Abstract
Background
The progression of pressure-overload left ventricular hypertrophy (LVH) to chronic heart failure (CHF) may involve a relative deficit in energy supply and/or delivery.
Methods and Results
We measured myocardial creatine kinase (CK) metabolite concentrations and adenosine triphosphate (ATP) synthesis through CK, the primary energy reserve of the heart, to test the hypothesis that ATP flux through CK is impaired in patients with LVH and CHF. Myocardial ATP levels were normal, but creatine phosphate levels were 35% lower in LVH patients (n= 10) than in normal subjects (n= 14, P< 0.006). Left ventricular mass and CK metabolite levels in LVH were not different from those in patients with LVH and heart failure (LVH+CHF, n= 10); however, the myocardial CK pseudo first-order rate constant was normal in LVH (0.36±0.04 s−1 in LVH versus 0.32±0.06 s−1 in normal subjects) but halved in LVH+CHF (0.17±0.06 s−1, P < 0.001). The net ATP flux through CK was significantly reduced by 30% in LVH (2.2 ± 0.7 μmol · g−1 · s−1, P < 0.011) and by a dramatic 65% in LVH+CHF (1.1±0.4 μmol · g−1 · s−1, P< 0.001) compared with normal subjects (3.1 ± 0.8 μmol · g−1 · s−1).
Conclusions
These first observations in human LVH demonstrate that it is not the relative or absolute CK metabolite pool sizes but rather the kinetics of ATP turnover through CK that distinguish failing from nonfailing hypertrophic hearts. Moreover, the deficit in ATP kinetics is similar in systolic and nonsystolic heart failure and is not related to the severity of hypertrophy but to the presence of CHF. Because CK temporally buffers ATP, these observations support the hypothesis that a deficit in myofibrillar energy delivery contributes to CHF pathophysiology in human LVH.
Keywords: hypertrophy, adenosine triphosphate, heart failure, creatine kinase, magnetic resonance spectroscopy, metabolism
Left ventricular hypertrophy (LVH) is, initially, an adaptive response to chronic pressure overload but is ultimately associated with a 10-fold greater likelihood of subsequent chronic heart failure (CHF).1,2 Because pressure-overload LVH increases energetic cost, a mismatch in myocardial energy supply and demand may contribute to the development of heart failure in LVH. Myocardial energetic demands are met primarily through mitochondrial adenosine triphosphate (ATP) production via oxidative phosphorylation.3 The creatine kinase (CK) reaction serves as the prime cardiac energy reserve, providing ATP during periods of increased demand by reversibly and rapidly converting adenosine diphosphate (ADP) and phosphocreatine (PCr) to ATP and creatine.4,5 The PCr/ATP ratio is one indicator of the energetic state of the myocardium and is reduced in animal models of myocardial hypertrophy,6,7 in human LVH,8–10 and in CHF.11,12 Despite the potential importance of the cardiac PCr/ATP ratio for understanding cardiac energetics, this ratio does not directly reflect the rate of ATP production through the CK reaction,7,13–15 which may be more important in the progression to CHF in patients with LVH.
In animal models of chronic pressure-overload LVH, 31P magnetic resonance spectroscopy (MRS) magnetization transfer techniques demonstrate significant decreases in CK flux that are larger than the changes in the cardiac PCr/ATP ratio.7,13 In both canine and swine models, the forward flux through CK is reduced by 30% to 50% in compensated LVH compared with normal hearts7,15 and by 60% in those that developed CHF.7 The abnormality in CK metabolism in those studies was related to the severity of hypertrophy.7 However the degree of hypertrophy was greater in failing than nonfailing hearts,7,16 and therefore, it was not possible to determine whether the reported metabolic changes were related to the degree of hypertrophy, the concomitant heart failure, or both. At least half of all patients with LVH-associated CHF have preserved systolic function,17 a condition sometimes referred to as “diastolic heart failure.” The metabolic abnormalities associated with nonsystolic CHF have not been reported previously.
We recently developed a high-speed magnetization transfer technique that enabled, for the first time, direct measures of ATP flux through CK in the human heart.18 A highly significant reduction in ATP synthesis from CK, even before any reduction in cardiac [ATP] can be detected, occurs in patients with nonischemic dilated cardiomyopathy (DCM) and mild-to-moderate CHF.18 Reduced ATP buffering by CK in DCM could potentially contribute to the systolic dysfunction.18 As yet, there are no reports of CK flux in the hypertrophied human heart, or indeed in any other condition that progresses to CHF. Because the underlying pathophysiology, signaling cascades, and energy demands of pressure-overload LVH differ from those of DCM, the extent to which ATP kinetics are altered in human LVH in the presence and absence of CHF is not known. We therefore used the same 31P MRS 4-angle saturation transfer (FAST) magnetization transfer technique to directly measure myocardial ATP turnover through CK in patients with compensated and failing LVH, as defined by a modified Framingham scoring system,19 to test the hypothesis that CK energy delivery is altered in human pressure-overload hypertrophy with or without associated CHF. The present study, is the first (to the best of our knowledge) to address whether changes in ATP kinetics in failing hypertrophied hearts are simply related to the degree of hypertrophy or are a characteristic of the progression to heart failure and whether they occur in both systolic and nonsystolic human heart failure.
Methods
Additional details are provided in the online-only Data Supplement.
