Abstract
Background
Increased activity of single ventricular L-type Ca2+-channels (L-VDCC) is a hallmark in human heart failure. Recent findings suggest differential modulation by several auxiliary β-subunits as a possible explanation.
Methods and Results
By molecular and functional analyses of human and murine ventricles, we find that enhanced L-VDCC activity is accompanied by altered expression pattern of auxiliary L-VDCC β-subunit gene products. In HEK293-cells we show differential modulation of single L-VDCC activity by coexpression of several human cardiac β-subunits: Unlike β1 or β3 isoforms, β2a and β2b induce a high-activity channel behavior typical of failing myocytes. In accordance, β2-subunit mRNA and protein are up-regulated in failing human myocardium. In a model of heart failure we find that mice overexpressing the human cardiac CaV1.2 also reveal increased single-channel activity and sarcolemmal β2 expression when entering into the maladaptive stage of heart failure. Interestingly, these animals, when still young and non-failing (“Adaptive Phase”), reveal the opposite phenotype, viz : reduced single-channel activity accompanied by lowered β2 expression. Additional evidence for the cause-effect relationship between β2-subunit expression and single L-VDCC activity is provided by newly engineered, double-transgenic mice bearing both constitutive CaV1.2 and inducible β2 cardiac overexpression. Here in non-failing hearts induction of β2-subunit overexpression mimicked the increase of single L-VDCC activity observed in murine and human chronic heart failure.
Conclusions
Our study presents evidence of the pathobiochemical relevance of β2-subunits for the electrophysiological phenotype of cardiac L-VDCC and thus provides an explanation for the single L-VDCC gating observed in human and murine heart failure.
Introduction
Homeostasis of intracellular Ca2+ concentration [Ca2+]i is essential for cardiac function and integrity; its dysregulation is a hallmark of advanced heart failure [1], [2]. Voltage-dependent L-type Ca2+-channels (L-VDCCs) are the source of trigger Ca2+ entering cardiomyocytes [3]. Data derived from numerous studies support an involvement of L-VDCC in pathological changes of [Ca2+]i in heart failure. Although still controversial, L-VDCC current density appears unchanged in failing cardiomyocytes [1], [4], [5]. Whole-cell currents are determined by a number of parameters, including number of channels, single-channel current amplitude and time spent in the open state. Therefore, altered number of active channels or activity of individual L-VDCC is not necessarily reflected by calcium current density. In fact, despite no change in whole-cell L-VDCC density (ICa), single-channel activity was significantly increased in ventricular myocytes from human end-stage failing hearts [6]. Chen et al. [7] showed attenuated ICa increase by (S)-BayK8644 in human failing myocardium whereas basal whole-cell currents were unchanged, indicating that single-channel activity is enhanced while channel density is lowered. These findings confirm the idea of an “electrophysiological heart-failure phenotype” of single L-VDCCs. The biochemical nature of this change in phenotype has not been delineated, although phosphorylation [8], [9] and dephosphorylation [10], [11] have been implicated. Activities of kinases and phosphatases not only change channel function but interfere with neurohumoral modulation of the L-VDCC; e.g. β-adrenergic regulation is blunted in heart failure possibly due to hyperphosphorylation of L-VDCCs [6], [7]. Using heterologous recombination we have shown that distinct subunit compositions of L-VDCC induce single-channel characteristics similar to the biophysical phenotype of “hyperphosphorylated” L-VDCC [12]. The latter suggests that changes in gene expression of L-VDCC subunits may form the basis of a heart-failure phenotype of L-VDCC. In mammalian hearts L-VDCCs are composed of an ion conducting pore (CaV1.2 or α1C), and two auxiliary subunits, an α2δ and a β-subunit. Most investigators agree that β-subunit diversity is of physiological and pathophysiological importance [13]–[18]. In fact, some studies have revealed altered β-subunit patterns in human heart failure [19], [20], suggesting that an altered β-subunit expression pattern is of functional relevance. Delineation of pathophysiological mechanisms in human heart is difficult because of wide inter-individual variance, including age, medication, state of disease etc. Human tissue also offers a limited choice of truly independent variables, such as time, disease stage and treatment options. Animal models offer control of any relevant factor to test pathophysiological concepts. We analyzed β-subunit gene expression in both human non-failing and failing hearts as well as in transgenic mice overexpressing the human CaV1.2 (α1C) subunit (tg CaV1.2). The latter was chosen because of phenotypical characteristics common with human heart failure, e.g. early blunting of β-adrenergic signaling, slow progression towards hypertrophy and calcium overload in failing myocytes [21], [22]. Most importantly, in young (non-failing; “Adaptive State”) tg CaV1.2 mice we previously found concordance of lowered β2-subunit expression and decreased activity of single L-VDCC [16]. In the present study we find an increase of single L-VDCC activity accompanied by enhanced expression of β2-subunits when these mice have entered the failing state (“Maladaptive State” ≥9 months of age). By examination of a new, double-transgenic mouse bearing both constitutive CaV1.2 and inducible β2-subunit overexpression in the heart we show a relationship between subunit expression and channel function.
Results
Gating parameters of single L-VDCC in failing human and transgenic myocardium
Single-channel activity of old (≥9 months, and in heart failure) tg CaV1.2 was significantly increased compared to young (4 months, no hypertrophy or heart failure) tg CaV1.2 [16] (Table 1). Henceforth, these animals are referred to as “young” and “old” tg CaV1.2, respectively. Peak ensemble average current (I peak) in old tg CaV1.2 mice was enhanced (−56±14 fA vs. −23±7 fA, p<0.05) due to an increased fraction of active sweeps, mean open time, and mean open probability, and a decrease of mean closed time (tclosed). Of further interest, the changes of peak current, fraction of active sweeps and open probability mirror findings obtained from single L-VDCC measurements in human cardiomyocytes from non-failing or failing idiopathic dilated cardiomyopathy (DCM) hearts, respectively [6] (Table 1).
Table 1. Single L-VDCC gating of young and old tg CaV1.2 resembles data obtained from human non-failing and failing ventricle.
| gating parameter | tg CaV1.2 | tg CaV1.2 | human LV | human LV |
| 4 months | ≥9 months | non-failing | failing (DCM) | |
| peak current Ipeak [fA] | −23±7 | −56±14* | −13±5 | −28±5* |
| fraction of active sweeps factive [%] | 53.7±5.3 | 71.7±8.2(*) | 26.4±5.3 | 56.7±8.0* |
| open probability Popen [%] | 4.4±1.2 | 6.4±0.9† | 3.2±1.3 | 6.1±1.6† |
| mean open time topen [ms] | 0.34±0.03 | 0.45±0.04* | 0.54±0.05 | 0.65±0.04 |
| mean closed time tclosed [ms] | 6.6±1.0 | 3.1±0.7* | 9.3±1.6 | 7.5±1.7 |
| number of experiments | 13 (13) | 11 (5) | 16 (12) | 9 (6) |
In a previous study [6] we found single-channel activity to be significantly increased in ventricular myocytes from human hearts failing due to idiopathic dilated cardiomyopathy compared to non-failing ventricles. In excellent agreement the present study reveals activity of single L-VDCC from ≥9 months old, i.e. failing murine hearts overexpressing the human CaV1.2 to be significantly increased compared to single-channel activity in 4 months old, i.e. non-failing young transgenics obtained in a previous study [16]. Charge carrier: 70 mM Ba2+; holding potential: −100 mV; test potential: +20 mV. Note that Schroder et al. [6] did not use a depolarizing bath solution, thus potentials are approximate values.
p<0.05 and (*)p = 0.07 in a Student's t-test; †p<0.05 in a Mann-Whitney test (performed when data failed normality test). Numbers of experiments given in parentheses indicate number of experiments with only one channel in the patch.
