Abstract
After the transection of the Schaffer collateral pathway in hippocampal slice cultures, reactive sprouting is induced in the CA3 area, and eventually synaptic transmission between areas CA1 and CA3 is restored. Using this model, we have studied the role of ionotropic glutamate receptors in the initiation of axonal sprouting and the regeneration of functional synapses. We show that neither reactive sprouting nor functional recovery of synaptic transmission occur in the presence of the non-N-methyl-d-aspartate (NMDA) receptor antagonist 6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione (CNQX). In contrast, the NMDA receptor antagonists methyl-10,11-dihydro-5-H-dibenzocyclohepten-5,10-imine (MK-801) or 3-(RS)-2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid (CPP) did not interfere with these processes. Moreover, we observed that the application of NMDA receptor antagonists induced massive axonal sprouting and an increase in the frequency of miniature excitatory postsynaptic currents in unlesioned cultures. Our results thus indicate that NMDA and non-NMDA receptors exert a differential effect on reactive sprouting and the recovery of synaptic transmission after injury in the hippocampus. Activation of non-NMDA receptors appears necessary for these processes to occur, whereas activation of NMDA receptors suppresses growth-associated protein -43 expression and axonal outgrowth.
It is widely believed that an injury to the axons of neurons in the adult mammalian central nervous system (CNS) results in irreversible damage because they are incapable of regenerating. There have, however, been several convincing demonstrations of regeneration after axonal injury under certain experimental conditions (1–4). The ability of axons in the adult CNS to regrow is thought to depend on the balance of growth-promoting and growth-inhibitory factors in the local environment (e.g., refs. 5–7). Functional recovery after injury requires not only axonal growth but also the reestablishment of appropriate synaptic connections (for review, see ref. 8).
Reactive sprouting and neosynaptogenesis have been studied extensively in the hippocampus (for review, see refs. 9–11). For example, reactive sprouting of the mossy fiber axons and of other axons occurs in response to disruption of perforant path inputs and subsequent deafferentation of dentate granule cells (e.g., refs. 10, 12). These collaterals form new functional synapses (13). More recently, it has become clear that hippocampal pyramidal cells are also capable of undergoing reactive sprouting in response to deafferentation resulting from destruction of area CA3 or Schaffer collateral transection (e.g., refs. 14–16). Restoration of synaptic transmission from area CA3 to area CA1 has been reported after transection of the Schaffer collateral pathway in organotypic hippocampal slice cultures (14).
We have previously reported that antibodies against the growth-associated protein of 43 kDa (GAP-43) (17) are a useful tool for visualizing the sprouting of pyramidal cell axons (16). Using this antibody, we demonstrated that new Schaffer collaterals were generated de novo by CA3 cells after transection in hippocampal slice cultures.
It is of importance to understand the sequence of events underlying the initiation of sprouting and the subsequent reestablishment of functional synapses. One important factor is likely to be the neurotransmitter released by the regenerating axon, i.e., glutamate in the case of CA3 pyramidal cell axons. There is considerable evidence, for example, that glutamate receptor activation is pivotal in establishing appropriate synaptic connections during development (e.g., refs. 18, 19). In addition, the development of reactive mossy fiber sprouting during kindling was recently shown to be reduced or blocked by antagonists of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors (20).
In this study, we addressed the question of whether sustained blockade of NMDA or non-NMDA receptors would affect reactive sprouting of CA3 cell axons and neosynaptogenesis.
MATERIALS AND METHODS
Preparation of Slice Cultures.
Slice cultures were prepared from 6- to 7-day-old Wistar rat pups, as previously described (21, 22). In brief, 400-μm-thick hippocampal slices were attached to glass coverslips, by using clotted chicken plasma, placed in sealed test tubes with serum containing medium, and maintained on a roller-drum in an incubator at 36°C for 14 days.
Schaffer Collateral Transection.
Lesions were made with a razor blade shard while bathing the cultures in saline containing tetrodotoxin (TTX, Latoxan, Rosans, France, 0.5 μM) and 10 mM Mg2+ to prevent excitotoxic injury. The cut extended from the alveolar surface at the border between areas CA1 and CA3 to the subiculum, producing two completely isolated halves (Fig. 1A). Control sister cultures were also exposed to TTX and 10 mM Mg2+. Cultures were then returned to normal medium.