Human Subjects
Studies were approved by The Johns Hopkins Institutional Review Board on human investigation, and all subjects gave informed consent. Fourteen healthy subjects (mean age 41 ± 7years; 2 women) without a history of hypertension, coronary artery disease, or diabetes mellitus were studied, including some previously reported who were contemporaneous with the current studies.18 All subjects with LVH (n=20; age 56 ± 12 years; 11 women) had stage II hypertension of at least 1-year duration with echocardiographic evidence of concentric hypertrophy (septal and posterior wall thickness > 1.2 cm). All patients had either a cardiac catheterization within the prior 12 months that demonstrated no significant coronary stenosis or a negative stress test. In these 20 patients, 10 had CHF (LVH+CHF), as defined with a modified Framingham scoring system,19 and 10 did not (LVH). CHF was diagnosed at the time of the patient’s hospitalization with 2 major criteria (paroxysmal nocturnal dyspnea or orthopnea, jugular venous distention, rales, cardiomegaly, radiographic pulmonary edema, S3 gallop, hepatojugular reflux, or > 4.5-lb weight loss in response to diuresis) or 1 major and 2 minor criteria (ankle edema, night cough, dyspnea on exertion, hepatomegaly, or pleural effusion).19 Because half of all patients with heart failure have preserved ejection fractions,17,20 LVH+CHF patients were subdivided into 2 groups: those with normal left ventricular systolic function (left ventricular ejection fraction ≥50% [“nonsystolic CHF”], n=5) and those with impaired left ventricular ejection fraction (left ventricular ejection fraction < 50% [“systolic CHF”], n=5), as defined by prior ejection fraction criteria.17 No magnetic resonance imaging/MRS studies were performed on patients during episodes of acute decompensated CHF.
Cardiac MRS
Subjects were positioned prone in a clinical broadband 1.5T General Electric (Milwaukee, Wis) magnetic resonance imaging scanner on a 31P MRS 6.5-cm receive, 20-cm transmit surface coil probe, and image-guided, 1-dimensional chemical shift imaging was used to spatially localize spectroscopic acquisitions (1-cm slice thickness) to the human heart. The FAST method was applied to measure ATP flux through the CK reaction with 2 pairs of 31P acquisitions with adiabatic pulse flip angles of 15° and 60°: 1 pair with the γ-ATP resonance at −2.7 ppm saturated, and the other pair with the same irradiation applied at +2.7 ppm (control saturation).18,21 A fifth 31P MRS data set was acquired with a 60° pulse, without selective saturation, to measure both the spillover of the saturation and phosphate metabolite concentrations. A cardiac 1H MRS data set was then acquired with a 60° pulse using the 31P MRS receiver coil to provide a tissue water reference for the concentration measurements.22 The image-guided spectroscopy examinations typically took a total of 60 to 75 minutes per subject.
[PCr] and [ATP] were calculated by 2 previously validated techniques that used water22 and phosphate23,24 as internal and external references, respectively, and the results were averaged for the myocardial slices in each subject.22–24 The water-reference concentration measures (μmol/g tissue) assumed substantially equivalent tissue water content among the groups.22 The pseudo first-order rate constant, Kfor, was calculated from the ratios of PCr signals in FAST data sets from equations 5, 6, and 9 of Bottomley et al,21 corrected for spillover irradiation based on the unsaturated 31P cardiac data. Kfor is effectively the fraction of the PCr pool that exchanges with ATP each second. The CK forward flux rate was calculated from the product Kfor[PCr] (in μmol · g−1 · s−1) for each subject.
Statistical Analysis
Continuous variables are presented as mean ± SD, whereas categorical variables are presented as either absolute counts or percentages. Statistical analysis of demographic variables was performed with an unpaired, 2-tailed Student t test. The Shapiro-Wilk test was used to test the normality of the key metabolic variables, and it could not reject the hypothesis that all these variables are normally distributed. Therefore, parametric testing was performed, and differences in means among groups were assessed by ANOVA followed by groupwise comparisons with the Tukey-Kramer adjustment to control the overall error rate. In all instances, statistical significance was assumed at P < 0.05.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline demographics are presented in Table 1. Subjects with LVH+CHF were significantly younger and had lower ejection fractions than those with LVH alone. Importantly, the degree of hypertrophy, as measured by the left ventricular mass index and by septal and posterior wall thickness, was the same in LVH and LVH+CHF patients.
TABLE 1.
Demographics
| LVH (n=10) | LVH+CHF (n=10) | |
|---|---|---|
| Gender, female:male, n | 6:4 | 5:5 |
| Age, y | 63±10 | 50±10* |
| Body surface area, m2 | 1.9±0.2 | 2.0±0.3 |
| Heart rate, bpm | 71±12 | 76±12 |
| Blood pressure, mm Hg | ||
| Systolic | 143±18 | 145±25 |
| Diastolic | 80±16 | 80±10 |
| Left ventricular mass index, g/m2 | 166±50 | 167±30 |
| Ejection fraction, % | 61±9 | 46±18* |
| Atrial fibrillation, % | 0 | 10 |
| Creatinine, mg/dL | 0.94±0.3 | 1.32±0.5 |
| Diabetes mellitus, % | 50 | 50 |
| History of tobacco use, % | 50 | 70 |
| Current tobacco use, % | 30 | 40 |
| Percentage with use of: | ||
| β-Blocker | 40 | 70 |
| Angiotensin-converting enzyme inhibitor | 60 | 30 |
| Angiotensin receptor blocker | 10 | 0 |
| Loop diuretic | 20 | 60 |
| Spironolactone | 0 | 10 |
| Digoxin | 0 | 0 |
Left ventricular mass index and ejection fraction were determined with echocardiography.
P < 0.05.