L-VDCC subunit expression in non-failing and failing human hearts
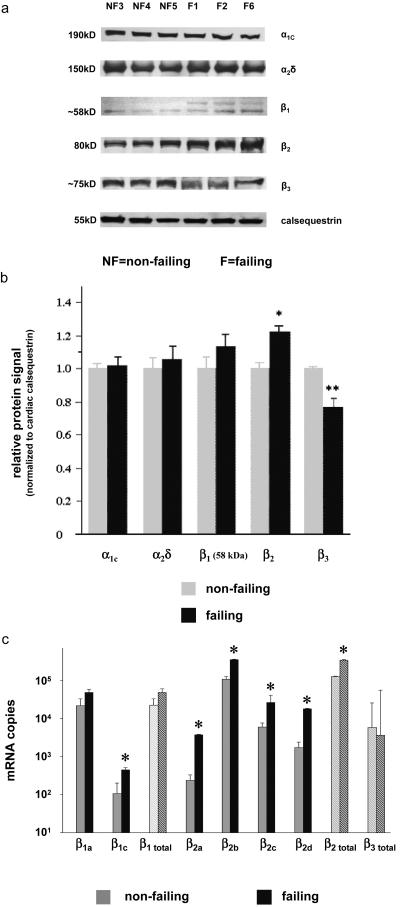
Protein expression of CaV1.2, α2δ, low molecular weight β1, and β3 was similar in non-failing and failing human myocardium, but we found a significant up-regulation of β2 (Figure 1a,b). There was no difference in gene expression of the CaV1.2, and the α2δ at the protein level (mRNA data not shown). At least two β1-subunit isoforms (β1a,c), four β2-subunit isoforms (β2a–d), and two β3-subunit isoforms (β3a,trunc) are expressed at relevant levels in human myocardium [12]. β1a (GenBank No NM_199247) and β1c (GenBank_199248) are sequence-identical except for replacement of exon 7a by exon 7b in β1c, consistent with previous work [23]. β2a–d isoforms differ only with respect to the N-terminal region (D1 domain). Quantitation by real-time PCR revealed an increased expression of β1c and all β2 isoforms in heart failure, in line with the protein data (Figure 1c).
Figure 1. Subunit expression of cardiac L-VDCC subunits in human myocardial specimens.
(a) Human specimens from non-failing (NF) and failing (F) myocardium (n = 4–5) were analyzed in immunoblots using specific polyclonal antibodies directed against the particular L-VDCC subunits. (b) L-VDCC subunit expression was normalized to cardiac calsequestrin protein expression in the same sample (number of NF/F specimens was always identical for each subunit; n = 5–8). Quantitative analysis of subunit protein expression is depicted as ratio of F vs. NF. * p<0.001; ** p<0.0001. (c) mRNA expression of β-subunit isoforms (NF: n = 5; F: n = 9–13) was measured by real time PCR, and always normalized to cardiac calsequestrin mRNA expression. * p<0.05.
L-VDCC subunit protein expression in old wild type and old tg CaV1.2 hearts
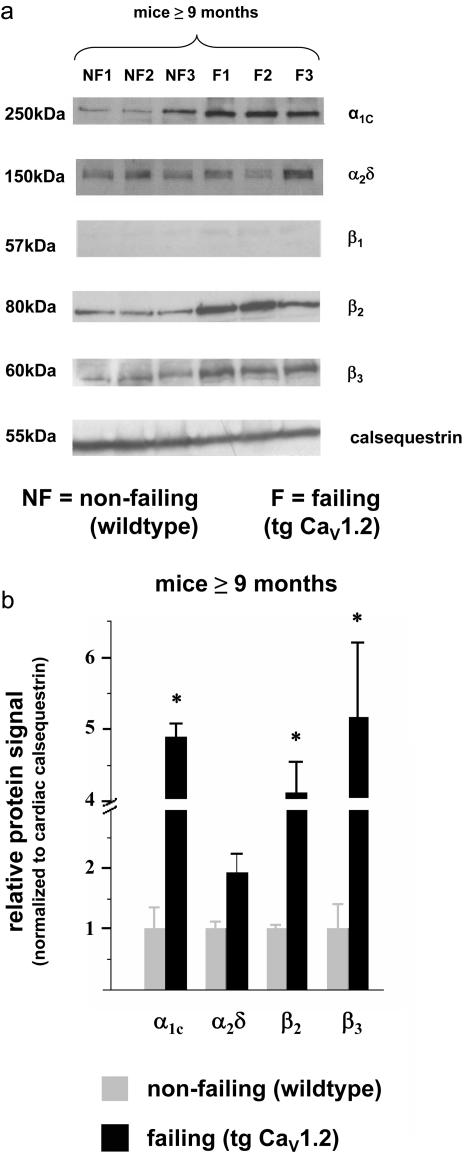
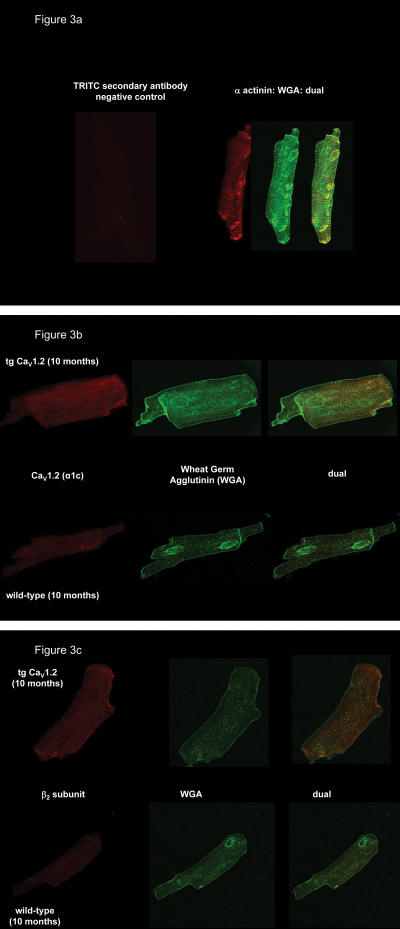
β1, β2 and β3 isoforms are expressed at the protein level in old CaV1.2 mouse heart, although expression of β1-subunit isoforms was faint. Compared to old wild type mice (≥9 months), the old tg CaV1.2 showed a significant up-regulation of β3-, and β2-subunits (Figure 2), in striking contrast to the down-regulation of β2-subunits we previously observed in young tg CaV1.2 [16]. In the old tg CaV1.2 mouse myocytes, up-regulated β2-subunits and overexpressed CaV1.2 both localize to the surface sarcolemma and the T-tubules (Figure 3), suggesting the functional relevance of altered expression levels.
Figure 2. Protein expression of cardiac L-VDCC subunits in old wild-type and tg CaV1.2 mice.
(a) Specimens from old wild-type mice and tg CaV1.2 in heart failure were analyzed in immunoblots using specific polyclonal antibodies directed against the particular L-VDCC subunits.
(b) Protein expression of L-VDCC subunits was always normalized to cardiac calsequestrin protein expression in the same sample. Quantitative analysis of subunit protein expression is depicted as ratio of 10 months old tg CaV1.2 vs. age-matched wild-type. β1 protein bands were faint, and thus not analyzed quantitatively (number of WT/old tg CaV1.2 specimens was always identical for each subunit; n = 4). * p<0.05.