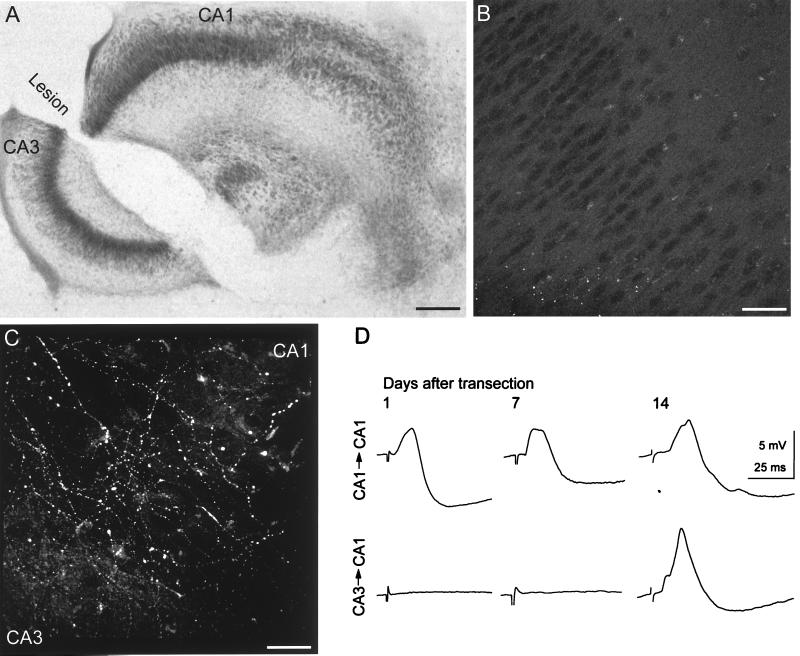
Figure 1.
Functional recovery after Schaffer collateral transection in hippocampal slice cultures. (A) Nissl-stained culture illustrating extent of the transection between areas CA3 and CA1. Bar = 1 mm. (B) Confocal image of background GAP-43-immunoreactivity within area CA3 after 14 days in vitro, i.e., at the time lesions were made. No labeled fibers can be seen. Bar = 60 μm. (C) Confocal image of the lesion site taken 14 days after lesion. Numerous GAP-43-immunoreactive fibers cross the lesion into area CA1. (D) Evoked synaptic responses recorded intracellularly from CA1 pyramidal cells in response to a stimulus in areas CA1 (Upper) or CA3 (Lower) at the indicated times after Schaffer collateral transection. Functional recovery across the lesion was observed 14 days after the lesion.
Pharmacological Treatment.
Twenty-four hours after the lesion, cultures were incubated in serum-containing medium containing either the competitive non-NMDA receptor antagonist 6-nitro-7-sulfamoylbenzo[f]quinoxaline-2,3-dione (NBQX, Tocris Cookson, Bristol, UK) or 6-cyano-7-nitroquinoxaline-2,3-dione (Tocris Cookson) or the competitive NMDA receptor antagonist methyl-10,11-dihydro-5H-dibenzocyclohepten-5,10-imine (MK-801, Tocris Cookson) or the noncompetitive NMDA receptor antagonists or 3-(RS)-2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid (CPP, 10 μM, a gift from Novartis, Basel, Switzerland). The media containing the substances were replaced daily. Control sister cultures received fresh control medium daily.
Immunohistochemistry.
The procedures used for immunohistochemistry were as described previously (16). The anti-GAP-43 monoclonal antibody (10E8/E7; ref. 17) was provided by K. Meiri (State University of New York Health Science Center, Syracuse, NY). An anti-rat glial fibrillary acidic protein antibody (GFAP, Boehringer Mannheim) was used to identify reactive astrocytes. Control and treated cultures were processed simultaneously.
Electrophoresis and Western Blotting for GAP-43 and Tubulin.