Conventional transaxial cardiac 1H images and corresponding myocardial 60° 31P FAST spectra from a patient with LVH+CHF are shown in Figure 1. The PCr and ATP peak areas with control irradiation are proportional to the concentrations of the metabolites, spillover effects notwithstanding. The fractional reduction in the PCr peak with [γ]-ATP saturated is proportional to the ATP flux through the CK reaction.
Figure 1.
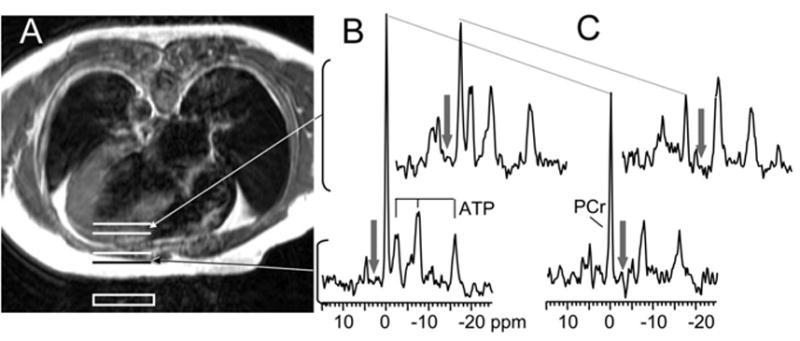
Axial spin-echo MR image (A) of a patient with LVH+CHF lying prone over a 31P surface coil (white box) and the corresponding localized 31P MR spectra from the chest (lower 2 spectra) and left ventricle (upper 2 spectra). The resonances derive from PCr and the γ-, α-, and β -phosphate resonances of ATP. The spectra were acquired with a 60° flip angle in the presence of chemically selective saturating irradiation (arrows) either in the control (B) or γ-ATP position (C). The decrease in the height of the PCr peak between control and γ-ATP saturation (dotted lines) is directly related to the rate of ATP synthesis through the CK reaction.
Metabolite concentrations, Kfor, and CK flux data are summarized in Table 2. Myocardial [ATP] was not significantly reduced in LVH or LVH+CHF compared with that in normal subjects. However, mean cardiac [PCr] was reduced by 30% in both LVH and LVH+CHF compared with values observed in normal subjects (Table 2). Therefore, myocardial PCr/ATP was reduced by 30% in both LVH and LVH+CHF compared with normal (1.9 ± 0.3 versus 1.3 ± 0.4 in all 20 patients, P < 0.002) but did not differ significantly between LVH groups with and without CHF (1.3 ± 0.3 in LVH versus 1.3 ± 0.5 in LVH+CHF, P = NS).
TABLE 2.
High-Energy Phosphate Concentrations and CK Flux
| Normal (n= 14) | LVH (n= 10) | LVH+CHF (n= 10) | |
|---|---|---|---|
| [PCr], μmol/g | 9.4±1.1 | 6.1±2.0* | 7.2±3.7 |
| [ATP], μmol/g | 5.5±1.3 | 4.7±1.3 | 5.0±1.1 |
| PCr/ATP | 1.9±0.3 | 1.3±0.3† | 1.3±0.5† |
| Kfor, s−1 | 0.32±0.06 | 0.36±0.04 | 0.17±0.06‡ |
| CK flux, μmol · g−1 · s−1 | 3.1 ± 0.8 | 2.2 ± 0.7§ | 1.1 ± 0.4|| |
P < 0.006 vs Normal,
P < 0.003 vs Normal.
P < 0.001 vs Normal and vs LVH.
P = 0.011 vs Normal.
P < 0.001 vs Normal and P < 0.003 vs LVH.
The kinetic data revealed more dramatic changes in the pseudo first-order rate constant and forward CK flux among the 3 groups than those seen in the absolute or relative pool sizes alone. The mean pseudo first-order rate constant, Kfor, was similar in LVH and in normal subjects (Figure 2A). However, because of the reduction in [PCr], the net CK flux was reduced by 30% in the LVH group (P = 0.011; Figure 2B). Moreover, Kfor was reduced by 50% in LVH+CHF compared with normal subjects (P < 0.001) and those with LVH alone (P < 0.001). Mean CK flux in LVH+CHF was reduced by 65% compared with that in normal subjects (P < 0.001; Table 2; Figure 2B) and by 30% compared with those with LVH (P < 0.003).
Figure 2.
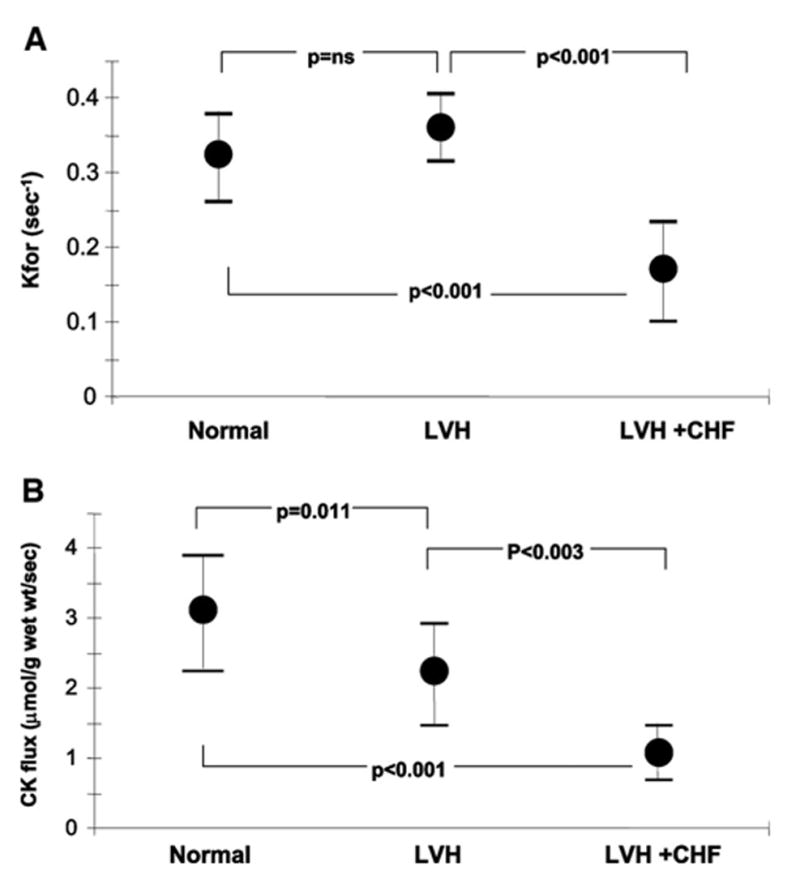
A, CK myocardial pseudo first-order rate constant (Kfor) for healthy subjects (Normal), patients with LVH, and others with LVH with CHF (LVH+CHF). Note that Kfor is not reduced by LVH but only in LVH+CHF. B, Net ATP flux through the CK reaction (CK flux) in healthy subjects (Normal), patients with LVH, and others with LVH with CHF (LVH+CHF).