Figure 3. Immunocytochemistry shows increased sarcolemmal expression of CaV1.2 and β2-subunits in ventricular myocytes from old tg CaV1.2 mice.
(a) Immunocytochemistry of cardiomyocytes isolated from 10 months old wild-type or tg CaV1.2 mice. Controls show no specific interaction for TRITC labeled secondary antibody and labeling of sarcolemma, T-tubules and intercalated discs by Oregon Green 488-conjugated wheat germ agglutinin (WGA).
(b,c) Primary antibodies directed against CaV1.2 and β2-subunits, respectively, demonstrate predominant t-tubular localization of the respective antigens in old wild-type and tg CaV1.2.
β-subunit-dependent modulation of CaV1.2 expressed in HEK293
The diversity of β-subunit expression patterns found in cardiomyocytes necessitated the functional characterization of each β-subunit isoform. Using HEK293 cells constitutively expressing the human CaV1.2 as a homologous recombination system we show that the gene as well as alternative splicing determines calcium channel gating, extending and elaborating on previous work [12]. This is highlighted by significant differences in peak current and open probability between β-subunits (Table 2), with β2a and β2b exerting the strongest effects. β2c or β2d as well as β1a, β1c, and β3a induced a minor to moderate increase in single-channel activity with no significant effects detected for closed times. Taken together, the data support the view that the single-channel phenotype of failing cardiomyocytes is caused by channel complexes containing β2a or β2b.
Table 2. Gating of single recombinant human CaV1.2 in HEK293 cells is differentially modulated by several coexpressed human cardiac auxiliary β-subunits.
| gating parameter | β1a | β1c | β2a | β2b | β2c | β2d | β3a | control |
| peak current Ipeak [fA] | −36±12 | −22±5 | −109±25* | −123±28* | −24±7 | −37±16 | −46±14* | −12±2 |
| fraction of active sweeps factive [%] | 53.3±10.5 | 32.5±5.2 | 57.6±6.6 | 58.7±9.1 | 45.5±14.6 | 64.1±8.2 | 48.9±6.4 | 46.9±6.3 |
| open probability Popen [%] | 2.1±0.6 | 2.4±0.5* | 6.9±1.4* | 8.3±1.9* | 1.8±0.6 | 3.2±1.3 | 2.8±0.8(*) | 1.1±0.2 |
| mean open time topen [ms] | 0.34±0.09 | 0.28±0.02* | 0.31±0.02* | 0.34±0.03* | 0.23±0.03 | 0.26±0.01* | 0.30±0.03* | 0.22±0.01 |
| mean closed time tclosed [ms] | 11.5±1.7 | 15.8±5.3 | 7.0±0.5* | 4.7±1.0* | 10.8±2.6 | 11.4±1.7 | 12.1±2.9 | 12.4±1.3 |
| number of experiments | 10 (6) | 11 (4) | 14 (6) | 16 (8) | 5 (4) | 9 (6) | 10 (8) | 8 (5) |
Single-channel gating parameters of human cardiac CaV1.2 stably expressed in HEK293 cells transiently cotransfected with several auxiliary β-subunits and a human cardiac α2δ-2-subunit. Coexpression with β2a and β2b reveals strongest stimulation of single channels compared to control cells without transfection of any β-subunit (ctr). Data were obtained by patch-clamp recordings using cell-attached configuration (charge carrier: 110mM Ba2+; holding potential: −100 mV; test potential: +10 mV for 150 ms). *p<0.05 and (*)p = 0.07 in Bonferroni-corrected post-hoc tests versus control, following one-way ANOVA. Numbers of experiments given in parentheses indicate number of experiments with only one channel in the patch.
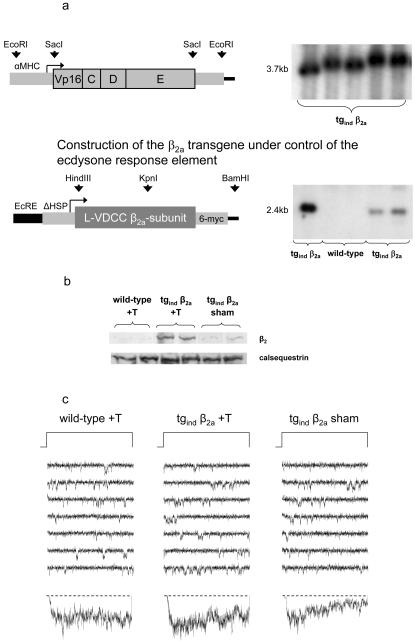
Generation of an inducible, heart-specific β2a-subunit overexpression mouse (tgind β2a)
Our functional analyses support the idea of pathophysiological relevance of β2-subunit up-regulation, but the parallel biophysical and biochemical changes in cardiomyocytes may still be coincidental. Rather than following the natural course of gene expression changes, transgene-controlled β2-subunit overexpression should prove its causative role in native tissue. A hybrid drosophila-bombyx ecdysone receptor (VgBmEcR) when coupled to an αMHC promoter should combine strictly drug-controlled, transgene-specific, and cardiac tissue-specific gene induction. We generated this double transgenic model of αMHC VgBmEcR and the (rat) β2a gene, hence referred to as tgind β2a, under control of the ecdysone response element (Figure 4a). Mice carrying both transgenic constructs developed normally and did not show any signs of developmental or cardiac dysfunction. In vivo induction with tebufenozide clearly increased cardiac β2-subunit expression in tgind β2a mice at the protein level (Figure 4b) proofing functionality of drug-controlled gene expression. However, the single L-VDCC phenotype after induction was not altered in this mouse when compared with either tebufenozide treated wild-type mice or sham-induced tgind β2a (Figure 4c, Figure 5a–e).
Figure 4. tg mouse model with an inducible cardiac overexpression of the β2a under control of a hybrid bombyx-ecdysone receptor.
(a) For cardiac-specific expression the hybrid bombyx-ecdysone receptor (VgBmEcR) was placed under the control of αMHC promoter (for details see Materials and Methods). Transgenic mice (tgind β2a) positive for the hybrid bombyx-ecdysone receptor and the construct of the ecdysone response element (EcRE) and the β2a, respectively, are identified in Southern blots. The radiolabeled probe specific for the coding sequence of VgBmEcR was generated by SacI digestion. It hybridized to a 3.7 kb band in transgene mouse genomic DNA digested by EcoRI digest; the radiolabeled DNA probe specific for the coding sequence of β2a was generated by HindIII/KpnI digest. It hybridized to a 2.4 kb band of genomic DNA digested with HindIII/BamHI in tgind β2a but not in WT.
(b) 48 h after treatment with the inducing drug tebufenozide (+T) Western-blot analysis with ventricular tissue from 4–5 month old mice reveals increased expression of β2-protein in tgind β2a compared to treated wild-type or sham-induced transgenics.
(c) Exemplary traces of single-channel recordings from murine ventricular myocytes. Induction of cardiac overexpression of the β2a (+T) does not alter single-channel behavior compared to either wild-type mice after treatment with the inducing drug or i.p-application of only the vehicle (water/oil-emulsion) to β2a-transgenic mice (“sham”). Data were obtained by patch-clamp recordings using cell-attached configuration (charge carrier: 70 mM Ba2+; holding potential: −100 mV; test potential: +20 mV for 150 ms). Bottom traces show ensemble average currents from the respective experiment.
