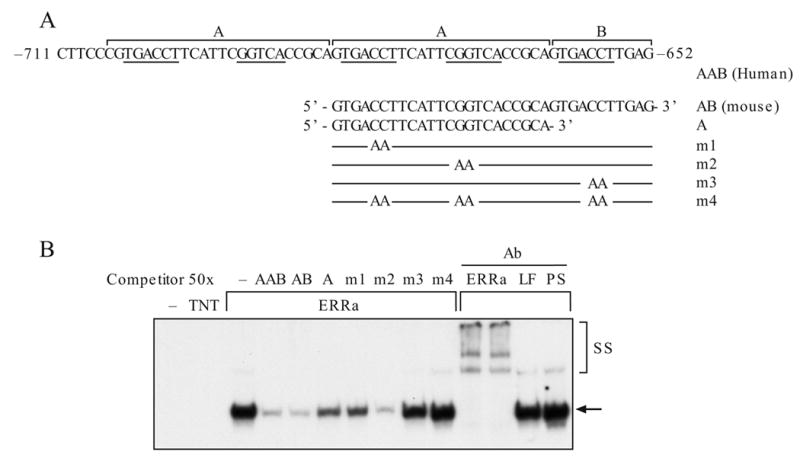
Detection of ERRα binding to AAB or AB by EMSA. A. Schematic presentation of the AAB element and the oligonucleotides used in competition. Location of the mutation(s) is indicated (Liu et al., 2005). B. Binding of ERRα to AB oligonucleotides. C. Binding of nuclear protein from HEC-1B cells to AB oligonucleotides. Specific binding was shown by oligonucleotides competition. D. Antibody supershifting analysis. One μl of antibody was added to the reaction mixture for supershift study. Arrow, protein-DNA complexes; SS, antibody supershifted complexes; TNT, in vitro transcription/translation mixture; LF, lactoferrin antibody; PS, rabbit serum; Ab, antibody.
GST pull down assays
The GST pull-down experiments with GST, GST-ERRα (full length and AF2 deletion) and GST-ERRγ (full length and AF2 deletion) expression plasmids were performed as described (Zhang and Teng, 2000). GST and GST-fusion proteins were prepared from Escherichia coli BL-21 cells after isopropyl-l-thio-β-D-galactopyranoside (IPTG) induction of an overnight culture. The bacteria were disrupted by sonication, and the fusion protein was isolated with 50% slurry of glutathione-Sepharose beads. Five μg of GST or GST fusion proteins were incubated with the in vitro transcribed/translated 35S-labeled ERRα, ERRγ, PGC-1α or SRC1 in binding buffer (50 mM potassium phosphate, pH7.5; 150 mM KCl; 1 mM MgCl2; 10% (v/v) glycerol; 1% (v/v) Triton X-100) for 1 h at 4°C. Binding of the 35S-labeled protein to GST-fusion protein was examined with SDS-PAGE and visualized by autoradiography.
Coimmunoprecipitation assay
HEC -1B cells were transfected with 3 μg/dish of flag-ERRα and myc-ERRγ for 24 h in 100 mm dishes. The cells were washed 3 times with ice-cold PBS and lysed with 1 ml/dish of RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% Na-deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 μg/ml each of aprotinin, leupeptin and pepstatin; 1 mM Na3VO4 and 1 mM NaF). After a pre-clearing step with protein A-agarose for 30 min, 400 μg of protein lysate were incubated with 10 μl of mouse monoclonal anti-Flag, anti-myc or IgG (as a negative control) antibodies overnight at 4°C. The antibody bound proteins were adsorbed to the protein A-agarose. The immunoprecipitated proteins were then resolved in 4–12% Nu-PAGE Bis-Tris gel system under denaturing condition (boiling and presence of SDS) with or without reducing agent (50 mM of DTT). After transferring the protein onto polyvinylidene difluoride membranes, the presence of flag-tagged ERRα and myc-tagged ERRγ was detected first with anti-myc antibody and then stripped and reprobed with anti-flag antibody.
Limited proteolysis assay
The in vitro transcribed/translated [35S]- methionine labeled ERRα or ERRγ (1 μl) was incubated with or without the AB or m4 DNA elements (500 ng) in 20 μl reaction buffer (50 mM Tris-HCl, pH 7.9, 40 mM KCl, 6% glycerol, 0.05% NP-40) for 20 min at room temperature. Trypsin (Sigma, St. Louis, MO) was added to a final concentration of 0.4 or 1.6 ng/μl. The incubation was carried out for 5 min at 30°C before stopped by adding the loading buffer and heated at 95° C for 10 min. The product was resolved by 4–12% Nu-PAGE Bis-Tris gel system of Novex. The gels were dried, exposed to x-ray film and visualized by autoradiography.
Chromatin immunoprecipitation (ChIP) assay
Commercial mouse monoclonal antibodies were obtained with the following sources: ERα (TE111.5D11) from NeoMarker (Fremont, CA); c-myc (9E10) from Santa Cruz Biotechnology (Santa Cruz, CA), flag (M2) from Sigma, SRC1 (SRC-1-1135, Clone 4) from Gene Tex, Inc. (San Antonio, Texas). The rabbit polyclonal antibodies against acetyl-histone H3 and acetyl-histone H4 were from Upstate Biotechnology (Charlottesville, VA) whereas HDAC1 (H-51), PGC-1α (H-300) and CBP (A-20) were from Santa Cruz Biotechnology. Purified normal rabbit IgG was from Sigma. The ChIP assay was performed according to the instructions of the ChIP Assay Kit (Upstate Biotechnology, Lake Placid, NY) with minor modifications. HEC-1B cells (in 100 mm culture dish) transfected with 2 μg of ERRα, ERRγ, PGC-1α or control empty vector pSG5 for 24 h and the proteins were cross-linked by incubation with 1% formaldehyde for 10 min at 37°C. Cells were washed with cold PBS buffer twice and disrupted in SDS lysis buffer containing the protease inhibitor cocktail (1mM PMSF, 1μg /ml aprotinin and 1μg /ml pepstatin A). Chromatin was sonicated to an average length of DNA of 200–1000 bp as verified by agarose gel electrophoresis. The sheared chromatin was diluted in ChIP dilution buffer and an aliquot of the solution reserved for input control. The chromatin was immunoprecipitated by antibodies as indicated. Briefly, after adding the antibodies (5 to 10 μg), chromatin solutions were gently rotated overnight at 4°C, protein A agarose slurry (containing sonicated salmon sperm DNA) was added and constantly rotated for one more hour at 4°C. The agarose beads were collected by centrifugation, washed and the antibody bound chromatin was released from the agarose beads. Finally, the DNA was purified by phenol/chloroform extraction and ethanol precipitation. The presence of the MHREs region was detected with forward and reverse primers (Table II) in PCR reaction (Liu et al., 2005). In addition, 4 kb upstream of the MHRE region was examined with forward and reverse primers and served as a negative control. The PCR conditions for ChIP assay were 94°C for 30s, 58 °C for 30s, 72° C for 30s for a total of 35 cycles.
RESULTS
Fasting induces ERRα, ERRγ and PGC-1α gene expression in mouse liver
To examine the expression levels of ERRα and ERRγ and PGC-1α in the same tissue sample, we performed Northern blot analyses using the Ambion human tissue blot that contains equally loaded polyA mRNA from various human tissues (Fig. 1A). We used equal amount of probes that were labeled to a similar specific activity in the hybridization. ERRα mRNA was detected in all tissue examined with 24h of exposure time, whereas the levels of ERRγ and PGC-1α mRNA were several fold less than that for ERRα, and they required 48h of exposure time to show the signals. Under these hybridization conditions, ERRγ and PGC-1α mRNA were not detected in some tissues such as spleen, lung and brain, whereas relatively low levels of ERRγ were present in skeletal muscle and pancreas. Despite the differential expression level, the ERRs and PGC-1α were coexpressed in the skeletal muscle, heart and kidney.
Figure 1.