Not only was LV mass similar in LVH and LVH+CHF (Table 1), but the abnormality in CK kinetics exhibited no significant correlation with the severity of hypertrophy, as measured by left ventricular mass index (Figure 3) and septal wall thickness (r2 < 0.03, data not shown). Half of the patients with LVH+CHF (n=5) had nonsystolic heart failure with preserved LVEF (62±8%, range 55% to 75%), whereas the others (n=5) did not (30±7%, range 20% to 35%; P < 0.001). LVH+CHF patients with nonsystolic heart failure and those with systolic heart failure had similar Kfor (0.15±0.07 versus 0.18±0.06 s−1, P = NS) and cardiac CK flux (1.02 ± 0.07 versus 1.19 ± 0.37 μmol · g−1 · s−1, respectively, P = NS). Accordingly, Kfor and CK flux did not correlate with ejection fraction (Figure 3). Thus, in hypertrophic hearts, a low Kfor appeared to be specific for the progression to heart failure and occurred without lower [ATP]. Highly significant reductions in the CK pseudo first-order rate constant and CK flux occur in hypertrophied patients with both systolic and nonsystolic heart failure and are not directly related to the severity of LVH per se.
Figure 3.
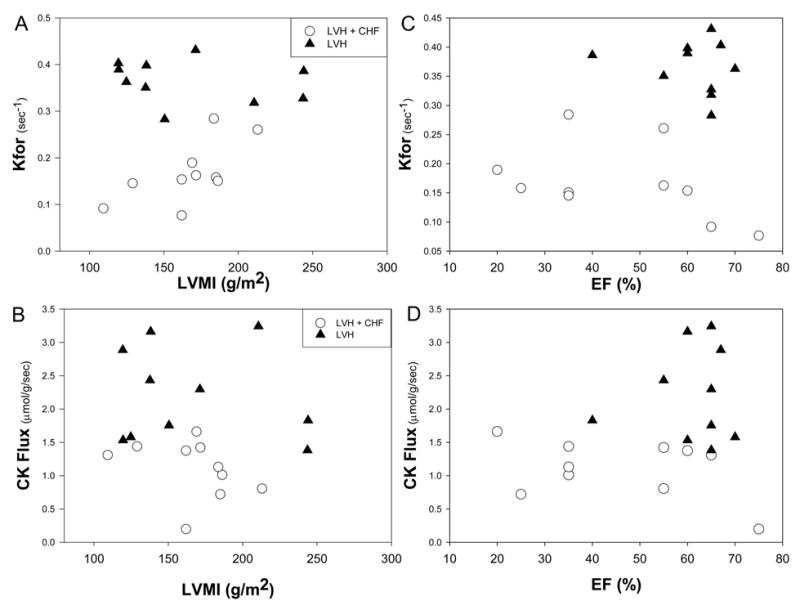
LVH severity, as indexed by left ventricular mass index (LVMI; A and B) and left ventricular ejection fraction (EF; C and D) versus ATP kinetics, as indexed by Kfor (above) and CK flux (below) for LVH (▲) and LVH+CHF (○). Neither Kfor nor CK flux correlated with the severity of LVH (r2 < 0.03 for both in all 20 patients) or with ejection fraction. It is the presence of CHF, not the severity of LVH, that is associated with reduced ATP synthesis through CK. Reduced CK flux occurs in failing hearts with impaired and preserved ejection fractions.
Discussion
The present study is the first to report in vivo measures of ATP flux through the CK reaction, the prime energy reserve, in hypertrophic human hearts. We found that in the absence of CHF, ATP flux through CK is reduced by 30% in LVH patients, and this reduction can be accounted for by a proportionate loss of substrate (PCr). Patients with a similar degree of LVH but who additionally had CHF exhibited no greater reductions in PCr and ATP than those without CHF. However, the CK pseudo first-order rate constant was markedly depressed in these CHF patients compared with that of normal subjects and of LVH patients without CHF. Consequently, myocardial CK flux in LVH patients with CHF declined to approximately one third that of normal subjects. These data provide direct evidence that a defect in the kinetics of ATP turnover through CK and not the pool sizes per se may distinguish failing and nonfailing human hypertrophic myocardium.