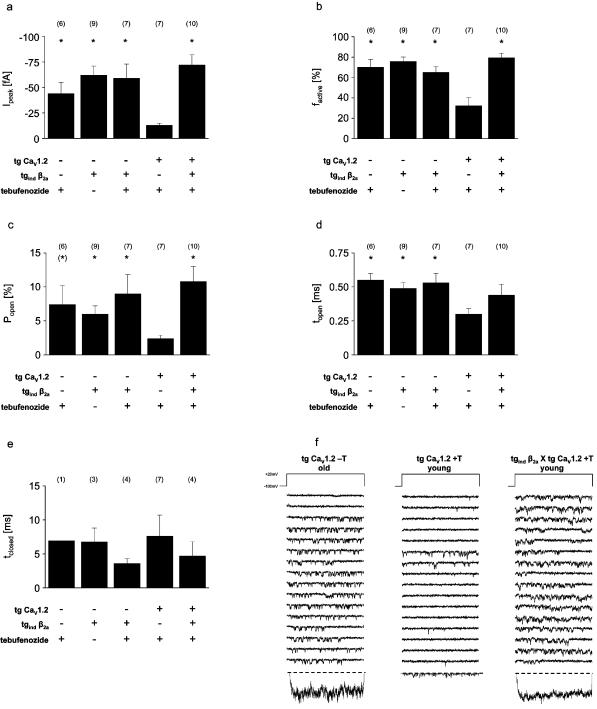
Figure 5. Gating of single L-VDCC in ventricular myocytes from mice showing cardiac overexpression of Ca2+-channel subunits.
(a–e) Single-channel gating parameters of ventricular L-VDCC from murine hearts. Compared to 4–5 months old mice showing a cardiac overexpression of the human CaV1.2 (tg CaV1.2), the inducing compound tebufenozide (T) significantly increased single L-VDCC activity in ventricular myocytes from age-matched double-transgenics (tg Cav1.2×tgind β2a, showing an additional inducible β2a-overexpression) 48 h after drug administration. Overexpression of the β2a-subunit without overexpression of the human Cav1.2 does not alter single-channel gating (cp. Figure 4c). Tebufenozide treatment does not affect single-channel gating in ventricular myocytes from wild-type mice. Data were obtained by patch-clamp recordings using cell-attached configuration (charge carrier: 70 mM Ba2+; holding potential: −100 mV; test potential: +20 mV for 150 ms). *p<0.05 and (*)p<0.09 compared to tg Cav1.2 in Student's t-test. Number of underlying experiments is given in parentheses.
(f) Exemplary traces of single-channel recordings from murine ventricular myocytes. Activity of single L-VDCC is clearly higher in old (≥9 months, failing) tg CaV1.2 compared to channels from young (4–5 months, non-failing) tg CaV1.2. Induction of β2-overexpression in hearts of young tgind β2a×tg CaV1.2 by tebufenozide mimicks the heart-failure phenotype of L-VDCC gating otherwise not observed until tg CaV1.2 enter the “Maladaptive State” at an age ≥9 months. T = tebufenozide. Data were obtained by patch-clamp recordings using the cell-attached configuration (charge carrier: 70 mM Ba2+; holding potential: −100 mV; test potential: +20 mV for 150 ms). Bottom traces show average currents from the respective experiment.
Characterization of L-VDCC activity in double transgenic (tg CaV1.2×tgind β2a) mice
Adaptive down-regulation of β2-subunit expression in the young tg CaV1.2 [16] sets the stage for analysis of a β2-subunit increase in native tissue. For this we crossbred tg CaV1.2 mice and tgind β2a mice. Consistent with the hypothesis of a functional predominance of β1-subunits in the young tg CaV1.2 mice, (N.B.: These animals are “Adaptive”, and in fact demonstrate hypercontractility, without failure) activity of single-channels was dramatically increased by β2-subunit induction in tg CaV1.2×tgind β2a 48 hours after induction (Figure 5) (e.g. peak current: −72±10 fA vs. −13±2 fA in tg CaV1.2 likewise treated with tebufenozide, p<0.05). This is evidence for our proposed relationship of structure and function of L-VDCC, at the single channel level, in ventricular myocytes ex vivo.
Discussion
The major, novel result of our study is the concomitant increase in β2-subunit expression and L-VDCC activity in three independent models: human dilated cardiomyopathy, old tg CaV1.2 mice spontaneously progressing into heart failure and young (“Adaptive State”) tg CaV1.2 mice with additional tissue-specific inducible overexpression of β2-subunits. We explain our results by the differential effects of the β-subunits, namely β2a and β2b as the most important modulators in recombinant assays.
The three β-subunits (β1–3) thus far known to be expressed in mammalian hearts have vastly different effects on current density [17], [24], kinetics [12], [24], and single-channel properties [12], [14], [17]. Beyond that, some β2-subunit isoforms have been implicated in mediating membrane targeting [3], [25], cardiomyocyte apoptosis [26], cell death [17], adrenergic regulation [27], [28] and modulation of CaM Kinase II [29], [30]. It is of interest that the pattern of auxiliary subunits [3], including the prevalence of β-subunit isoforms varies among species, with β2-subunits predominating in small rodents like rats [17], and mice [31], and additional relevant expression of β1- and β3-subunits in humans [13], [19], [23].
In our study the homologous recombination of human L-VDCC subunits indicates the biophysical relevance of β2a and β2b isoforms for up-regulation of single L-VDCC activity. The increase of β2-subunit gene expression in human heart failure suggests that these L-VDCC subunits form the basis of the ”heart-failure phenotype” of single L-VDCC found in human hearts [6]. Young tg CaV1.2 animals were chosen as a known example of dynamic adaptation of β2-subunit expression in heart. The functional relevance of this adaptation is illustrated by whole-cell current density, that is increased by only 50% [22], [32], while CaV1.2 protein expression and density of single channels show a 3-fold increment in these transgenic hearts [16], [32]. This apparent discrepancy is explained by an up-regulation of β1- but substantial down-regulation of the β2-subunit expression [16]. This gene expression is characteristic for the “Adaptive phase” of the model [3], [22] putatively limiting calcium overload at the younger ages. We now demonstrate that when this tg Cav1.2 mouse enters into the “Maladaptive state” with overt heart failure at ≥9 months of age, both single L-VDCC activity and β2-subunit expression increase, mimicking alterations of channel structure and biophysics in terminal human heart failure. Thus, the old tg CaV1.2 mouse may be regarded as a heart-failure model in which a primary calcium (current) overload can no longer be effectively counterbalanced by adaptive mechanisms, i.e. β-subunit expression. The transcriptional mechanisms underlying this bidirectional control of β2-subunit expression, however, remain to be elucidated in the context of changes in β1 and β3 subunit expression in human and old tg CaV1.2 mouse heart failure.
As a novel and first approach to induce an increased β2-subunit overexpression in intact animals, rather than in isolated cells [17], [24], [26] we generated a mouse model of cardiospecific inducible β2a-subunit expression (tgind β2a). Induction of β2-overexpression in this mouse model did not affect overall single L-VDCC gating significantly. As a more recent study indicates that an 1:1-stoichiometry of pore-forming α1- and auxiliary β-subunit may be sufficient for modulation of channel gating [33], we assume that most calcium channel pores are saturated with native β-subunits in the induced tgind β2a. However, mean closed time was lower in induced tgind β2a suggesting that a portion of overexpressed β2a exerts functional action similar to the recombinant channel data presented. To prove our concept that β2-subunit expression underlies the activity of single L-VDCC of the heart-failure phenotype we crossbred tgind β2a with tg CaV1.2 mice [21], [22]. Induction of β2a-subunit gene expression in the young double tg mice (tg CaV1.2×tgind β2a) led to a premature increase of single L-VDCC activity. This confirms our theory, derived from recombinant channel data, in the relevant tissue ex vivo.