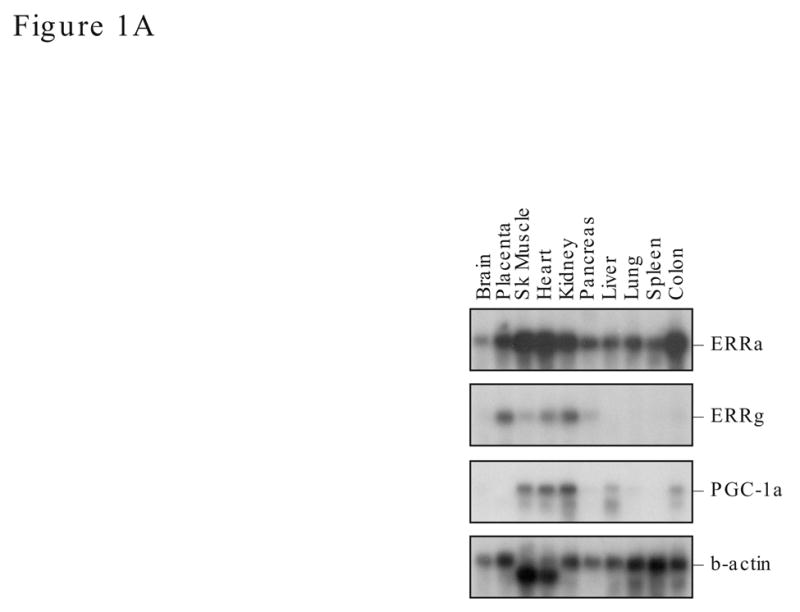
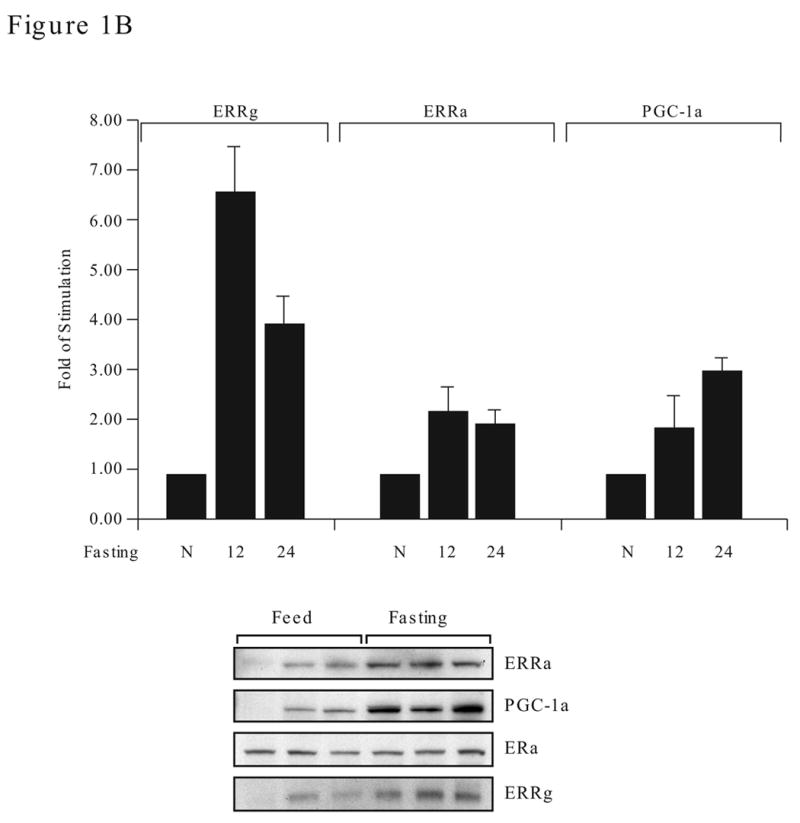
Expression of ERRα, ERRγ and PGC-1α in human tissues and mouse liver during fasting. A. Northern blot analyses of human tissues. ERRα, ERRγ, PGC-1α and β-actin probes (between 200–300 bp in length) were labeled to a similar specific activity and used in sequential hybridization to the FirstChoiceTM Northern Human Blot I from Ambion (2 μg polyA RNA /lane). The hybridization time for all four probes was 16 h, however, the exposure time varies. The x-ray film for ERRγ and PGC-1α were exposed for 48 h while ERRα was exposed for 24 h and β-actin for 2 h. B. ERRα, ERRγ and PGC-1α expression in mouse liver during fasting. Adult female mice (4 per group) were fed (N), fasted for 12 h (12) or 24 h (24). Total liver RNA was prepared from the individual mouse and analyzed by real time PCR with specific primers to ERRα, ERRγ or PGC-1α (upper). Data is presented as fold of stimulation from 4 mice ± SE. Liver nuclear protein lysates (30 μg protein) were individually prepared and the presence of ERRα, ERRγ, PGC-1α and ERα were detected by Western blot analyses with their respective antibodies (lower). The antibody dilution and exposure time as follow: ERRα, 1:1000 and 2 min; ERRγ, 1:200 and 1 min; PGC-1α, 1:500 and 5 min; ERα, 1:2000 and 5 min. There were three mice per experimental group and the length of fasting was 24 h.
During fasting, the demand for energy production through the mitochondria oxidation phosphorylation pathway in liver is increased and the expression of PGC-1α and ERRα is induced to meet the needs for executing the energy balance program (Knutti and Kralli, 2001; Puigserver and Spiegelman, 2003). To examine whether the expression of ERRγ is also affected by fasting, we investigated its expression in liver by real time PCR from fed (control) and fasting mice. It was interesting to find that the mRNA of ERRγ was induced 6-fold after the food was removed for 12 h and down to 4-fold by 24 h. Although the absolute value of ERRα mRNA in the fasting mice was higher than ERRγ, the fold of induction was higher in ERRγ whereas the ERRα and PGC-1α were both increased steadily and maintained at 2–3 fold higher than the fed mice (Fig. 1B, upper panel). Fasting also increased the production of ERRα, ERRγ and PGC-1α proteins in liver (Fig. 1B, lower panel) but had no effect on the ERα protein, which remained constant in the level of fed and fasting (24h fasting) mice. This study demonstrated that in addition to ERRα and PGC-1α, the expression of ERRγ is also regulated by fasting.
Functional and physical interaction of ERRα and ERRγ on the ERRα gene promoter
We have previously shown that ERRα gene is regulated by PGC-1α and ERRγ in uterine cells (Liu et al., 2005). To investigate whether coexpression of ERRα and ERRγ has any effect on the activity of ERRα gene promoter, we cotransfected various amounts of ERRα and ERRγ expression constructs individually or in combination with the human ERRα MHRE-reporter (AAB-Luc) into human endometrial carcinoma HEC-1B cells. In agreement with our previous findings, overexpression of ERRγ increased the AAB-Luc activity nearly 35 times higher than overexpression of ERRα (Fig. 2A). However, the ERRγ-stimulated activity was reduced rather than enhanced by ERRα coexpression. This effect was not due to the different expression levels of the ERRα or ERRγ constructs in transfected cells because both expression constructs either alone or together produced comparable levels of protein as detected by myc antibody in Western blotting analyses (Fig. 2A, insert).
Figure 2.