Myocardial High-Energy Phosphate Metabolism and the Role of CK
Myocardial ATP synthesis via oxidative phosphorylation is directly related to myocardial oxygen consumption, which is not decreased but is normal or increased at rest in animal models of pressure-overload LVH15,16 and in patients with pressure-overload LVH.25 In addition, detailed studies of deoxymyoglobin have failed to detect evidence of an oxygen deficit in LVH.26 Thus, if there is a metabolic energy defect associated with LVH, it is not obviously related to mitochondrial ATP synthesis via oxidative phosphorylation but may lie elsewhere.
The CK reaction is important in energy metabolism because of its ability to rapidly buffer ATP and to transfer high-energy phosphates between sites of production and utilization. Specific cytosolic and mitochondrial isoforms of CK maintain high ATP/ADP ratios at the myofibrillar sites of utilization and low ATP/ADP ratios at the mitochondrial sites of production. The CK cytosolic isozyme shift to more “fetal” isoforms in LVH models7,14,15 and in human LVH4 is postulated to offer a thermodynamic advantage. CK isoform analysis could not be performed in the present noninvasive study of stable ambulatory patients with LVH because there was no clinical indication for the requisite cardiac biopsy. Recent studies in CK knockout mice, however, suggest similar kinetic characteristics among the CK isoforms.27
CK Metabolite Levels in LVH
The myocardial PCr/ATP ratio has been used for more than 2 decades to index myocardial energy metabolism, and the reduction in PCr/ATP observed in LVH here is consistent with published results in animal models7,15 and in patients with LVH and heart failure.8–10 However, a reduced PCr/ATP is not specific to CHF, because patients with LVH but no heart failure can also have reduced PCr/ATP10,28 (Table 2).
Measurements of absolute high-energy phosphate pools, rather than their ratios, have been proposed as a more discerning measure of energy metabolites.9,29,30 However, the ATP pool was reported as nearly normal30 or only modestly reduced4,9 in compensated hypertrophy, and the current observations are consistent with those prior reports.
ATP Turnover Through CK in LVH
The rate of turnover of high-energy phosphate pools may be more physiologically important than the relative or absolute size of the pools themselves. The similar relative and absolute metabolite pool sizes in LVH and LVH+CHF reveal the potential pitfalls in trying to infer CK metabolism from single measures of PCr/ATP or pool sizes alone, as is commonly done. The present findings are consistent with prior in vitro31 and in vivo7,15 CK flux measurements in pressure-overload animal models of LVH with 31P nuclear magnetic resonance magnetization saturation techniques. In compensated canine and porcine LVH, the fraction of the PCr pool exchanging with ATP, Kfor, was normal.7,15 Because there was a 30% to 50% loss of [PCr], the forward CK flux was reduced by that amount in those settings, as observed in the present study in human LVH. In hypertrophied animal hearts that progressed to failure, there was a significant 35% decline in Kfor that further reduced CK flux by 55% to 60% below normal.7 Thus, the present data in human LVH and LVH+CHF are consistent with these prior animal studies in that we observed progressive decreases in CK flux for the compensated and failing hypertrophied myocardium but found reduced Kfor only in failing myocardium (Table 2).
Additionally, our observations allow new insights not available from prior studies in animals. First, these are original kinetic observations in human LVH. Second, hypertrophy was more severe in failing hearts than in nonfailing hearts in the animal models,7,15 and it was concluded that the reduction in energetics was related to the severity of hypertrophy. Unlike animal models, in which the stimulus is abrupt, human pressure-overload LVH due to hypertension often develops over a longer time period and in the presence of antihypertensive therapies. This clinical setting for the first time provided the ability to determine the effect of heart failure on CK kinetics in LVH, because patients with and without CHF had similar degrees of hypertrophy (Table 1). Thus, although LVH results in a loss of PCr (Table 2), reduced kinetics is closely tied to the presence of heart failure and not to the severity of LVH (Figure 3).
These observations in LVH also provide novel insights and extend results from those previously reported for human DCM.18 First, reductions in PCr/ATP and [PCr] are greater here in LVH and LVH+CHF (30%) than in DCM (10% to 20%) by these methods.11,18 Second, the relative reduction in cardiac CK flux from normal values was greater in the present study in LVH+CHF (65%) than in DCM (50%).18 Indeed, the failing hypertrophic hearts studied here have the greatest deficits in CK flux yet studied in humans. Third, these data provide the first information on CK energy turnover in diastolic heart failure with preserved systolic function and demonstrate that reduced CK flux is common to both systolic and nonsystolic human heart failure.