Hippocampal cultures (n = 10 for each condition; repeated in four series of cultures) were freed from the plasma clot, pooled, and lysed in buffer containing 1% Nonidet P-40, 1% sodium cholate, 0.05% SDS, 20 mM Tris (pH 7.5), and 100 mM NaCl. Protein concentration was determined using the Bradford dye-binding assay (Bio-Rad), according to the manufacturer’s protocol, with BSA as the standard. Fifteen micrograms of total protein was separated by SDS/PAGE (10%) under reducing conditions and semi-dry blotted onto poly(vinylidene difluoride) membranes (Millipore). Membranes were incubated with anti-GAP-43 antibodies (1:400 dilution of the 10E8/E7 hybridoma supernatant), followed by a horseradish peroxidase-conjugated anti-mouse secondary antibody (1:2,500, Amersham Pharmacia). The immunoreactive proteins were detected with an enhanced chemiluminescence kit (ECL, Amersham Pharmacia) according to the manufacturer’s protocol, by using ECL-Hyperfilm (Amersham Pharmacia). The same blot was rescreened with antibodies directed against the juvenile form of β-tubulin (Sigma; 1:500). A parallel gel, run under the same conditions, was silver stained to control for equal protein loading (ref. 23). Quantification of signal intensities in Western blots was performed with a densitometer using mcid-m2 software (Imaging Research, St. Catherine’s, ON, Canada).
Electrophysiology.
Cultures were placed in a recording chamber attached to the stage of an inverted microscope and perfused with saline containing: 149 mM Na+; 146.3 mM Cl−; 2.7 mM K+; 3.8 mM Ca2+; 1.5 mM Mg2+; 11.6 mM HCO3−; 0.4 mM H2PO4−; 5.6 mM glucose; and 10 mg/l phenol red (pH 7.4). Sharp microelectrode recordings were made in current-clamp mode from CA1 pyramidal cells by using a 1 M potassium methylsulfate filling solution. Synaptic potentials were evoked with 155 mM NaCl-containing electrodes placed within area CA1 or CA3, by using intensities of 0.5 to 10 μA. Cells were held at −60 to −65 mV to facilitate discrimination of excitatory and inhibitory postsynaptic potentials [excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs)]. All recordings were carried out at room temperature. For whole-cell recordings, the extracellular saline was prepared as above, but with 2.5 mM CaCl2/2 mM MgCl2/0.5 μM tetrodotoxin/50 μM picrotoxin (Fluka)/100 μM CPP. CA1 pyramidal cells were identified by using infrared differential interference contrast microscopy optics, and recordings were performed by using patch-clamp electrodes (3–5 MΩ) filled with a solution containing: 140 mM potassium gluconate; 10 mM KCl; 5 mM Hepes; 1.1 mM EGTA; 4 mM MgCl2; 10 mM phosphocreatine, pH 7.25; 285 mOsm. Membrane currents were filtered at 2 kHz and sampled at 4 kHz. All recordings were performed at a holding potential of −70 mV. Series resistance (typically between 7 and 15 MΩ) was regularly checked, and cells with more than a 10% change were excluded from the analysis. Miniature excitatory postsynaptic currents (mEPSCs) were detected and analyzed off-line (24, 25). To construct cumulative amplitude histograms, 100 events were randomly selected from each neuron. To obtain the mean mEPSC waveform from each neuron, all events were aligned on the rising phase and averaged. The averaged mEPSCs were fitted with a single exponential function to determine the decay time-constant. The mean mEPSC waveform for a given treatment was obtained from the averaged mEPSC from each cell. All data are reported as mean ± SEM. Statistical comparisons were made by using two-tailed unpaired Student’s t test. The Kolmogorov–Smirnov test was used to compare cumulative distributions.
RESULTS
Regeneration After Schaffer Collateral Transection.
The protein GAP-43 is highly expressed in growing and regenerating axons of the peripheral and central nervous system (17). GAP-43 immunocytochemistry was therefore used to detect newly formed axons. GAP-43-immunoreactive fibers were detected only in the first few days after the hippocampal slices were placed in culture (16), and no GAP-43-immunoreactive fibers were apparent in cultures maintained 14 days in vitro (Fig. 1B), i.e., at the time the transections were made.