Such deliberate overexpression of β2-subunit in vivo, when carried forward in a chronic manner, hopefully will pave the way for understanding the progression of heart failure if these alterations in single L-VDCC gating lead to decompensation at an earlier age of the animal. This knowledge will have direct implications because pharmacological agents which modulate L-VDCC function are in everyday clinical practice and have been shown to be beneficial in various clinical trials targeting different populations [34], [35]. We wish to emphasize that, at this point in our studies, we show a relationship between electrophysiological parameters that is consistent with heart failure. In order to prove this, it is necessary to chronically imbue the young animals with a heart-specific increase in the β2 subunit and follow their transition to heart failure at specific age points as they mature. These experiments are ongoing but will require considerable time.
Materials and Methods
Materials
Non-failing and failing human left ventricular specimens were obtained from explanted hearts not transplanted for technical reasons (n = 5), or from orthotopic heart transplantation recipients (n = 8). Heart failure patients were in NYHA class III–IV (17–63 y, 3 females) mean duration of symptomatic heart failure ranged from 9–60 months, peak oxygen exercise capacity was 13.3–15.5 ml kg−1 min−1 at time of listing. Heart transplant recipients were ambulatory at time of operation and received treatment with inhibitors of the angiotensin-system (100%), β-blockers (75%), aldosterone antagonist (50%), diuretics (62.5%).
Animals
Mice with cardiac-specific heterozygous overexpression of the human Cav1.2 [21] were bred with non transgenic littermates. These animals were bred with the tgind β2a as described below. Non transgenic littermates served as WT controls in this study.
The study was approved by the local institutional committees.
Quantitative analysis of L-VDCC subunit mRNA expression in human myocardium
Real-time PCR:
Quantitation was performed with the iCycler iQ real-time PCR detection system using primer/fluorescent probe concentrations of 200 nmol/L either in 1× iQ Supermix (β2a–d) or 1× iQ SyBr Green when no fluorescent probes was used (β1a,b, β3, cardiac calsequestrin) (all Bio-Rad®). Both iQ Supermix and SyBr Green based real-time PCR were followed by gel electrophoresis confirming amplification of singular products of expected size. In addition, SyBr Green based real RT-PCR was followed always by melt-curve analysis (70°C–94°C at 0.5°C steps) verifying the similarity of standard and specimen amplification product melting point. Quantitation was performed using intra-assay standard curves of the specific templates. Templates were cloned from human non-failing left ventricle mRNA reversely transcribed by iScript (Biorad®), identity to published sequences was confirmed at both strands.
Human β1 template:
PCR-cloning by sense primer 5′-CTCAAGGGCTACGAGGTTAC-3′ and antisense primer 5′-GTGTTTGGACTGAGACTTTCC-3′ (GenBank NM_000723; resp. positions: 864-883, and 1247-1227), with 94°C (3 min), and 40 cycles of 51°C (30 sec), 72°C (20 sec), and 94°C (20 sec). Real-time PCR was set up with the cloning primers and run at 94°C (3 min), and 40 cycles of 50°C (30 sec), 72°C (20 sec), and 94°C (20 sec). Correlation: ≥0.992, efficiency: ≥93.4%.
Human β1 isoform templates:
PCR cloning of the β1a by sense primer 5′-GCCTCGGCTCCAGCAAA-3′ and antisense primer 5′-CTCACCAAGCTCAGCCTCTTC-3′ (GenBank NM_199247; resp. positions 692-708; 855-835), of the β1c by the same sense but different antisense primer 5′-CTCTGTCGACTTCTGCTTCTGTTT-3′ (GenBank NM_000723; position 807-784), with 94°C 3 min, 40 cycles 56°C (30 sec), 72°C (20 sec), 94°C (20 sec). Real-time PCR was set up with the respective cloning sense primer and antisense primers, and the fluorescent probe 5′-6FAM-CTCCAGTTCCAGTCTGGGAGATGTGGT XT p (GenBank NM_000723; 720-746) and run at 94°C (3 min), and 40 cycles of 53°C, and 94°C (each 30 sec). Correlation: ≥0.990, efficiency: ≥90.6%.
Human β2a–d isoform templates:
PCR cloning of β2a–d isoforms was by isoform specific sense primers:
β2a: 5′-GCATCGCCGGCGAGTA-3′, (GenBank423189, position: 21-36);
β2b: 5′-GACAGACGCCTTATAGCTCCTCAA-3′ (GenBank AF285239; position: 7-30);
β2c: 5′-AGTGGACTGGACCTGCTGAA-3′ (GenBank AF423190; position: 13-32);
β2d: 5′-GCCGCCGCACAGTCATAT-3′ (GenBank AF423191; position: 109-126);
always with the same antisense primer 5′-CGGTCCTCCTCCAGAGATACAT-3′ (GenBank AF423189; position: 109-89). Real-time PCR was set up with the respective cloning primers, and the fluorescent probe 5′-6FAM-ATGGACGGCTAGTGTAGGAGTCTGCCGA XT p (GenBank NM_000723; position: 79-52) and run at 94°C (3 min), and 40 cycles of 56.5°C, and 94°C (each 30 sec). Correlation was ≥0.995, efficiency ≥90.4%.
Template of human β3 and human cardiac calsequestrin:
see [12].
Cloning of human β1-, β2-, β3-splice variants and insertion into bicistronic eukaryotic expression vectors
β1-subunits:
Full length β1-subunit isoform sequences were cloned using two pairs of sequence specific primers: 1st sense primer 5′-CCTCTCCATGGTCCAGAAGACCAGCA-3′ and 1st antisense primer 5′-CAAATAAAGCTTTCTGCATCATGTCTGTAA-3′, and 2nd sense primer 5-TTACAGACATGATGCAGAAAGCTTTATTTG-3′ and 2nd anti-sense primer 5′-GCGCCCACTACATGGCATGTTCCT-3′ (GenBank NM_199247; resp. positions: 147-173; 1048-1019; 1019-1048; 1732-1709). PCR with the 1st primer pair yielded two amplification products due to alternative splicing of exon 7a and exon 7b. Full length message of β1A with either exon 7a or 7b was reassembled in pIRES2-EGFP opened with BglII/SmaI site using the internal HindIII restriction site.
β2-subunits:
Full length β2-subunit isoform sequences were cloned using two pairs of sequence specific primers derived from GenBank sequences. N-terminal coding sequences for β2-subunit splice variants were generated using isoform specific primer pairs. β2a: sense primer 5′-CTCTTCATGCAGTGCTGCGGGCTGGT-3′ and antisense primer 5′- ACTTCCGCTAAGCTTGACCTTGTG-3′ (GenBank U95019; resp. positions: 496-521, 1397-1374); β2b: sense primer 5′-ATGCTTGACAGACGCCTTATAGCT-3′ and same antisense primer (GenBank AF285239; resp. positions: 1-24, 899-876); β2c: sense primer 5′-ATGAATCAGGGGAGTGGACTGGAC-3′ and same antisense primer (GenBank AF423190; resp. positions: 1-24, 977-894); β2d: sense primer 5-ATGGTCCAAAGGGACATGTCCAAG-3′ and same antisense primer (GenBank AF423191; resp. positions: 1-24, 1061-1038). The C-terminal fragment was amplified by sense primer 5′-CACAAGGTCAAGCTTAGCGGAAGT-3′ and antisense primer 5′-GGCAAAACTCATTGGGGGAT-3′ (GenBank U95019; resp. positions: 1374-1397, 2327-2308). Amplification was performed in cDNA reverse transcribed (Revert Aid Kit, MBI Fermentas®) from mRNA isolated from non-failing human left-ventricular myocardium using Trizol (Invitrogen®) and the poly(A)tract kit (Promega®). PCR conditions always were 40 cycles of 94°C, 58°C, 72°C, (each 1 min); and 5 min 72°C. Amplification products were visualized by UV protected 0.8% agarose gel-electrophoresis, extracted (Perfect Gel Clean-up, Eppendorf-Vaudaux®), and subcloned into pCR2.1-TOPO (Invitrogen®). Sequences of cloned fragments were determined on both strands (MWG-Biotech®). For eukaryotic expression full length β2-subunit isoforms were reassembled in the pcDNA3 polylinker region (Invitrogen®) opened by BamHI/NotI using the internal HindIII restriction site. Full length coding sequences were inserted by T4 DNA ligation of N-terminal β2-subunit isoform fragments cut by BamHI (pCR2.1 restriction site) and HindIII (internal restriction site contained in all β2-subunit isoforms) and of the C-terminal fragment cut by HindIII and NotI (pCR2.1 restriction site). Full-length coding sequences of β2 isoforms were inserted by T4 DNA ligation into pIRES2-EGFP opened by EcoRI restriction.