Mechanisms Contributing to Altered CK Kinetics in LVH+CHF
In general, reductions in CK flux may be due to a loss of total enzyme activity (Vmax), altered substrate ratios, or allosteric modification of the enzyme. Because PCr is a substrate for the forward CK reaction, reduced [PCr] lowers CK flux in human LVH without CHF. However, reduced [PCr] cannot account for the further decline in CK flux observed in LVH+CHF, because PCr is not further depleted with CHF (Table 2). The larger flux reduction in human CHF arises from a decrease in Kfor. Total in vitro CK enzyme activity is preserved in LVH models without heart failure7,15 but is reduced in those with heart failure7 and in LVH patients at the time of cardiac surgery.4 These observations support the view that the decrease in Kfor observed in the present study in hypertrophic hearts that failed was at least in part due to a loss of CK enzyme. Total myocardial creatine content is 40% to 50% lower in patients with LVH+CHF than in asymptomatic LVH patients,32 despite the similar PCr content (Table 2). Taken together, the CK equilibrium reaction33 predicts lower [ADP] in LVH+CHF than in LVH. This, as well as less CK enzyme (Vmax), likely contributes to the lower Kfor and CK flux in LVH+CHF hearts.33
Consequences of Altered CK Kinetics in LVH+CHF
Because cardiac CK flux is important for temporal ATP buffering, it appears unlikely that a significant reduction in CK flux would be adaptive or protective in LVH or LVH+CHF. Reducing CK flux in normal animal hearts by PCr depletion impairs contractile function34 and eliminates survival after infarction.35 Genetic CK knockout reduces in vivo contractile reserve,36 and knockout of both the M- and mitoisoforms of CK also limits contractile recovery after ischemia.37 However, subcellular reorganization38 and increased activity of other ATP buffers and phosphotransfer networks,39 such as adenyl kinase, occur after CK knockout and serve to attenuate the consequences of CK loss in those settings. Other recent work demonstrates that PCr depletion, and hence reduced CK capacity, adversely affects actinomyosin function even when [ATP] is normal.40
Would a reduction in CK flux of the magnitude observed in LVH+CHF be sufficient to limit mechanical function in the hypertrophied heart? Because CK is important for buffering cardiac ATP over time, one can calculate the impact of a 65% to 70% loss of CK buffering capacity. In the steady state, ATP synthesis rates must match those of ATP utilization to keep [ATP] constant during the cardiac cycle.41–43 The mean basal rate of ATP production via oxidative phosphorylation is ≈0.4 μmol · gww−1 · s−1 (where gww indicates gram of wet weight) in the normal human heart.44,45 Because roughly 75% of ATP utilization occurs by peak force generation in each cardiac cycle,46,47 one can conservatively anticipate that 75% of ATP utilization occurs during the first 150 ms of each contraction in the normal heart. The difference between this early ATP requirement (0.75 × 0.4 to ≈0.30 μmol/gww, assuming a heart rate of 60 bpm) and the amount provided by de novo ATP synthesis during the same time period (0.4μmol gww−1 · s−1 × 0.15 s to ≈0.06 μmol/gww) must be met by temporal ATP buffers, of which CK is primary. To provide the requisite ATP (0.24 μmol/gww) in 0.15 second, a CK flux rate of at least at 1.6 μmol · gww −1 · s−1 is required. The observation that most patients with LVH and no CHF symptoms have mean CK flux (2.2±0.7 μmol · gww−1 · s−1) above this level and those with CHF have CK flux (1.1±0.4 μmol · gww−1 · s−1) at or below this level is therefore consistent with a hypothesis that the reduction in CK flux in LVH+CHF is of sufficient magnitude to compromise the ATP buffering capacity over time and thereby limit ATP availability to the myofibrils. This mechanism would impact both systolic and diastolic function. Because ATP demand is higher in pressure-overload hypertrophied hearts than in normal hearts, temporal buffering by CK may be even more important and the consequences of reduced CK flux more limiting in LVH than in the normal hearts used in these calculations. Note that this mechanism would also mean that the hypertrophied heart is more metabolically susceptible to stressors such as increased chronotropic and inotropic demand and ischemia. Glucose and glycogen provide another rapid source of ATP in many tissues, including muscle.48 In this regard, the “substrate switch” toward increased reliance on glucose observed in some heart failure models and strategies to increase glucose utilization in hypertrophic hearts49 may also act as an additional rapid source of ATP during the cardiac cycle when the CK reservoir falls in CHF.
The present observations are consistent with but do not prove that inadequate ATP delivery via the CK reaction contributes to heart failure in patients with pressure-overload LVH. Unfortunately, a means to increase CK activity in the failing human heart has yet to be identified, and thus, the contractile consequences of such a metabolic intervention are unknown. Many genetic and phenotypic factors, including neuroendocrine, intracellular signaling cascades, extracellular changes, and mechanical remodeling, have all been shown to be associated with the progression to failure in hypertrophic hearts.50,51 The present metabolic findings do not diminish the importance of these factors but rather offer the added perspective that reduced ATP supply may act as both an initiating event and a consequence. Inadequate ATP availability would initiate and accentuate the adverse consequences of energy-dependent pathways.50 Conversely, factors that increase energy demand, such as adrenergic stimulation and biomechanical remodeling, exaggerate any energetic deficit.51 Future studies to define the interactions between inadequate energy delivery and well-characterized pathways that contribute to the pathophysiology of LVH and CHF are needed.
Conclusions
These results demonstrate that it is the kinetics of CK energy rather than the metabolite pool sizes or relative ratios that distinguishes compensated and failing hypertrophic human hearts. The findings are consistent with prior animal studies and demonstrate for the first time that reduced ATP kinetics is related to the development of heart failure rather than to the severity of hypertrophy. The reduction in CK flux in LVH+CHF is greater than that previously reported in human DCM, is similar in systolic and nonsystolic heart failure, and may be of a sufficient magnitude to impair the temporal buffering of ATP at the myofibrils. This provides a potential metabolic mechanism contributing to the systolic and diastolic dysfunction in failing human hypertrophied myocardium and provides a rationale to guide new investigations into means of augmenting CK flux in hypertrophied failing hearts.
Supplementary Material
Acknowledgments
We thank Dr Robert G. Shulman for several helpful discussions about temporal ATP buffering and acknowledge statistical assistance from Drs Glenn Hirsch and Shenghan Lai. Tricia Steinberg, RN, assisted with patient enrollment.
Footnotes
The online-only Data Supplement is available at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.106.613646/DC1.
Clinical trial registration information—URL: http://www.clinicaltrials.gov. Unique identifier: NCT00181259.
This work was supported by grants HL61912, HL63030, and HL56882 and by the Donald W. Reynolds Foundation.