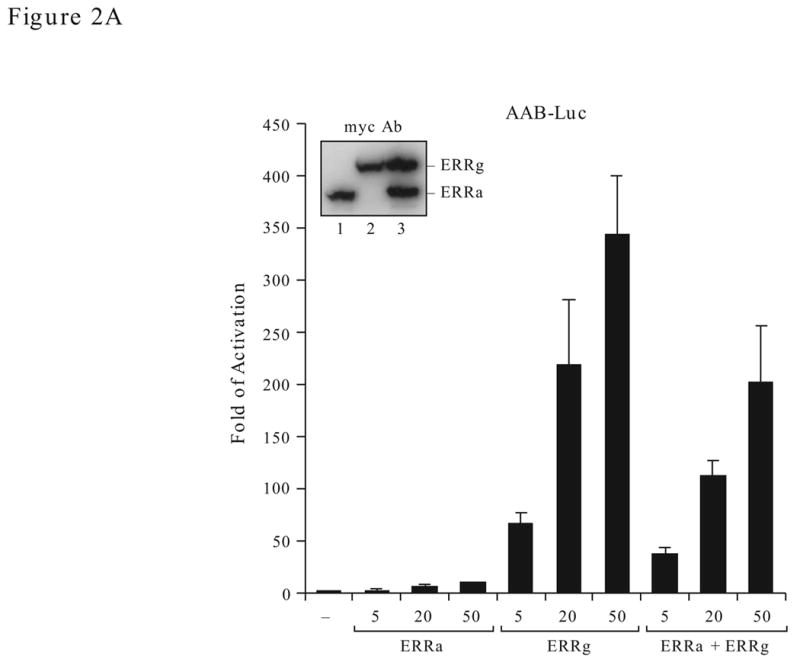
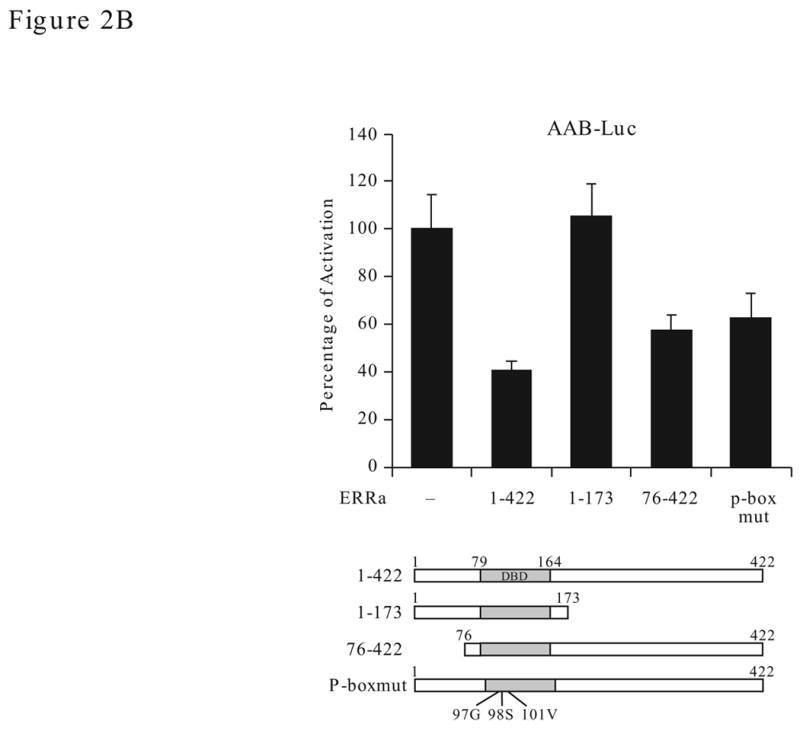
Effect of ERRα and ERRγ on the AAB activity. A. Dose effect. The AAB-Luc reporters were cotransfected with myc-ERRα or myc-ERRγ individually or together (1:1 ratio) at the varying concentration (as indicated) into HEC1-B cells. Twenty-four h after transfection, cells were collected and reporter Luc activities measured. The firefly luciferase reporter activities were normalized to Renilla luciferase activities and plotted as fold of activation against vector control. The results are reported as the mean ± S.D. from a minimum of three independent experiments with duplicates for each experiment. Expression level of the transiently transfected ERRα and ERRγ expression constructs were verified with myc antibody by Western blotting (insert). B. Effect of ERRα on ERRγ transactivation function with AAB. Mutant ERRα (either truncation or mutation) were cotransfected with ERRγ expression vector (or empty vector) and the AAB-Luc reporters into HEC-1B cells. The Luc activities were determined 24 h later. Schematic presentation of the ERRα constructs is shown at the bottom. 1–422, full-length ERRα; 1–173, N-terminal region of the ERRα (1–173 amino acid); 76–422, C-terminal region of the ERRα (76–422 amino acid); P-boxmut, mutation made at the P-box of the DBD. Data is presented as means ± SE from three experiments with duplicated samples.
This repression effect of ERRα on ERRγ-stimulated activity could come from competition for binding to the MHRE (either AAB or AB). By using various ERRα mutant constructs (Fig. 2B, bottom), we examined whether competition could be the cause of repression on ERRγ-stimulated activity. It was interesting to find that the ERRα mutant lacking the N-terminus (76–422) or defective in DNA binding (p-box mut) still repress ERRγ stimulated activity on the AAB-Luc. The repression effect was lost when the 174–422 region of the ERRα was deleted even though the mutant constructs still retain the DBD region (1–173). These results suggest that the repression function of ERRα lies beyond the DNA binding and the mechanism of repression may be more complicated than simple competition for binding to DNA.
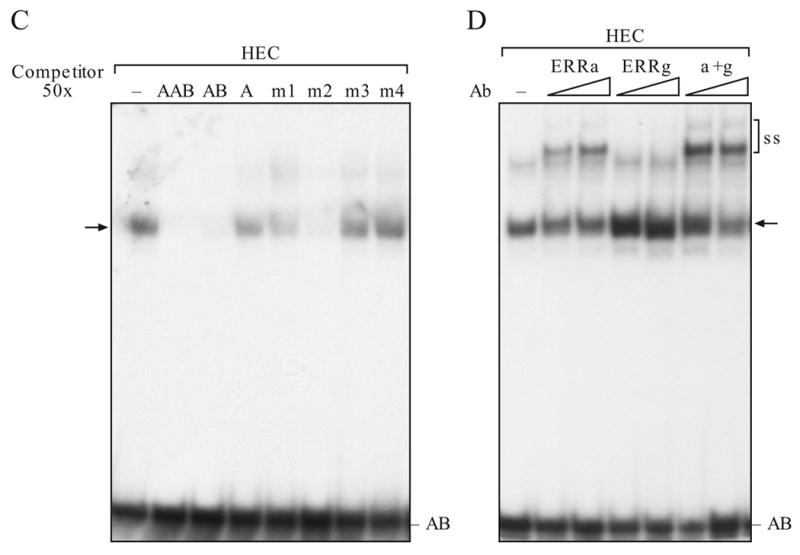
In the electrophoresis mobility shift assay (EMSA), we found that the in vitro transcribed/translated ERRα and ERRγ but not ERα, ERβ or RORα bound AAB (data not shown). The binding specificity was demonstrated by competition and antibody supershift (SS) experiments. Excessive unlabeled (molar ratio of 50x) wild-type oligonucleotides (AAB and AB, Fig. 3A) competed efficiently for binding with ERRα (Fig. 3B) on the AB element. Interestingly, m2 competed efficiently with ERRα binding. Nevertheless, the competition efficiency was significantly reduced with m1 and m3 and with the short A element (A). When all three potential ERRs binding sites were mutated (m4), the mutant oligonucleotides lost the ability to compete. In the presence of antibody, the AB-ERRα complexes could be supershifted, while non-relevant antibody (LF) and pre-immune serum (PS) did not affect the mobility of the band. These observations are similar to the EMSA study of ERRγ binding with the AB element (Liu et al., 2005). Whether the endogenous ERRα and ERRγ bind AB in the context of nuclear protein extract was also investigated (Fig. 3C). Using nuclear protein extract of HEC-1B cells, we found one retarded protein-AB element complex. The wild-type and mutant oligonucleotides competed with this complex with similar efficiency as the ERRα or ERRγ proteins produced in vitro (Fig. 3C and Liu, 2005). Furthermore, the antibody to ERRα supershifted this complex (SS) (Fig. 3D), which indicates the presence of ERRα in the nuclear protein extract. We did not detect any supershifted band by the antibody to ERRγ, however, a reduced retarded band (arrow) and slightly increase of supershifted band (ss) were found when both antibodies were present. The findings suggest that abundant ERRα and less ERRγ are present in the nuclear protein-AB complex. Non-relevant serum did not supershift the complex (data not shown). These studies suggested that both ERRα and ERRγ bind specifically and at the similar location on the AB element.
To investigate whether ERRα and ERRγ compete for binding to the AB element or they can bind simultaneously, we co-transcribed/translated the ERRα and myc-tagged ERRγ in vitro which produced protein products of different sizes. Binding of these protein to the AB element were examined. We found that ERRα and ERRγ bind individually or with each other (α/γ) on the AB (Fig. 4A) and the 1xERE (Fig. 4B) elements. Interestingly, ERRα binds 1xERE more efficiently than ERRγ does, although they both bind the AB element equally well (compare Fig. 4A and B). It is not clear whether ERRα and ERRγ bind as homodimer or heterodimers in the present study, more detailed EMSA are required since the AB element consist of three ERE half site for potential bindings. However, on the 1x ERE, regardless of the weak binding, ERRγ formed a heterodimer with the ERRα. ERα did not heterodimerize with ERRα (Zhang and Teng, 2000; Zhang and Teng, 2001) or ERRγ (Razzaque et al., 2004) and served as a control (Fig. 4C).
Figure 4.
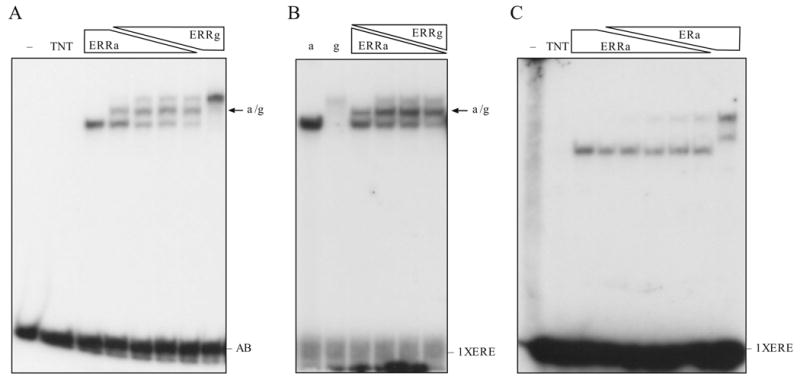
Binding characteristic of ERRα and ERRγ on AB or 1X ERE oligonucleotides. A. ERRα and ERRγ binding to AB oligonucleotides. In vitro transcribed/translated ERRα and myc-tagged ERRγ were used. The heterodimer form is indicated. B. ERRα and ERRγ binding to 1x ERE. C. ERRα and ERα binding to 1x ERE. Relative ratio of the receptors produced in vitro was described in the Material and Methods.