Three to seven days after lesioning, GAP-43-immunoreactive fibers with visible growth cones were apparent within area CA3, but not in area CA1, as reported previously (16). At these times, only a small number of GFAP-immunoreactive cells were visible at the edges of the lesion (not shown).
Fourteen days after the lesion, numerous GFAP-immunoreactive cells had formed a bridge across the lesion cavity. Coincident with the formation of this bridge, the GAP-43-immunoreactive axons extending from area CA3 were seen to grow on top of the GFAP-immunoreactive cells and to enter area CA1 (Fig. 1C). GAP-43-immunoreactive axons could be detected to extend up to 1.4 mm into area CA1 beyond the lesion. In none of four cultures examined in which GFAP-positive bridges were absent could axons be seen to cross the lesion.
Functional Recovery After Transection.
Electrophysiological techniques were used to establish whether new fibers formed functional synapses with CA1 cells. A stimulating electrode was placed in area CA3, and intracellular recordings were made from CA1 pyramidal cells. In no culture examined 1 or 7 days after the lesion could evoked synaptic responses be detected (n = 4 and 6 cultures, respectively) (Fig. 1D). When the stimulating electrode was then moved into area CA1 while recording from the same CA1 pyramidal cell, a small EPSP followed by an IPSP could always be evoked, indicating the cells were capable of responding to synaptically released transmitter, had any Schaffer collateral connection been present. The Schaffer collateral connections between area CA3 and CA1 had therefore been fully transected. By 14 days after lesion, in contrast, evoked EPSP/IPSP sequences were recorded from CA1 pyramidal cells in response to stimulation across the lesion in 11 of 14 cultures tested (Fig. 1D).
We thus conclude that transection of mature CA3 cell axons leads to the de novo sprouting of GAP-43-immunoreactive axon collaterals that reinnervate area CA1 and that functional synapses are then formed with both pyramidal cells and inhibitory interneurons, as reported previously (14).
Effects of Glutamate Receptor Antagonists on Lesioned Cultures.
The role of glutamate receptors in functional recovery from lesions was tested with chronic application of glutamate receptor antagonists to lesioned cultures. Lesions were made in cultures maintained >14 days in vitro. Cultures were then allowed to recover for 24 hr before the addition of the antagonists for 14 days, i.e., until the time point when recovery was readily detectable in untreated lesioned cultures.
Functional recovery did not occur in any of 11 cultures treated with the selective non-NMDA receptor antagonist NBQX (20 μM) (Fig. 2A). EPSP/IPSP sequences in CA1 pyramidal cells could be elicited only when stimulating within area CA1 but not from area CA3. In contrast, functional recovery was seen in six of nine lesioned cultures treated with the selective NMDA receptor antagonist MK-801 (20 μM) (Fig. 2A). Comparable effects were found after application of 20 μM APV (recovery in three of four lesioned cultures).
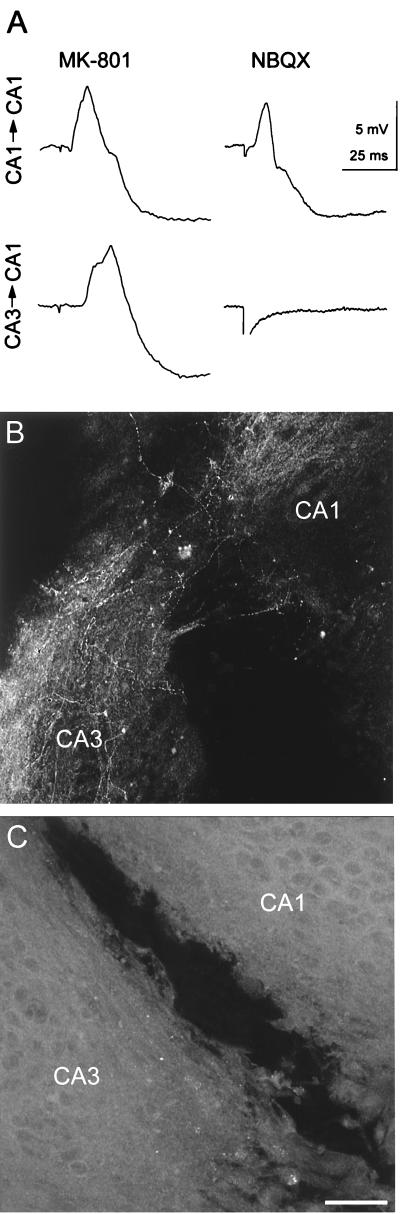
Figure 2.