Drs Smith, Bottomley, Schulman, Gerstenblith, and Weiss received salary support from the grants listed above but have no other relevant financial conflicts of interest.
References
- 1.Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980;69:576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Ann Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 4.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313:1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- 5.Wallimann T. Bioenergetics: dissecting the role of creatine kinase. Curr Biol. 1994;4:42–46. doi: 10.1016/s0960-9822(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 6.Massie B, Schaefer S, Garcia J, McKirnan MD, Schwartz GG, Wisneski JA, Weiner MW, White FC. Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation. 1995;91:1814–1823. doi: 10.1161/01.cir.91.6.1814. [DOI] [PubMed] [Google Scholar]
- 7.Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576. doi: 10.1161/01.cir.103.11.1570. [DOI] [PubMed] [Google Scholar]
- 8.Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976. doi: 10.1016/0140-6736(91)91838-l. [DOI] [PubMed] [Google Scholar]
- 9.Okada M, Mitsunami K, Inubushi T, Kinoshita M. Influence of aging or left ventricular hypertrophy on the human heart: contents of phosphorus metabolites measured by 31P MRS. Magn Res Med. 1998;39:772–782. doi: 10.1002/mrm.1910390515. [DOI] [PubMed] [Google Scholar]
- 10.Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van der Wall EE, de Roos A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation. 1999;99:2261–2267. doi: 10.1161/01.cir.99.17.2261. [DOI] [PubMed] [Google Scholar]
- 11.Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J. 1991;122:795–801. doi: 10.1016/0002-8703(91)90527-o. [DOI] [PubMed] [Google Scholar]
- 12.Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GAJ, Lackner K, Ertl G. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease: altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 13.Bittl JA, Balschi JA, Ingwall JS. Contractile failure and high-energy phosphate turnover during hypoxia: 31P-NMR surface coil studies in living rat. Circ Res. 1987;60:871–878. doi: 10.1161/01.res.60.6.871. [DOI] [PubMed] [Google Scholar]
- 14.Smith SH, Kramer MF, Reis I, Bishop SP, Ingwall JS. Regional changes in creatine kinase and myocyte size in hypertensive and nonhypertensive cardiac hypertrophy. Circ Res. 1990;67:1334–1344. doi: 10.1161/01.res.67.6.1334. [DOI] [PubMed] [Google Scholar]
- 15.Ye Y, Wang C, Zhang J, Cho YK, Gong G, Murakami Y, Bache RJ. Myocardial creatine kinase kinetics and isoform expression in hearts with severe LVH hypertrophy. Am J Physiol. 2001;281:H376–H386. doi: 10.1152/ajpheart.2001.281.1.H376. [DOI] [PubMed] [Google Scholar]
- 16.Gong G, Liu J, Liang P, Guo T, Hu Q, Ochiai K, Hou M, Ye Y, Wu X, Mansoor A, From AH, Ugurbil K, Bache RJ, Zhang J. Oxidative capacity in failing hearts. Am J Physiol. 2003;285:H541–H548. doi: 10.1152/ajpheart.01142.2002. [DOI] [PubMed] [Google Scholar]
- 17.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 18.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 20.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 21.Bottomley PA, Ouwerkerk R, Lee RF, Weiss RG. The four-angle saturation transfer (FAST) method for measuring creatine kinase reaction rates in vivo. Magn Reson Med. 2002;47:850–863. doi: 10.1002/mrm.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottomley PA, Atalar E, Weiss RG. Human cardiac high-energy phosphate metabolite concentrations by 1D-resolved NMR spectroscopy. Magn Reson Med. 1996;35:664–670. doi: 10.1002/mrm.1910350507. [DOI] [PubMed] [Google Scholar]
- 23.Bottomley PA, Hardy CJ, Roemer PB. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med. 1990;14:425–434. doi: 10.1002/mrm.1910140302. [DOI] [PubMed] [Google Scholar]
- 24.Yabe T, Mitsunami K, Inubushi T, Kinoshita M. Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy. Circulation. 1995;92:15–23. doi: 10.1161/01.cir.92.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Laine H, Katoh C, Luotolahti M, Yki-Jarvinen H, Kantola I, Jula A, Takala TO, Ruotsalainen U, Iida H, Haaparanta J, Nuutila P, Knuuti J. Myocardial oxygen consumption is unchanged but efficiency is reduced in patients with essential hypertension and left ventricular hypertrophy. Circulation. 1999;100:2425–2430. doi: 10.1161/01.cir.100.24.2425. [DOI] [PubMed] [Google Scholar]
- 26.Bache RJ, Zhang J, Murakami Y, Zhang Y, Cho YK, Merkle H, Gong G, From AH, Ugurbil K. Myocardial oxygenation at high workstates in hearts with left ventricular hypertrophy. Cardiovasc Res. 1999;42:616–626. doi: 10.1016/s0008-6363(98)00332-0. [DOI] [PubMed] [Google Scholar]
- 27.Saupe KW, Spindler M, Hopkins JAC, Shen W, Ingwall JS. Kinetic, thermodynamic, and developmental consequences of deleting creatine kinase isoenzymes from the heart. J Biol Chem. 2000;275:19742–19746. doi: 10.1074/jbc.M001932200. [DOI] [PubMed] [Google Scholar]