The GST-pull-down assay demonstrated a direct protein-protein interaction between ERRα and ERRγ (Fig. 5A). In addition, ERRα and ERRγ interaction was also demonstrated by coimmunoprecipitation (IP/IB) (Fig. 5B). We consistently detected a larger complex at the 100–120 kDa regions with either myc-ERRγ and flag-ERRα specific antibodies. The large complex could be reduced to the expected protein size of 53 or 64 kDa for ERRα and ERRγ, respectively, in the presence of reducing agent (DTT) suggesting that the ERRs may form higher order complexes in vivo with bisulfite bonds that are not dissociable by boiling or detergent. These results clearly showed that ERRα and ERRγ interact in the cell and they may bind the response element as a heterodimer or even as a multimer.
Figure 5.
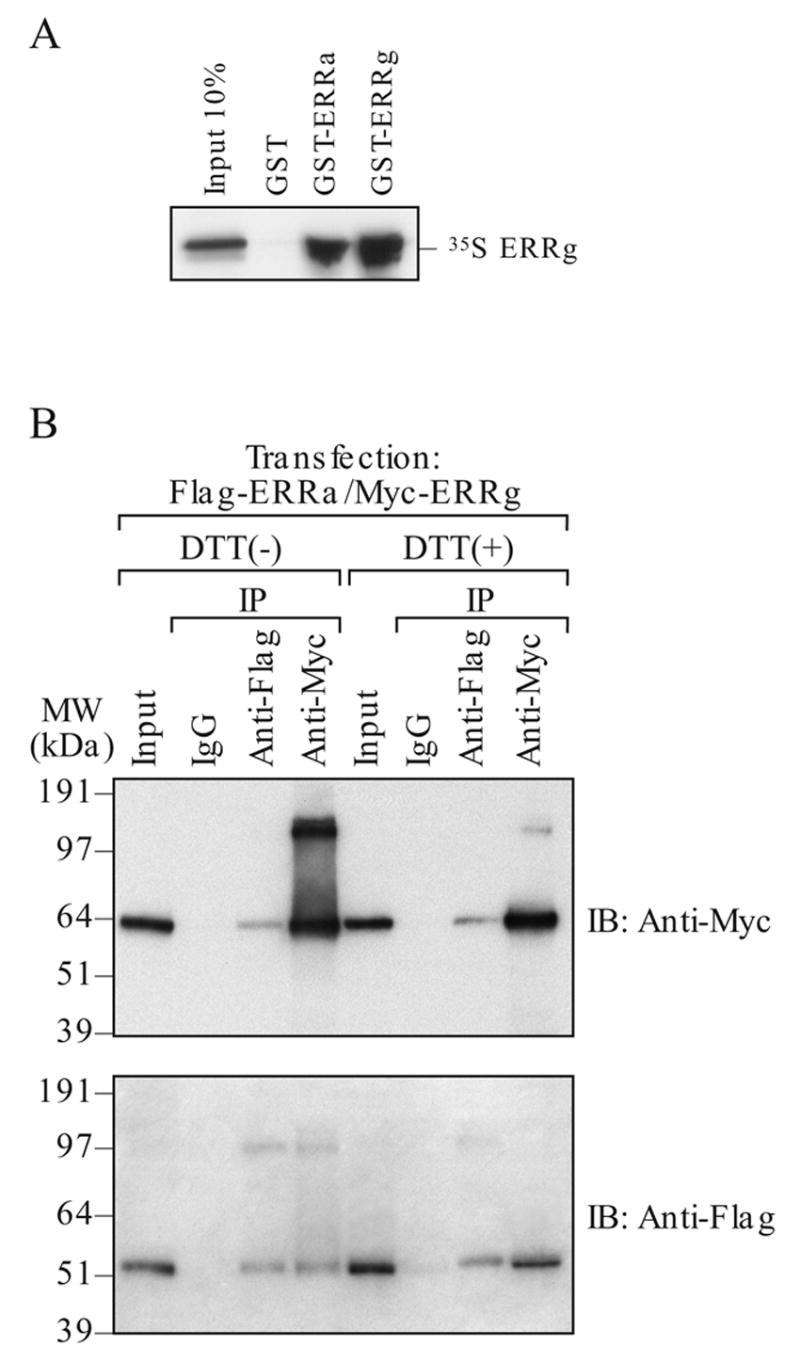
Detection of ERRα and ERRγ interaction in vitro and in vivo. A. GST-pull down assay. In vitro transcribed/translated 35S- ERRγ was pull-down with GST- linked ERRα or ERRγ. B. Coimmunoprecipitation. HEC-1B cells were transfected with either flag-ERRα or myc-ERRγ and the protein complex was immunoprecipitated (IP) by indicated antibody (either flag or myc). The complex was resolved on a denatured SDS-PAGE either with or without the presence of reducing agent (5 mM DTT). The protein was detected by immunoblotting (IB) with indicated antibody (either flag or myc).
Differential molecular characteristics of the ERRα and ERRγ
Recent findings of several laboratories demonstrate that the sequence of DNA binding element is important in regulating the ERRγ transactivation function (Razzaque et al., 2004; Sanyal et al., 2004). Consistent with these reports, we found that the AAB of the ERRα gene and the synthetic 3x ERE which contain multiple ERE half sites, are very sensitive to ERRγ stimulation whereas 1x ERE and the NRRE of the MCAD gene are less responsive (Fig 6A). In fact, a 10-fold difference can be observed in HEC-1B or commonly used cervical carcinoma HeLa cells. On the contrary, ERRα did not show preference for these response elements and consistently activated all response elements at a lower level. To study whether the DNA sequence could mediate the ERRs’ structural change, we use limited proteolysis assay. The radiolabeled ERRs were bound to the wild type or mutant AB elements and subjected to limited proteolysis by increasing doses of trypsin as described in the Methods. ERRγ yields different trypsin digestion pattern (Fig. 6B, right panel, arrows) when bound to the AB element as oppose to the unbound protein or in the presence of mutated AB element (m4), an occurrence not shared with ERRα (Fig. 6B, left panel). Moreover, ERRγ strongly interacted with coactivator SRC1, and the interaction required AF2 domain as the mutant construct that lacks the AF2 domain (ΔAF2) interacted poorly with the coactivators (Fig. 6C). In contrast, SRC1 interacted weakly with ERRα and the AF2 domain did not play a critical role in the interaction. Surprisingly, PGC-1α interacted equally with ERRα and ERRγ, and the AF2 domains are not required for the interaction suggesting that the binding of PGC-1α to ERRs at a region that is different from that used by other coactivators. In contrast, the AF2 domain of the ERRα was reported to be required for PGC-1α binding (Huss et al., 2002). Our deletion construct contains extra three amino acids at the C-terminus. Whether this difference between the two deletion constructs can influence PGC-1α interaction warrants further investigation.
Figure 6.