Glutamate receptor antagonists and functional recovery after lesions. (Upper) Evoked synaptic responses recorded intracellularly from CA1 pyramidal cells in response to a stimulus in areas CA1 or CA3 at the indicated times after 14 days of treatment with either 20 μM MK-801 or 20 μM NBQX. Functional recovery was thus observed when NMDA, but not non-NMDA, receptors were blocked. There was no significant difference between the average latencies of control and MK-801 treated cultures (P > 0.05; unpaired two-tailed t test). (Lower) Confocal images of GAP-43-immunoreactive fibers at the lesion site in cultures treated for 14 days with (B) 20 μM MK-801 or (C) 20 μM NBQX. Blocking non-NMDA receptors prevented the lesion-induced appearance of GAP-43-immunoreactive fibers. Bar = 60 μm.
Lack of functional recovery after transection in NBQX-treated cultures could have resulted from a failure of axons to sprout or from an inability of the sprouted axons to form functional synapses. GAP-43 immunohistochemistry was used to distinguish between these possibilities. Consistent with the physiological results, numerous GAP-43-immunoreactive fibers were readily detected in area CA3 of lesioned cultures treated for 7 or 14 days with MK-801 (20 μM) (Fig. 2B) (all of 10 and 11 cultures, respectively), with maximal staining at 7 days after lesion. In contrast, GAP-43-immunoreactive fibers were never seen either within area CA3 or crossing the lesion in cultures treated with the non-NMDA receptor antagonist NBQX (20 μM) for 3–14 days (all of 12 cultures), although a GFAP-positive bridge was present in the lesion cavity (Fig. 2C). Identical effects were also found after application of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (n = 5 cultures). We conclude that lesion-induced sprouting does not occur when non-NMDA receptors are blocked after the lesion.
Induction of Axonal Sprouting by NMDA Receptor Antagonists in Unlesioned Cultures.
In lesioned cultures, GAP-43-immunoreactive fibers originate only in area CA3 and not in area CA1 (16). In lesioned cultures treated with MK-801, however, we were surprised to observe that numerous GAP-43-immunoreactive fibers were present in area CA1 as well as area CA3. We therefore asked whether application of MK-801 or APV to unlesioned cultures would per se increase GAP-43 levels. Indeed, MK-801 (20 μM), APV (20 μM) or CPP (10 μM) treatment resulted in the appearance of many GAP-43-immunoreactive fibers in cultures maintained for >14 days in vitro. This effect could be seen as early as 3 days after treatment (Fig. 3A). Processes were labeled in both areas CA3 and CA1, and immunoreactivity was maximal after 7 days of treatment (Fig. 3B). Large numbers of fibers could be seen growing out of the cultures into the surrounding plasma clot from areas CA3 and CA1 and extending for distances of up to 150 μm (not shown). Similar fiber outgrowth is seen in cultures within the first week after explantation but is never seen in cultures maintained for >14 days in vitro. After 14 days of continuous treatment, immunoreactive fibers were no longer apparent (Fig. 3C). Consistent with the immunohistochemical results, Western blot analysis revealed that GAP-43 protein content in cultures treated with MK-801 (20 μM) for 7 days increased by 281 ± 22% when compared with control level (n = four experiments) (Fig. 3F). The levels of another axonal protein, β-tubulin III (26), were also highly elevated after treatment with MK-801 (20 μM) for 7 days compared with either control cultures or cultures treated with NBQX (20 μM) for 7 days, in which levels were barely detectable (n = four experiments).