β3-subunits:
Full length coding sequence was excised by EcoRI/XhoI and inserted into pIRES2-dsRed2 opened with EcoRI/SmaI.
human α2δ-2:
Full length coding sequence was obtained from Klugbauer et al. [36] excised by restriction with HindIII/XhoI and inserted into pIRES2-dsRed2 opened with NheI blunt/XhoI.
Western-blot analysis of Ca2+-channel subunits
Protein expression levels of the L-VDCC subunits were assayed by Western-blot analysis of human and mouse cardiac ventricular protein samples. Briefly, protein extracts were obtained by homogenizing frozen heart tissue in buffer (5% SDS, 50 mM TRIS-HCl, pH 7.4, 250 mM sucrose, 75 mM urea, and 10 mM DTT containing complete protease inhibitor cocktail tablet from Roche) using a Teflon homogenizer. The homogenate was denatured by incubation at 95°C (2 min) followed by centrifugation at 16,000g (5 min); supernatants (containing membrane fractions and cytosolic proteins) were collected for analysis. Protein was quantified using Bicinchoninic acid (BCA) Protein Assay (Pierce®). For CaV1.2 and α2δ-1 Western blots, 60 µg, and for β2 and β3 Western blots, 150 µg of total protein were separated on a 8% and 12% SDS-PAGE gel (BioRad®). Gels were transferred to nitrocellulose membranes (Amersham®) according to standard wet transfer procedure. L-VDCC subunits were detected using the following antibodies: anti-human CaV1.2 against the II-III loop (generous gift from Dr. Hannelore Haase, Max-Delbrück Center, Berlin, Germany; [37], [38]); anti-β2 (generous gift from Dr. Adolfo Cuadra (Dr. M. Hosey), Northwestern University, Chicago, USA; [39]); anti-β3 and anti-α2δ−1 (Alomone;[40], [41]), and anti-calsequestrin (Santa Cruz;[42]). The anti-β1 (Swant; cp. [16]) stains a band at ∼57 kDa in the membrane of human skeletal muscle (data not shown) where β1a is pre-dominant [43] suggesting that the antibody detects a β1a in human and mouse myocardium. In the present study this antibody detected an additional band of ∼65 kDa in murine myocardium and ∼70 kDa in human heart. Though our present mRNA data indicate that there are two β1-isoforms in cardiac tissue we cannot exclude a cross-reaction of the antibody with β3-subunits since the second band is quite close to the band detected by the β3-specific antibody from Alomone (see above). Thus we decided to avoid any quantitation of this “high molecular band” detected in murine and human cardiac tissue, respectively.
Immunofluorescence analysis of Ca2+-channel subunits
Ventricular myocytes were freshly isolated from 10 month old tg CaV1.2 and controls as previously described, stored in Kraft-Bruehe solution and plated on laminin-coated with poly-L-lysine and 50 µg/ml mouse laminin (Invitrogen®) coverslips for 1 h at 37° C, 5% CO2. After incubation myocytes were washed with relaxation buffer (mM: 100 KCl, 5 EGTA, 5 MgCl2, 0.25 dithiothreitol (DTT) in PBS, pH 6.8). Myocytes were then fixed in pre-cooled (−20°C) methanol/acetone (1∶1) for 5–10 min at 4°C. To prevent non-specific binding, myocytes were blocked with 10% normal donkey serum (Sigma®) in PBS overnight (labeling buffer) [44]. Primary antibodies were diluted in labeling buffer and incubated with myocytes overnight at 4°C. Primary antibody dilutions for different subunits of the L-VDCC studied were: 1∶200 for α1C (Alomone®) and 1∶500 for β2. In the case of Wheat Germ Agglutinin (WGA) labeling, myocytes were incubated overnight at 4°C with Oregon Green 488-conjugated WGA (Molecular Probes®) at a concentration of 1 µg/ml. WGA selectively binding to N-acetyl-d-glucosamine in glycoproteins was used to label the peripheral sarcolemma, the T-tubules and the intercalated disks. After overnight incubation, myocytes were washed with PBS and incubated with secondary antibody in PBS-0.1% BSA for 1 h at room temperature. Secondary antibody for the study of the L-VDCC subunits was tetramethylrhodamine-isothiocyanate (TRITC)-conjugated donkey anti rabbit antibody at 1∶400 dilution (Jackson ImmunoResearch®, USA). For negative control experiments, myocytes were kept in labeling buffer overnight without primary antibody and only incubated with secondary antibody at the same concentration. After washing the cells with PBS, coverslips were mounted on slides using Gel/Mount aqueous mounting media (Biomeda®) and images were acquired on a Nikon PCM 2000 laser confocal scanning microscope as 0.5 mm “optical sections” of the stained cells, keeping gain and background values constant through the different samples.
Generation of transgenic mice with inducible cardiac overexpression of β2a
Recent modifications in the Drosophila ecdysone receptor revealed better regulation of gene expression in mammalian cells, however, the dependence on steroidal ligand activation (i.e. ponasteron) with its potential additional effects on gene expression remains. The ecdysone receptor from Bombyx mori is activated by the non-steroidal ligand tebufenozide (effective drug in MIMIC, Dow AgroSciences®, Munich, Germany) without known specific interaction in mammalian cells. This construct regulated β-galactosidase expression in HEK293 cells at concentrations of 1 µM tebufenozide as effectively as the Drosophila ecdysone receptor (data not shown). For our experiments we intraperitoneally injected 9 mg tebufenozide (i.e. twice the dose leading to maximum serum concentration of the drug) 48h before isolation of cardiac myocytes. The hybrid drosophila-bombyx ecdysone receptor (VgBmEcR) was constructed by fusion of the binding and transactivation domain of the modified drosophila system (pVgRXR, Invitrogen®) to the ligand binding domain of the bombyx ecdysone receptor (BmEcR in pBSII KS+BmB1 = ecdysone receptor type B1 of Bombyx mori, obtained from Fujiwara H., Tokyo, Japan) using the restriction enzyme BsrGI and NotI. The coding sequence of VgBmEcR was set under control of the promoter of αMHC for cardio-specific expression. The αMHC promoter was excised from pBlue-MHCβ1ARSV40polyA (obtained from Stefan Engelhardt, Wuerzburg, Germany) using DraI and PvuII and inserted into pVgBmEcR opened with the same enzymes. The ecdysone-regulated plasmid pInd-β2a was constructed by excision of the β-galactosidase coding sequence from pInd-LacZ (Invitrogen®) using HindIII and XbaI and insertion of the coding sequence for rat β2a excised from pCR3-β2a-6myc (obtained from A.J. Chien, Chicago, USA) with the same restriction enzymes. The linearized coding sequence of constructs were injected simultaneously into embryonic stem cells, and mice transgenic for the VgBmEcR and the β2a-subunit were identified by PCR using construct-specific primers (not depicted), and by Southern blot using αMHC-VgBmEcR and β2a specific probes labeled radioactively. Mouse DNA was obtained from mice 3 weeks post delivery and digested with EcoRI for proof of αMHC-VgBmEcR-, and HindIII/BamHI for Ind-β2a -genomic integration. Probes specific for αMHC-VgBmEcR and Ind-β2a were obtained by SacI and KpnI/HindIII restriction of the respective coding sequences. Probes were radio-labeled with α-32P-CTP using the Klenow fragment. Animals positive for integrated coding sequences were identified by 3.7 kb hybridization signal for αMHC-VgBmEcR and a 2.4 kb signal for Ind-β2a (Figure 4a).