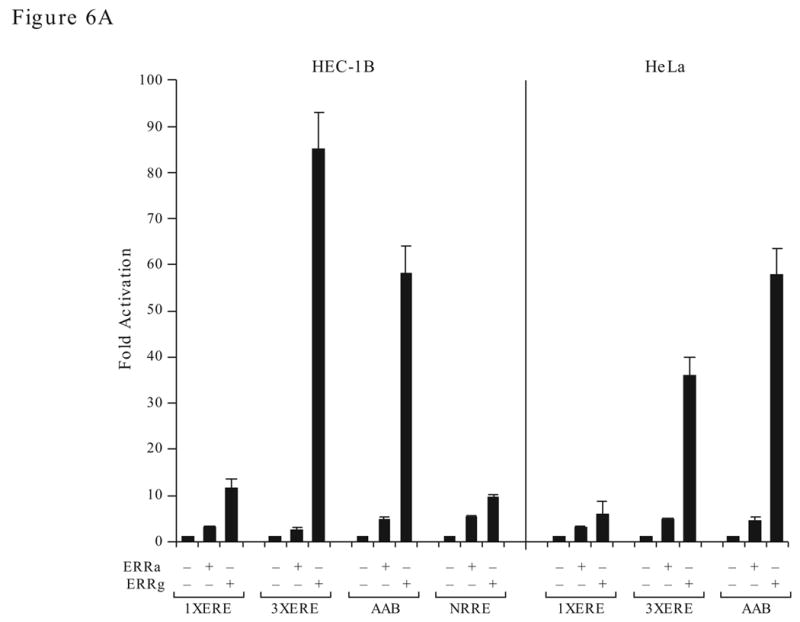
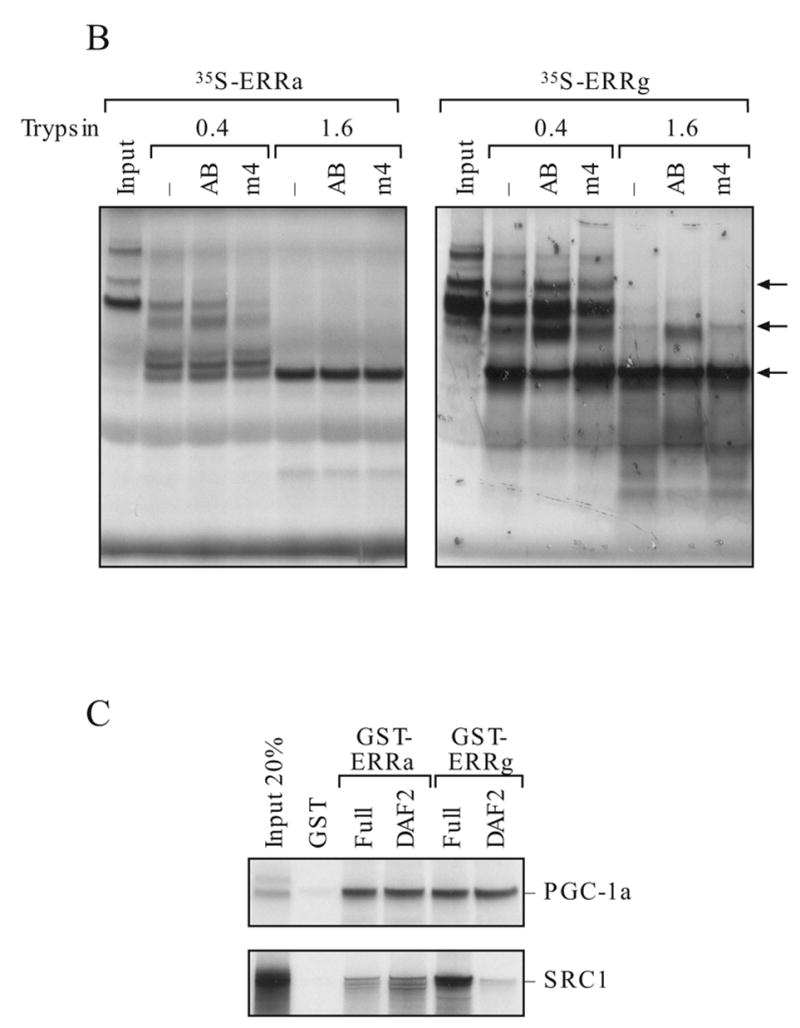
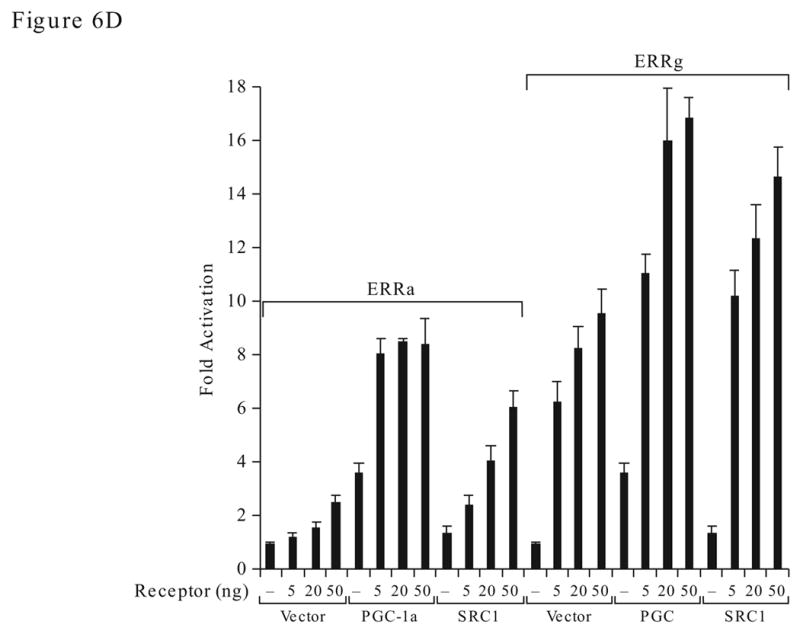
Differential molecular characteristics of the ERRα and ERRγ. A. Effect of response element on the transactivation activity of ERRγ and ERRα. Transfection study was preformed in HEC-1B (left) and HeLa (right) cells. B. Effect of trypsin digestion on ERRα and ERRγ with or without bound to the AB oligonucleotides. C. Detection of coactivator interaction with ERRγ and ERRα by GST-pull down assay. AF2, AF2 domain deletion; full, the full-length protein. D. Selectivity of ERRγ and ERRα in coactivator utilization. Fold activation is against the empty expression vector.
Next, we investigated whether the coactivator interactions could be reflected in the ERRα and ERRγ activation function. We transfected a constant level of coactivator with increasing concentration of either ERRα or ERRγ expression constructs in the HEC-1B cells. We found that the transactivation activity of ERRα was stimulated 7–8 fold by PGC-1α and 3–5 fold by SRC1 (Fig. 6D). ERRγ strongly activated the AAB-Luc reporter without additional coactivator, presumably via the endogenous factors. However, overexpression of PGC-1α and SRC1 further stimulated the AAB-Luc activity. PGC-1α alone stimulated the AAB-Luc 4-fold while SRC1 was inactive. Thus indicates that PGC-1α is an effective coactivator in the stimulation of AAB-Luc activity and that the endogenous ERRα or ERRγ serves as the DNA binding partner for PGC-1α.
Effect of ERRα and ERRγ expression on the chromatin structure and coactivator recruitment at the MHRE region
We used chromatin immunoprecipitation assay (ChIP) to determine whether binding of ERRα to the MHRE chromatin is affected by increasing expression of ERRγ or vice versa in the HEC-1B cells. ERRα and ERRγ were both detected on the MHRE chromatin of HEC-1B cells. Overexpression of either ERRα or ERRγ increased its own binding without affecting the occupancy of the other (Fig. 7A, compare the relative value under the band). These results suggest that the binding of ERRα and ERRγ to the MHRE is not mutually exclusive, which is consistent with the aforementioned in vitro EMSA study (Fig. 4A). Furthermore, the potential of ERRα and ERRγ to form a heterodimer or even multimer on the MHRE region is also agreeable with the coimmunoprecipitation study (Fig. 5B). It was interesting to find that the chromatin structure of MHRE was modified towards a transcriptionally active state through increasing acetylation of histone 3 and 4 (Ac-H3 and Ac-H4) and decreasing histone deacetylase 1 (HDAC1) occupancy by overexpression of either ERRα or ERRγ (Fig. 7B). In contrast, ERRγ expression increased SRC1 and CBP recruitment, whereas ERRα did not. These results support the protein-protein interaction study where ERRγ interacts with SRC1 stronger than with ERRα (Fig. 6C). This result could, in part, explain the strong activation activity of ERRγ on the MHRE. Modification of chromatin structure and coactivator or corepressor recruitment are not observed at the upstream non-relevant region of the ERRα gene. Previously, we have shown that PGC-1α enhances ERRγ interaction with MHRE both in vitro and in vivo (Liu et al., 2005). The present ChIP study demonstrated again that PGC-1α promotes endogenous ERRγ binding and SRC1 recruitment to the MHRE 24 h after transfection. Interestingly, overexpression of PGC-1α had no effect on ERRα binding to the same region at the time point examined (Fig. 7C). These ChIP studies combined with the functional study (Fig. 2) and coactivator interaction (Fig. 6C) imply that ERRγ is a strong activator in the regulation of ERRα gene expression, whereas ERRα involves in chromatin modification and modulates the activity of ERRγ on the MHRE.
Figure 7.