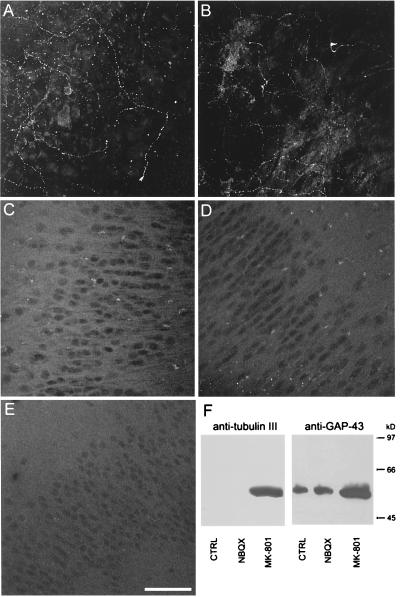
Figure 3.
Effects of glutamate receptor antagonists on GAP-43-immunoreactivity. Confocal images of GAP-43-immunoreactivity in area CA3 of unlesioned cultures treated with 20 μM MK-801 for 3 (A), 7 (B), or 14 days (C) in CA1 after 14 days of MK-801 treatment. Confocal images of GAP-43-immunoreactivity in area CA3 of cultures treated with 20 μM NBQX for 7 days (D), and in area CA1 (E). Bar = 70 μm. (F) GAP-43- and β-tubulin III-immunoreactive protein levels in response to MK-801 and NBQX treatment for 7 days in total lysates from hippocampal slice cultures. Detergent-solubilized cell lysates were separated on a 15% SDS-polyacrylamide gel and immunoblotted with anti-GAP43 and anti-tubulin antibodies. Note the increase in both GAP-43- and β-tubulin-immunoreactivity in MK-801-treated cultures compared with either control or NBQX-treated sister cultures.
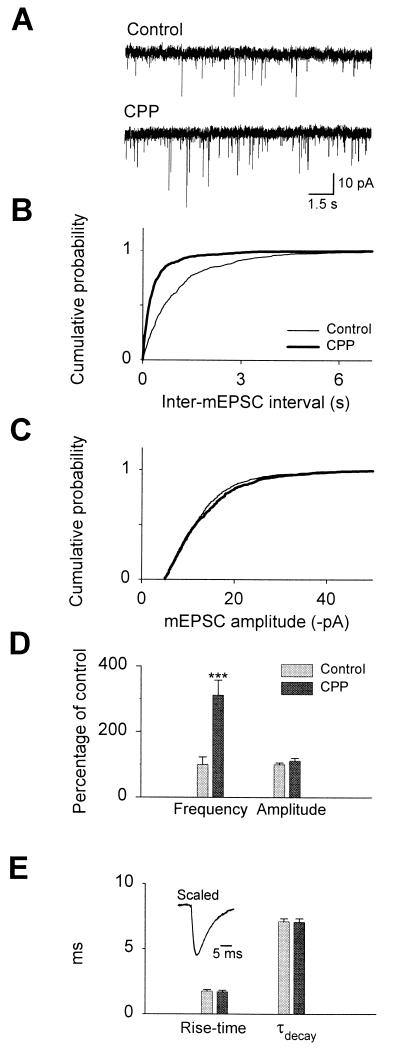
Electrophysiological analysis of mEPSCs was consistent with an NMDA receptor antagonist-induced sprouting and the formation of new synaptic connections. The frequency of (S)-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated mEPSCs markedly increased after 14 days of treatment (control: 1.44 ± 0.33 Hz vs. CPP 5.41 ± 0.66 Hz; n = 10; P < 0.005; Fig. 4 A and D), with no significant change in the mean mEPSC amplitude; Fig. 4C (control −13.3 ± 0.6 pA vs. CPP −14.7 ± 1.2 pA; n = 10; Fig. 4A) or kinetics (10–90% rise-time in control: 1.82 ± 0.07 ms vs. CPP: 1.71 ± 0.11 ms, P > 0.05; control decay time-constant: 7.1 ± 0.22 ms vs. CPP: 7.07 ± 0.25 ms, P > 0.05; n = 10; Fig. 4E).
Figure 4.