Cell culture and co-transfection
Cell culture and transient co-transfection were done as described [12], [45]. In brief, HEK293 cells were stably transfected with the full-length CaV1.2-subunit (GenBank NM_000719) cloned from human heart [46]. Cells were seeded in polystyrene Petri dishes (9.6 cm2, Falcon®, Heidelberg, Germany) at a density of 1–2⋅104 cells cm−2 and transiently co-transfected with the cDNA plasmids encoding the different human cardiac β2 splice variants together with human cardiac α2δ-2-subunit [36]. Lipofection was carried out 24–36 h after plating by incubating (3–6 h) with SuperFect (Qiagen®) and the respective plasmids at a DNA mass ratio of 3∶3∶1 [12], [45]. Transfected cells were grown on Petri dishes in Dulbecco′s modified Eagle's medium (DMEM, Biochrom KG®, Berlin, Germany) supplemented with 10% fetal bovine serum (Sigma®, Deisenhofen, Germany), penicillin (10 units ml−1) and streptomycin (10 µg ml−1, both from Biochrom®). Electrophysiological recordings in GFP-positive cells were obtained 48–72 h after transfection.
Isolation of ventricular myocytes
Single ventricular myocytes were isolated from murine hearts by enzymatic dissociation using the method described earlier [47]. In brief, hearts were perfused with a collagenase solution (Worthington type I and II, 75 U l−1) in a Langendorff setup and subsequently cut into small chunks. Myocytes were harvested by pouring the suspension through cheesecloth.
Single-channel recording
Single-channel recordings were performed by using the cell-attached configuration of the patch-clamp method as described earlier [47]. Cells were placed in disposable Petri dishes containing 3 ml of a high-potassium depolarizing solution (mM: 25 KCl, 120 K-glutamate, 2 MgCl2, 10 Hepes, 2 EGTA, 10 dextrose, 1 CaCl2, 1 Na2-ATP; pH 7.3 with KOH). Patch pipettes (borosilicate glass, 6–8M) were filled with pipette solution for myocytes (mM): 70 BaCl2, 110 sucrose and 10 Hepes; for HEK cells (mM): 110 BaCl2, 10 Hepes; pH 7.4 with TEA-OH. Ba2+ currents were elicited by voltage steps (150 ms at 1.66 Hz) from −100 mV to +20 mV (native channels) or +10 mV (recombinant channels) (≥180 sweeps per experiment). Data were sampled at 10 kHz and filtered at 2 kHz (3 dB, four-pole Bessel) by using an Axopatch 200A amplifier (Axon Instruments®, Foster City, USA). PCLAMP software (CLAMPEX 5.5.1, FETCHAN, and PSTAT 6) was used for data acquisition and analysis (Axon Instruments®, Foster City, USA). Signal-noise ratio and adequate resolution of openings were similar to previous work [12], [16], [47], as confirmed by comparison of, e.g. data from wild-type mice.
Data analysis and statistics of single-channel recordings
Linear leak and capacity currents (averaged non-active sweeps) were digitally subtracted. Openings and closures were identified by the half-height criterion. The fraction of active sweeps within a patch (factive), the open probability within active sweeps (Popen), and the peak value of single-channel ensemble average currents (Ipeak) were determined as described [16]. Where necessary, these parameters were corrected for the number of channels in a patch, as described [6]. For comparisons unpaired Student's two-tailed t-test or Mann-Whitney test was used where appropriate. Throughout, a level of p<0.05 was considered significant. Values are given as mean±SEM.
Acknowledgments
We gratefully acknowledge Jens Reifenrath for expert assistance with animal breeding, and Sigrid Kirchmann for excellent technical help. We are grateful to Dr. Snezena Petrovic for allowing us to perform the immunocytochemistry experiments in her laboratory.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Grant Support: Herzig S, DFG HE1578/13-1, ZMMK A5; Schwartz A, NIH (NHLBI), R01 HL079599-01; Schwartz A., Bodi I. T32-HL07382-30; Hullin R, Schweizer Herzstiftung, Katharina Huber-Steiner Stiftung; Hein L, DFG SFB355 TPC10.
References
- 1.Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res. 1999;42:270–283. doi: 10.1016/s0008-6363(99)00017-6. [DOI] [PubMed] [Google Scholar]
- 2.Shannon TR, Bers DM. Integrated Ca2+ management in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:28–38. doi: 10.1196/annals.1302.003. [DOI] [PubMed] [Google Scholar]
- 3.Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A. The L-type calcium channel in the heart: the beat goes on. J Clin Invest. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard S, Leclercq F, Lemaire S, Piot C, Nargeot J. Ca2+ currents in compensated hypertrophy and heart failure. Cardiovasc Res. 1998;37:300–311. doi: 10.1016/s0008-6363(97)00273-3. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee R, Spinale FG. L-type calcium channel abundance and function with cardiac hypertrophy and failure: a review. J Mol Cell Cardiol. 1998;30:1899–1916. doi: 10.1006/jmcc.1998.0755. [DOI] [PubMed] [Google Scholar]
- 6.Schroder F, Handrock R, Beuckelmann DJ, Hirt S, Hullin R, et al. Increased availability and open probability of single L-type calcium channels from failing compared with nonfailing human ventricle. Circulation. 1998;98:969–976. doi: 10.1161/01.cir.98.10.969. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, et al. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 8.Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases. Am J Physiol Cell Physiol. 2001;281:C1743–1756. doi: 10.1152/ajpcell.2001.281.6.C1743. [DOI] [PubMed] [Google Scholar]
- 9.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 10.Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- 11.Matthes J, Jager A, Handrock R, Groner F, Mehlhorn U, et al. Ca2+-dependent modulation of single human cardiac L-type calcium channels by the calcineurin inhibitor cyclosporine. J Mol Cell Cardiol. 2004;36:241–255. doi: 10.1016/j.yjmcc.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Hullin R, Khan IF, Wirtz S, Mohacsi P, Varadi G, et al. Cardiac L-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J Biol Chem. 2003;278:21623–21630. doi: 10.1074/jbc.M211164200. [DOI] [PubMed] [Google Scholar]
- 13.Hullin R, Singer-Lahat D, Freichel M, Biel M, Dascal N, et al. Calcium channel beta subunit heterogeneity: functional expression of cloned cDNA from heart, aorta and brain. Embo J. 1992;11:885–890. doi: 10.1002/j.1460-2075.1992.tb05126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary beta2 subunit splice variants on L-type calcium channel gating. Biophys J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, et al. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 16.Groner F, Rubio M, Schulte-Euler P, Matthes J, Khan IF, et al. Single-channel gating and regulation of human L-type calcium channels in cardiomyocytes of transgenic mice. Biochem Biophys Res Commun. 2004;314:878–884. doi: 10.1016/j.bbrc.2003.12.174. [DOI] [PubMed] [Google Scholar]