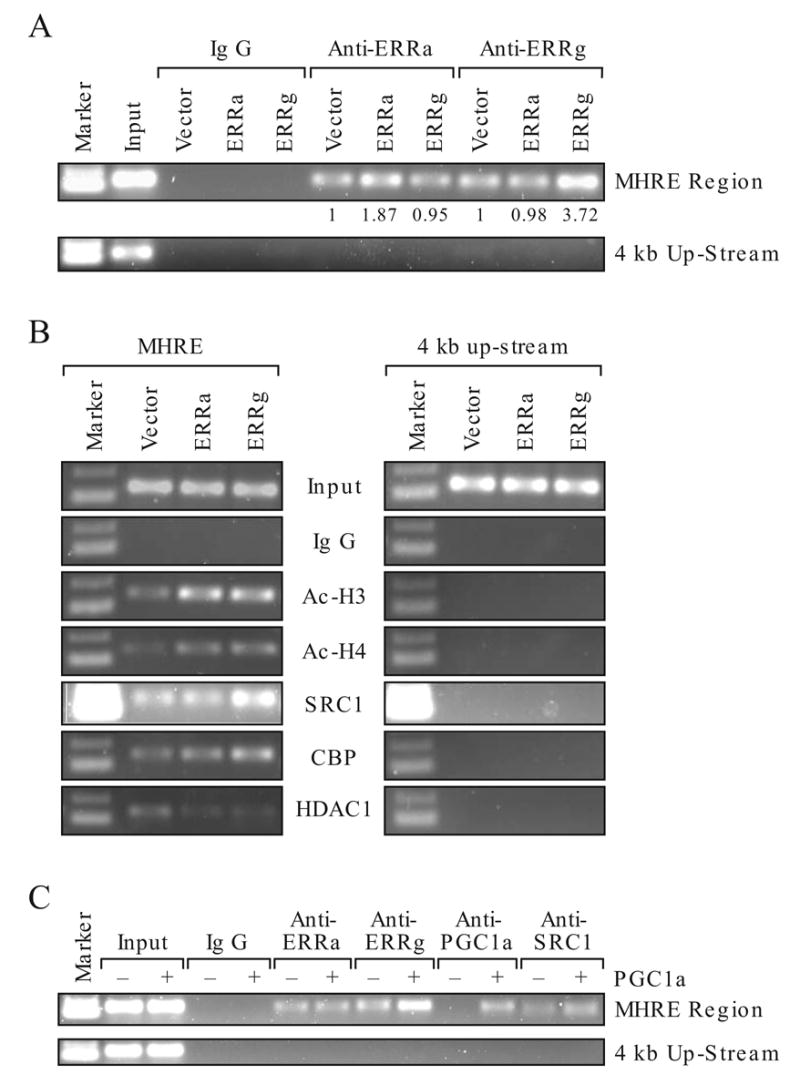
Effect of ERRα and ERRγ on MHRE chromatin structure. Chromatin was immunoprecipitated by indicated antibodies in HEC-1B cells. The MHRE and none relevant region at 4 kb upstream from the ERRα gene were detected by PCR. Antibody to ERRα, ERRγ, PGC-1α, acetylated histone 3 (Ac-H3), acetylated histone 4 (Ac-H4), histone deacetylase 1 (HDAC1), coactivator CBP and SRC1 were used. A. Detection of ERRα and ERRγ on the MHRE chromatin by ChIP assay. Empty vector, ERRα or ERRγ was overexpressed in the HEC-1B cells. The presence of ERRs on the MHRE was detected by ChIP with specific antibody to either ERRα or ERRγ. Intensity of the band was scanned in an Innotech Chemilmager 5500 with signal spot densitometry according to the user’s manual. Vector sample is set as 1. B. Effect of ERRα and ERRγ overexpression on the histone modification and coregulator recruitment at the MHRE chromatin region. C. Effect of PGC-1α overexpression on the ERRα, ERRγ binding and SRC1 recruitment to the MHRE chromatin region.
Discussion
In this report, we found that ERRγ, a close family member of ERRα stimulates ERRα gene expression whereas its own gene product represses ERRγ’s activity. The ERRs have different biochemical and molecular characteristics. When bind to MHRE, ERRγ but not ERRα recruits SRC1 and CBP. This finding is consistent with the functional study that ERRγ is a stronger activator than ERRα on regulation of its own gene expression. Importantly, PGC-1α enhances ERRγ occupancy on the chromatin of the ERRα gene at the MHRE region.
Expression of ERRα, ERRγ and PGC-1α in metabolically active tissues such as skeletal muscle, heart and kidney has been reported by many laboratories (Bonnelye et al., 1997; Heard et al., 2000; Hong et al., 1999; Huss et al., 2002; Ichida et al., 2002; Knutti et al., 2000; Puigserver et al., 1998; Sanyal et al., 2002; Shi et al., 1997; Shigeta et al., 1997; Sladek et al., 1997), however, they were examined individually. The Northern blot analyses of the present study examined the coexpression of ERRs and PGC-1α in the same poly A RNA preparation of various human tissues. Indeed, ERRα is most abundant in many tissues that require high-energy output in particular the kidney and heart where ERRγ and PGC-1α are also highly expressed. In these tissues, the relative ratio of ERRα, ERRγ and PGC-1α may be important in executing the metabolic program that involves the PGC-1α and ERRα and ERRγ. Recently, mounting evidence demonstrates that PGC-1α functions as a master regulator in executing the energy balance program including the up-regulation of ERRα gene expression (Knutti and Kralli, 2001; Puigserver and Spiegelman, 2003). ERRα cooperates with PGC-1α in stimulation of genes involved in the mitochondrial biogenesis, mitochondria oxidative phosphorylation (Huss et al., 2004; Schreiber et al., 2004), and the mitochondria structure (Cartoni et al., 2005). However, a physiological role for ERRγ in energy metabolism is not established. It is known that ERRγ is a strong activator of selective response elements (Razzaque et al., 2004; Sanyal et al., 2004), interacts with PGC-1α (Hentschke et al., 2002; Huppunen et al., 2004; Huss et al., 2002) and stimulates the ERRα gene promoter via the MHRE (Liu et al., 2005). Furthermore, treatment of HEC-1B cells with ERRγ siRNA significantly reduced the ERRα mRNA expression (Liu et al., 2005). Our current data showed that ERRγ expression in liver is also under the influence of physiological cue such as fasting. These studies support the hypothesis that ERRγ is one of the major regulators of ERRα gene expression. Since the expression of ERRα in selective tissues is influenced by circadian rhythm (Horard et al., 2004), basal levels of ERRα may fluctuate during the 24 h fasting period with maximum in the afternoon. It is not known whether PGC-1α or ERRγ are also under circadian regulation. These factors may influence the level of ERRα, ERRγ and PGC-1α mRNA measured during fasting. Nonetheless, four mice in the control group were killed in the afternoon at the highest level of circadian behavior and significant induction of ERRs and PGC-1α were detected in both 12 and 24h fasting mice. Taken together, ERRγ may involve in regulation of the energy balance program at least by enhancing the ERRα expression. Whether ERRγ regulates the same ERRα downstream target genes in mitochondria oxidative phosphorylation or mitochondria biogenesis requires further investigation.
ERRα and ERRγ share high sequence homology at their DNA binding domain (98% identity) and bind response elements in a similar fashion (Fig. 3 and Liu 2005), yet ERRγ shows high variability in its capacity to stimulate the different response elements, whereas ERRα does not (Fig. 6A). For example, ERRγ binds the consensus steroidogenic factor 1 response element (SF-1RE) and thyroid hormone response element (TRE), but it only activates the SF-1RE moderately and is inactive on the TRE, whereas ERRα activates both elements (Heard et al., 2000; Sanyal et al., 2002). Additionally, the osteopontin (OPN) gene promoter is very sensitive to ERRα stimulation but not to ERRγ (Bonnelye and Aubin, 2005; Huppunen et al., 2004). These studies demonstrate that the ERRα and ERRγ have very different molecular and biochemical characteristics. Indeed, our molecular study showed that the conformation of ERRγ is influenced by binding to the MHRE (Fig. 6B) and the changes of conformation could lead to the recruitment of coactivators or release of corepressors, thus affecting its transactivation function. The biological implication of ERRγ conformational change upon binding to different response elements is not clear but may be important to its functional roles, especially since it has a broad range of binding specificity (Razzaque et al., 2004). Another major difference between ERRα and ERRγ is that ERRγ interacts strongly with SRC1, a coactivator not only required for the transactivation function of nuclear receptors but also needed to increase the potency of PGC-1α (Puigserver et al., 1999). Specifically, the AF2 domain of the ERRγ is required for the protein-protein interaction with coactivators. On the other hand, ERRα interacts with the coactivator weakly whether AF2 domain is present suggesting, that the region of receptors and coactivators interaction is different between ERRα and ERRγ. Interestingly, both ERRα and ERRγ bind PGC-1α identically. The differences in coactivator interaction are also reflected in their coactivation function, in which PGC-1α is the most potent coactivator for both ERRα and ERRγ whereas SRC-1 is a potent coactivator for ERRγ. Since SRC-1 is ubiquitously expressed, it may explain why ERRγ actively stimulates the ERRα gene expression in the absence of PGC-1α in several cell lines tested (Fig. 6D and (Liu et al., 2005)).