- 28.Jung W, Sieverding L, Breuer J, Hoess T, Widmaier S, Schmidt O, Bunse M, van Erckelens F, Apitz J, Lutz O, Dietze GJ. 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation. 1998;97:2536–2542. doi: 10.1161/01.cir.97.25.2536. [DOI] [PubMed] [Google Scholar]
- 29.Bottomley PA. MR spectroscopy of the human heart: the status and the challenges. Radiology. 1994;191:593–612. doi: 10.1148/radiology.191.3.8184033. [DOI] [PubMed] [Google Scholar]
- 30.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P SLOOP magnetic resonance spectroscopy. J Am Coll Cardiol. 2002;40:1267–1274. doi: 10.1016/s0735-1097(02)02160-5. [DOI] [PubMed] [Google Scholar]
- 31.Bittl JA, Ingwall JS. Intracellular high-energy phosphate transfer in normal and hypertrophied myocardium. Circulation. 1987;74(suppl I):I-96–I-101. [PubMed] [Google Scholar]
- 32.Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, Takahashi M, Morikawa S, Inubushi T, Nakamura Y, Kinoshita M, Horie M. Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol. 2003;42:1587–1593. doi: 10.1016/j.jacc.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Bittl JA, DeLayre J, Ingwall JS. Rate equation for creatine kinase predicts the in vivo reaction velocity: 31P NMR surface coil studies in brain, heart and skeletal muscle of the living rat. Biochemistry. 1987;26:6083–6090. doi: 10.1021/bi00393a021. [DOI] [PubMed] [Google Scholar]
- 34.Zweier JL, Jacobus WE, Korecky B, Brandejs-Barry Y. Bioenergetic consequences of cardiac phosphocreatine depletion induced by creatine analogue feeding. J Biol Chem. 1991;266:20296–20304. [PubMed] [Google Scholar]
- 35.Horn M, Remkes H, Stromer H, Dienesch C, Neubauer S. Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation. 2001;104:1844–1849. doi: 10.1161/hc3901.095933. [DOI] [PubMed] [Google Scholar]
- 36.Crozatzier B, Badoul T, Boehm E, Ennezat PV, Guenoun T, Su J, Veksler V, Hittinger L, Ventura-Clapier R. Role of creatine kinase in cardiac excitation-contraction coupling: studies in creatine kinase-deficient mice. FASEB J. 2002;16:653–660. doi: 10.1096/fj.01-0652com. [DOI] [PubMed] [Google Scholar]
- 37.Spindler M, Meyer K, Stromer H, Leupold A, Boehm E, Wagner H, Neubauer S. Creatine kinase-deficient hearts exhibit increased susceptibility to ischemia-reperfusion injury and impaired calcium homeostasis. Am J Physiol. 2004;287:H1039–H1045. doi: 10.1152/ajpheart.01016.2003. [DOI] [PubMed] [Google Scholar]
- 38.Kaasik A, Veksler V, Boehm E, Novotova M, Minajeva A, Ventura-Clapier R. Energetic crosstalk between organelles: architectural integration of energy production and utilization. Circ Res. 2001;89:153–159. doi: 10.1161/hh1401.093440. [DOI] [PubMed] [Google Scholar]
- 39.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 40.Ogut O, Brozovich FV. Creatine phosphate consumption and the acto-myosin crossbridge cycle in cardiac muscles. Circ Res. 2003;93:54–60. doi: 10.1161/01.RES.0000080536.06932.E3. [DOI] [PubMed] [Google Scholar]
- 41.Robitaille PM, Merkle H, Lew B, Path G, Hendrich K, Lindstrom P, From AHL, Garwood M, Bache RJ, Ugurbil K. Transmural high energy phosphate distribution and response to alterations in workload in the normal canine myocardium as studied with spatially localized 31P NMR spectroscopy. Magn Reson Med. 1990;16:91–116. doi: 10.1002/mrm.1910160110. [DOI] [PubMed] [Google Scholar]
- 42.Kantor HL, Briggs RW, Metz KR, Balaban RS. Gated in vivo examination of cardiac metabolites with 31P nuclear magnetic resonance. Am J Physiol. 1986;251:H171–H175. doi: 10.1152/ajpheart.1986.251.1.H171. [DOI] [PubMed] [Google Scholar]
- 43.Bottomley PA, Herfkens RJ, Smith LS, Bashore TM. Altered phosphate metabolism in myocardial infarction: P-31 MR spectroscopy. Radiology. 1987;165:703–707. doi: 10.1148/radiology.165.3.2961004. [DOI] [PubMed] [Google Scholar]
- 44.Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang S-C, Kofoed KF, Weismueller S, Czernin J, Phelps ME, Schelbert H. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon11]acetate. J Nucl Med. 1998;39:272–280. [PubMed] [Google Scholar]
- 45.Ganz W, Tamura K, Marcus HS, Ronoso R, Yoshida S, Swan HJC. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation. 1971;44:181–195. doi: 10.1161/01.cir.44.2.181. [DOI] [PubMed] [Google Scholar]
- 46.Monroe RG. Myocardial oxygen consumption during ventricular contraction and relaxation. Circ Res. 1964;14:294–300. doi: 10.1161/01.res.14.4.294. [DOI] [PubMed] [Google Scholar]
- 47.Gibbs CL, Wendt IR, Kotsanas G, Young IR. The energy cost of relaxation in control and hypertrophic rabbit papillary muscles. Heart Vessels. 1990;5:198–205. doi: 10.1007/BF02058690. [DOI] [PubMed] [Google Scholar]
- 48.Shulman RG, Rothman DL. Lactate, glycogen and fatigue. In: Shulman RG, Rothman DL, editors. Metabolomics by In Vivo NMR. West Sussex, England: Wiley & Sons; 2005. [Google Scholar]
- 49.Liao R, Jain M, Cui L, D’Agostino L, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2003;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 50.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 51.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