- 17.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, et al. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakami M, Yamamura H, Suzuki T, Kang MG, Ohya S, et al. Modified cardiovascular L-type channels in mice lacking the voltage-dependent Ca2+ channel beta3 subunit. J Biol Chem. 2003;278:43261–43267. doi: 10.1074/jbc.M211380200. [DOI] [PubMed] [Google Scholar]
- 19.Haase H, Kresse A, Hohaus A, Schulte HD, Maier M, et al. Expression of calcium channel subunits in the normal and diseased human myocardium. J Mol Med. 1996;74:99–104. doi: 10.1007/BF00196785. [DOI] [PubMed] [Google Scholar]
- 20.Hullin R, Asmus F, Ludwig A, Hersel J, Boekstegers P. Subunit expression of the cardiac L-type calcium channel is differentially regulated in diastolic heart failure of the cardiac allograft. Circulation. 1999;100:155–163. doi: 10.1161/01.cir.100.2.155. [DOI] [PubMed] [Google Scholar]
- 21.Muth JN, Yamaguchi H, Mikala G, Grupp IL, Lewis W, et al. Cardiac-specific overexpression of the alpha(1) subunit of the L-type voltage-dependent Ca(2+) channel in transgenic mice. Loss of isoproterenol-induced contraction. J Biol Chem. 1999;274:21503–21506. doi: 10.1074/jbc.274.31.21503. [DOI] [PubMed] [Google Scholar]
- 22.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca(2+)-dependent transgenic model of cardiac hypertrophy: A role for protein kinase Calpha. Circulation. 2001;103:140–147. doi: 10.1161/01.cir.103.1.140. [DOI] [PubMed] [Google Scholar]
- 23.Collin T, Wang JJ, Nargeot J, Schwartz A. Molecular cloning of three isoforms of the L-type voltage-dependent calcium channel beta subunit from normal human heart. Circ Res. 1993;72:1337–1344. doi: 10.1161/01.res.72.6.1337. [DOI] [PubMed] [Google Scholar]
- 24.Wei SK, Colecraft HM, DeMaria CD, Peterson BZ, Zhang R, et al. Ca(2+) channel modulation by recombinant auxiliary beta subunits expressed in young adult heart cells. Circ Res. 2000;86:175–184. doi: 10.1161/01.res.86.2.175. [DOI] [PubMed] [Google Scholar]
- 25.Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 27.Bunemann M, Gerhardstein BL, Gao T, Hosey MM. Functional regulation of L-type calcium channels via protein kinase A-mediated phosphorylation of the beta(2) subunit. J Biol Chem. 1999;274:33851–33854. doi: 10.1074/jbc.274.48.33851. [DOI] [PubMed] [Google Scholar]
- 28.Ganesan AN, Maack C, Johns DC, Sidor A, O'Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamp TJ, Hu H, Marban E. Voltage-dependent facilitation of cardiac L-type Ca channels expressed in HEK-293 cells requires beta-subunit. Am J Physiol Heart Circ Physiol. 2000;278:H126–136. doi: 10.1152/ajpheart.2000.278.1.H126. [DOI] [PubMed] [Google Scholar]
- 30.Grueter CE, Abiria SA, Dzhura I, Wu Y, Ham AJ, et al. L-type Ca2+ channel facilitation mediated by phosphorylation of the beta subunit by CaMKII. Mol Cell. 2006;23:641–650. doi: 10.1016/j.molcel.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Serikov V, Bodi I, Koch SE, Muth JN, Mikala G, et al. Mice with cardiac-specific sequestration of the beta-subunit of the L-type calcium channel. Biochem Biophys Res Commun. 2002;293:1405–1411. doi: 10.1016/S0006-291X(02)00396-0. [DOI] [PubMed] [Google Scholar]
- 32.Song LS, Guia A, Muth JN, Rubio M, Wang SQ, et al. Ca(2+) signaling in cardiac myocytes overexpressing the alpha(1) subunit of L-type Ca(2+) channel. Circ Res. 2002;90:174–181. doi: 10.1161/hh0202.103230. [DOI] [PubMed] [Google Scholar]
- 33.Dalton S, Takahashi SX, Miriyala J, Colecraft HM. A single CaVbeta can reconstitute both trafficking and macroscopic conductance of voltage-dependent calcium channels. J Physiol. 2005;567:757–769. doi: 10.1113/jphysiol.2005.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packer M, O'Connor CM, Ghali JK, Pressler ML, Carson PE, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996;335:1107–1114. doi: 10.1056/NEJM199610103351504. [DOI] [PubMed] [Google Scholar]
- 35.Figulla HR, Gietzen F, Zeymer U, Raiber M, Hegselmann J, et al. Diltiazem improves cardiac function and exercise capacity in patients with idiopathic dilated cardiomyopathy. Results of the Diltiazem in Dilated Cardiomyopathy Trial. Circulation. 1996;94:346–352. doi: 10.1161/01.cir.94.3.346. [DOI] [PubMed] [Google Scholar]
- 36.Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase H, Pfitzmaier B, McEnery MW, Morano I. Expression of Ca(2+) channel subunits during cardiac ontogeny in mice and rats: identification of fetal alpha(1C) and beta subunit isoforms. J Cell Biochem. 2000;76:695–703. doi: 10.1002/(sici)1097-4644(20000315)76:4<695::aid-jcb17>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 38.Schotten U, Haase H, Frechen D, Greiser M, Stellbrink C, et al. The L-type Ca2+-channel subunits alpha1C and beta2 are not downregulated in atrial myocardium of patients with chronic atrial fibrillation. J Mol Cell Cardiol. 2003;35:437–443. doi: 10.1016/s0022-2828(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 39.Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, et al. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–30044. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- 40. www.alomone.com/System/UpLoadFiles/Dgallery/Docs/ACC-008.pdf. [Google Scholar]
- 41. www.alomone.com/System/UpLoadFiles/Dgallery/Docs/ACC-015.pdf. [Google Scholar]
- 42. www2.scbt.com/Datasheets_list/SC-16571.pdf. [Google Scholar]
- 43.Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, et al. Molecular heterogeneity of calcium channel beta-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- 44.Gao T, Puri TS, Gerhardstein BL, Chien AJ, Green RD, et al. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- 45.Erhardt JA, Hynicka W, DiBenedetto A, Shen N, Stone N, et al. A novel F box protein, NFB42, is highly enriched in neurons and induces growth arrest. J Biol Chem. 1998;273:35222–35227. doi: 10.1074/jbc.273.52.35222. [DOI] [PubMed] [Google Scholar]
- 46.Schultz D, Mikala G, Yatani A, Engle DB, Iles DE, et al. Cloning, chromosomal localization, and functional expression of the alpha 1 subunit of the L-type voltage-dependent calcium channel from normal human heart. Proc Natl Acad Sci U S A. 1993;90:6228–6232. doi: 10.1073/pnas.90.13.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foerster K, Groner F, Matthes J, Koch WJ, Birnbaumer L, et al. Cardioprotection specific for the G protein Gi2 in chronic adrenergic signaling through beta 2-adrenoceptors. Proc Natl Acad Sci U S A. 2003;100:14475–14480. doi: 10.1073/pnas.1936026100. [DOI] [PMC free article] [PubMed] [Google Scholar]