How ERRα represses the ERRγ-stimulated MHRE activity is unclear, nonetheless, we can rule out the competition for binding to the DNA since ERRα mutants deficient in DNA binding (p-box mut) retain the repression function, whereas the N-terminus containing the DNA binding domain lost the repression function. These study suggest that the repression function of the ERRα lies at the C-terminal region. In EMSA study, both ERRα and ERRγ were present on the MHRE although it was not clear whether they form heterodimers. Moreover, the ERRα and ERRγ interact with each other in vitro and in vivo. It has been shown that heterodimerization between ERRα and ERRγ inhibits the transactivation function of each other, in contrast to the homodimerization which is needed for their transcriptional activity (Horard et al., 2004; Huppunen and Aarnisalo, 2004). It is also known that one of the dimerization interfaces of ERRα resides in the C-terminal region (Horard et al., 2004), implicating that the heterodimerization between the ERRs is one of the mechanism for ERRα repression. We detected both ERRα and ERRγ on the chromatin of MHRE by ChIP assay and showed that overexpression of ERRγ significantly increases its occupancy on the chromatin of MHRE without affecting the ERRα presence, and vice versa (Fig. 7A). MHRE is composed of multiple ERE half sites with various spacing and orientations (Liu et al., 2003) and the two reversed ERE half sites (TGACCTTCA) separated with 14 bp are important for ERRs binding and transcriptional activity (Fig. 3 and reference (Liu et al., 2003)). It is unusual for a nuclear receptor to bind two ERE half site with such wide space in between. Nonetheless, ERRγ has been reported to bind a broad range of sequences, including the monovalent binding sites (Razzaque et al., 2004), whereas ERRα also binds variety of response sequences (Barry et al., 2006; Johnston et al., 1997) including the NRRE-1 of the medium chain acyl-coenzyme A dehydrogenase (MCAD) gene at sites with 14 bases in the middle (Maehara et al., 2003). Recently, Giguere’s laboratory demonstrated that a single nucleotide change in ERRα binding site can affect the mode of PGC-1α activation of its target promoters (Barry et al., 2006). Thus, the binding characteristics of ERRs may influence the recruitment of coactivators and the transactivation activity.
Although ERRα and ERRγ have very different transcriptional activities on the MHRE of ERRα gene they both modified the chromatin towards an active state by increasing acetylation of histone 3 and histone 4, and decreasing the presence of histone deacetylase 1. The most striking difference between ERRα and ERRγ lies in their ability to recruit SRC1. The presence of SRC1 on the MHRE chromatin is significantly increased by ERRγ, but not by ERRα. Interestingly, ectopic expression of PGC-1α enhances ERRγ occupancy at the MHRE region and increases the SRC1 presence as well. With PGC-1α overexpression, homodimerization of ERRγ may be increased whereas heterodimeriztion of ERRα and ERRγ may be decreased, thus providing an explanation for the activation of ERRα gene by ERRγ. In conclusion, this study shows that ERRγ has an important role in regulation of the ERRα gene expression. It is currently not known whether ERRγ also regulates the expression of genes involved in the fatty acid metabolic pathway and energy balance program. Because of the strong activation function of ERRγ and the fact that it is able to activate expression of the ERRα gene in the absence of PGC-1α, ERRγ might be a potential target for developing drugs that treat diabetic and metabolic-related human diseases.
Acknowledgments
We thank Dr. A. Jetten and Dr. R. DiAugustine for reading the paper and providing valuable comments. We acknowledge Dr. D. Liu for participating in the initial study. This research was supported by the Intramural Research Program of NIH, the National Institute of Environmental Health Sciences (NIEHS). The paper was edited by the NCI, CCR Fellows Editorial Board and Jessica Martin.
Abbreviations
- ERRα and ERRγ
estrogen-related receptor alpha and gamma
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator
- MHRE
multi-hormone response elements
- HEC-1B
human endometrial carcinoma cell line
- EMSA
electrophoresis mobility shift assay
- ChIP
chromatin immunoprecipitation assay
- SRC1
steroid receptor coactivator 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barry JB, Laganiere J, Giguere V. A Single Nucleotide in an Estrogen-rRelated Receptor a Site Can Dictate Mode of Binding and Peroxisome Proliferator-Activated Receptor g Coactivator 1a Activation of Target Promoters. Molecular Endocrinology. 2006;20:302–310. doi: 10.1210/me.2005-0313. [DOI] [PubMed] [Google Scholar]
- Bonnelye E, Vanacker JM, Spruyt N, Alric S, Fournier B, Desbiens X, Laudet V. Expression of the estrogen-related receptor 1 (ERR-1) orphan receptor during mouse development. Mech Dev. 1997;65:71–85. doi: 10.1016/s0925-4773(97)00059-2. [DOI] [PubMed] [Google Scholar]
- Bonnelye E, Aubin JE. Estrogen Receptor-Related Receptor {alpha}: A Mediator of Estrogen Response in Bone. J Clin Endocrinol Metab. 2005;90:3115–3121. doi: 10.1210/jc.2004-2168. [DOI] [PubMed] [Google Scholar]
- Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. Mitofusins 1/2 and ERR{alpha} expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee NRN. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97:161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci U S A. 2001;98:8880–8884. doi: 10.1073/pnas.151244398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disch DL, Rader TA, Cresci S, Leone TC, Barger PM, Vega R, Wood PA, Kelly DP. Transcriptional control of a nuclear gene encoding a mitochondrial fatty acid oxidation enzyme in transgenic mice: role for nuclear receptors in cardiac and brown adipose expression. Mol Cell Biol. 1996;16:4043–4051. doi: 10.1128/mcb.16.8.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Yang N, Segui P, Evans RM. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–94. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- Giguere V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, Moras D, Renaud JP. Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell. 2002;9:303–313. doi: 10.1016/s1097-2765(02)00444-6. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Heard DJ, Norby PL, Holloway J, Vissing H. Human ERR gamma, a third member of the estrogen receptor- related receptor (ERR) subfamily of orphan nuclear receptors: Tissue-specific isoforms are expressed during development and in the adult. Molecular Endocrinology. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Hentschke M, Susens U, Borgmeyer U. PGC-1 and PERC, coactivators of the estrogen receptor-related receptor gamma. Biochem Biophys Res Commun. 2002;299:872–879. doi: 10.1016/s0006-291x(02)02753-5. [DOI] [PubMed] [Google Scholar]
- Hentschke M, Susens U, Borgmeyer U. Domains of ERRgamma that mediate homodimerization and interaction with factors stimulating DNA binding. Eur J Biochem. 2002;269:4086–4097. doi: 10.1046/j.1432-1033.2002.03102.x. [DOI] [PubMed] [Google Scholar]
- Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274:22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- Horard B, Castet A, Bardet PL, Laudet V, Cavailles V, Vanacker JM. Dimerization is required for transactivation by estrogen-receptor-related (ERR) orphan receptors: evidence from amphioxus ERR. J Mol Endocrinol. 2004;33:493–509. doi: 10.1677/jme.1.01538. [DOI] [PubMed] [Google Scholar]
- Horard B, Rayet B, Triqueneaux G, Laudet V, Delaunay F, Vanacker JM. Expression of the orphan nuclear receptor ERRalpha is under circadian regulation in estrogen-responsive tissues. J Mol Endocrinol. 2004;33:87–97. doi: 10.1677/jme.0.0330087. [DOI] [PubMed] [Google Scholar]
- Huppunen J, Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRgamma. Biochem Biophys Res Commun. 2004;314:964–970. doi: 10.1016/j.bbrc.2003.12.194. [DOI] [PubMed] [Google Scholar]
- Huppunen J, Wohlfahrt G, Aarnisalo P. Requirements for transcriptional regulation by the orphan nuclear receptor ERRgamma. Mol Cell Endocrinol. 2004;219:151–160. doi: 10.1016/j.mce.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Huss JM, Kopp RP, Kelly DP. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J Biol Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- Huss JM, Torra IP, Staels B, Giguere V, Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida M, Nemoto S, Finkel T. Identification of a specific molecular repressor of the peroxisome proliferator-activated receptor gamma Coactivator-1 alpha (PGC-1alpha) J Biol Chem. 2002;277:50991–50995. doi: 10.1074/jbc.M210262200. [DOI] [PubMed] [Google Scholar]
- Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, Kraus RJ, Mertz JE. Estrogen-related receptor alpha 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen- response elements. Mol Endocrinol. 1997;11:342–352. doi: 10.1210/mend.11.3.9897. [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B. Evidence for Ligand-independent Transcriptional Activation of the Human Estrogen-related Receptor {alpha} (ERR{alpha}): CRYSTAL STRUCTURE OF ERR{alpha} LIGAND BINDING DOMAIN IN COMPLEX WITH PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR COACTIVATOR-1{alpha} J Biol Chem. 2004;279:49330–49337. doi: 10.1074/jbc.M407999200. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci U S A. 2003;100:12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutti D, Kaul A, Kralli A. A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol. 2000;20:2411–2422. doi: 10.1128/mcb.20.7.2411-2422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12:360–365. doi: 10.1016/s1043-2760(01)00457-x. [DOI] [PubMed] [Google Scholar]
- Kraus RJ, Ariazi EA, Farrell ML, Mertz JE. Estrogen-related receptor alpha 1 actively antagonizes estrogen receptor-regulated transcription in MCF-7 mammary cells. J Biol Chem. 2002;277:24826–24834. doi: 10.1074/jbc.M202952200. [DOI] [PubMed] [Google Scholar]
- Laganiere J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguere V. A Polymorphic Autoregulatory Hormone Response Element in the Human Estrogen-related Receptor {alpha} (ERR{alpha}) Promoter Dictates Peroxisome Proliferator-activated Receptor {gamma} Coactivator-1{alpha} Control of ERR{alpha} Expression. J Biol Chem. 2004;279:18504–18510. doi: 10.1074/jbc.M313543200. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Gladwell W, Teng CT. Estrogen Stimulates Estrogen-Related Receptor {alpha} Gene Expression Through Conserved Hormone Response Elements. Endocrinology. 2003;144:4894–4904. doi: 10.1210/en.2003-0432. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhang Z, Teng CT. Estrogen-related receptor-gamma and peroxisome proliferator-activated receptor-gamma coactivator-1alpha regulate estrogen-related receptor-alpha gene expression via a conserved multi-hormone response element. J Mol Endocrinol. 2005;34:473–487. doi: 10.1677/jme.1.01586. [DOI] [PubMed] [Google Scholar]
- Maehara K, Hida T, Abe Y, Koga A, Ota K, Kutoh E. Functional interference between estrogen-related receptor alpha and peroxisome proliferator-activated receptor alpha/9-cis-retinoic acid receptor alpha heterodimer complex in the nuclear receptor response element-1 of the medium chain acyl-coenzyme A dehydrogenase gene. J Mol Endocrinol. 2003;31:47–60. doi: 10.1677/jme.0.0310047. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, Castoria G, de Falco A, Di Domenico M, Galdiero M, Nola E, Chambon P, Auricchio F. In vitro phosphorylation and hormone binding activation of the synthetic wild type human estradiol receptor. J Steroid Biochem Mol Biol. 1991;38:407–413. doi: 10.1016/0960-0760(91)90328-3. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Handschin C, Arlow D, Xie X, St Pierre J, Sihag S, Yang W, Altshuler D, Puigserver P, Patterson N, Willy PJ, Schulman IG, Heyman RA, Lander ES, Spiegelman BM. Err{alpha} and Gabpa/b specify PGC-1{alpha}-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci U S A. 2004;101:6570–6575. doi: 10.1073/pnas.0401401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- Razzaque MA, Masuda N, Maeda Y, Endo Y, Tsukamoto T, Osumi T. Estrogen receptor-related receptor gamma has an exceptionally broad specificity of DNA sequence recognition. Gene. 2004;340:275–282. doi: 10.1016/j.gene.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Kim JY, Kim HJ, Takeda J, Lee YK, Moore DD, Choi HS. Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. J Biol Chem. 2002;277:1739–1748. doi: 10.1074/jbc.M106140200. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Matthews J, Bouton D, Kim HJ, Choi HS, Treuter E, Gustafsson JA. Deoxyribonucleic acid response element-dependent regulation of transcription by orphan nuclear receptor estrogen receptor-related receptor gamma. Mol Endocrinol. 2004;18:312–325. doi: 10.1210/me.2003-0165. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor ERRalpha. J Biol Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen-related receptor {alpha} (ERR{alpha}) functions in PPAR{gamma} coactivator 1{alpha} (PGC-1{alpha})-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101:6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Shigeta H, Yang N, Fu K, O’Brian G, Teng CT. Human estrogen receptor-like 1 (ESRL1) gene: genomic organization, chromosomal localization, and promoter characterization. Genomics. 1997;44:52–60. doi: 10.1006/geno.1997.4850. [DOI] [PubMed] [Google Scholar]
- Shigeta H, Zuo W, Yang N, DiAugustine R, Teng CT. The mouse estrogen receptor-related orphan receptor alpha 1: molecular cloning and estrogen responsiveness. J Mol Endocrinol. 1997;19:299–309. doi: 10.1677/jme.0.0190299. [DOI] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguere V. The orphan nuclear receptor estrogen-related receptor alpha is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol. 1997;17:5400–5409. doi: 10.1128/mcb.17.9.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugi M, Su L, Karlsberg K, Yuan YC, Chen S. Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003;1:981–991. [PubMed] [Google Scholar]
- Teng CT, Walker MP, Bhattacharyya SN, Klapper DG, DiAugustine RP, McLachlan JA. Purification and properties of an oestrogen-stimulated mouse uterine glycoprotein (approx. 70 kDa) Biochem J. 1986;240:413–422. doi: 10.1042/bj2400413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay GB, Kunath T, Bergeron D, Lapointe L, Champigny C, Bader JA, Rossant J, Giguere V. Diethylstilbestrol regulates trophoblast stem cell differentiation as a ligand of orphan nuclear receptor ERR beta. Genes Dev. 2001;15:833–838. doi: 10.1101/gad.873401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker JM, Bonnelye E, Chopin-Delannoy S, Delmarre C, Cavailles V, Laudet V. Transcriptional activities of the orphan nuclear receptor ERR alpha (estrogen receptor-related receptor-alpha) Mol Endocrinol. 1999;13:764–773. doi: 10.1210/mend.13.5.0281. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Murray IR, Qian J, Busch BB, Stevens WC, Jr, Martin R, Mohan R, Zhou S, Ordentlich P, Wei P, Sapp DW, Horlick RA, Heyman RA, Schulman IG. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc Natl Acad Sci U S A. 2004;101:8912–8917. doi: 10.1073/pnas.0401420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, Evans RM. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol. 1999;13:2151–2162. doi: 10.1210/mend.13.12.0381. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhou D, Chen S. Modulation of aromatase expression in the breast tissue by ERR alpha-1 orphan receptor. Cancer Res. 1998;58:5695–5700. [PubMed] [Google Scholar]
- Yang C, Chen S. Two organochlorine pesticides, toxaphene and chlordane, are antagonists for estrogen-related receptor alpha-1 orphan receptor. Cancer Res. 1999;59:4519–4524. [PubMed] [Google Scholar]
- Yang N, Shigeta H, Shi H, Teng CT. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem. 1996;271:5795–5804. doi: 10.1074/jbc.271.10.5795. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teng CT. Estrogen receptor-related receptor alpha 1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem. 2000;275:20837–20846. doi: 10.1074/jbc.M001880200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Teng CT. Estrogen receptor alpha and estrogen receptor-related receptor alpha1 compete for binding and coactivator. Mol Cell Endocrinol. 2001;172:223–233. doi: 10.1016/s0303-7207(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen K, Shih JC, Teng CT. Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol Endocrinol. 2006;20:1547–1561. doi: 10.1210/me.2005-0252. [DOI] [PubMed] [Google Scholar]
- Zuercher WJ, Gaillard S, Orband-Miller LA, Chao EY, Shearer BG, Jones DG, Miller AB, Collins JL, McDonnell DP, Willson TM. Identification and structure-activity relationship of phenolic acyl hydrazones as selective agonists for the estrogen-related orphan nuclear receptors ERRbeta and ERRgamma. J Med Chem. 2005;48:3107–3109. doi: 10.1021/jm050161j. [DOI] [PubMed] [Google Scholar]