Differential effect of CPP on mEPSC frequency and amplitude. (A) Representative recordings from control (Left) and 100 μM CPP-treated (Right) cultures. (B) Cumulative distributions for inter-mEPSC intervals and (C) mEPSC amplitudes recorded from control and CPP-treated cultures. (D) Average mEPSC frequencies and amplitudes expressed as percentage of values in untreated control sister cultures. (E) Average mEPSC 10–90% rise-times and decay time-constants. (Inset) Averaged mEPSC waveforms (scaled and superimposed) from all control and CPP-treated cells.
The induction of sprouting was specific for NMDA receptor antagonists. No immunocytochemical labeling of fibers was apparent in either area CA1 or CA3 at any point in cultures treated for 3–14 days with NBQX (Fig. 3 D and E), and Western blot analysis revealed a decrease in GAP-43 content to 15 ± 4% of the control level after 7 days of treatment (n = four experiments) (Fig. 3F).
DISCUSSION
We have observed that transection of Schaffer collateral axons in hippocampal slice cultures leads to the initiation of axonal sprouting by CA3 pyramidal cells within 3 days of the injury, regrowth of the axons into area CA1, and the reestablishment of functional synaptic transmission after 14 days. We have confirmed and extended previous studies (14) by showing that the Schaffer collateral axons were mature at the time the lesion was made, in that they expressed little or no detectable GAP-43. Furthermore, we have shown that regenerating fibers traverse the apparently inhospitable conditions in the lesion cavity via GFAP-immunoreactive cells. The regeneration proceeded somewhat slower in our experiments than in those of Stoppini et al. (14). This may reflect differences in the substrate within the lesion (i.e., glass vs. Teflon-coated nitrocellulose membranes) or the expanse of the lesion. The extensive ingrowth into area CA1 in these cultures suggests, as well, that growth-repulsive factors (e.g., ref. 5) are either not heavily expressed or that they do not hinder regrowing Schaffer collateral axons. We noted also that GAP-43-immunoreactive fibers were not seen to enter the dentate gyrus, suggesting the release in area CA1 of some target-derived attractant for regrowing CA3 cell axons.
Effects of Glutamate Receptor Antagonists on Regeneration and Synaptogenesis.
Lesion-induced axonal sprouting by CA3 pyramidal cells could not be detected with the GAP-43 antibody at any time after application of the non-NMDA glutamate receptor antagonist NBQX. Because no GAP-43-immunoreactive fibers were seen shortly after the lesion, it appears that NBQX prevented the induction of the sprouting response, rather than later steps during regeneration. The mechanisms controlling reactive sprouting thus appear to be different for pyramidal cells and dentate granule cells. In the latter cell type, NMDA receptor antagonists prevent sprouting of supragranular mossy fibers in vivo in response to a kindling stimulation protocol (20). In addition, the creation of vacant synaptic territory by deafferentation induces sprouting from dentate granule cells (12) but not from CA1 pyramidal cells (16).
The mechanisms underlying this effect are not known. One possibility is that NBQX decreases the amount of action potential discharge in the CA3 cells by decreasing excitatory synaptic drive and that this decrease in activity depresses the sprouting response. This seems unlikely, however, because lesion-induced sprouting of CA3 cells was observed in the presence of tetrodotoxin (not shown). Alternatively, lesion-induced axonal sprouting may depend on the release of neurotrophic factors and may require non-NMDA receptor activation, as suggested previously (27).
Induction of Axonal Sprouting by NMDA Receptor Antagonists.
We have unexpectedly observed that NMDA receptor antagonists induce axonal sprouting by CA3 and CA1 cells in unlesioned cultures. Four lines of evidence indicate that MK-801 did not merely increase the GAP-43 content of existing axons, but rather induced the sprouting of new axon collaterals. First, numerous axons could be seen radiating out from MK-801-treated cultures into the surrounding plasma clot. Such axons are seen in control cultures during synaptogenesis in the first few days in vitro, but are never seen in cultures maintained for >14 days. Second, some GAP-43-immunoreactive fibers possessed growth cones and often followed unusual meandering trajectories, like those regenerating after Schaffer collateral transection (16). Third, the content of β-tubulin III was also elevated in Western blots, indicating that increased expression of axonal proteins is not limited to GAP-43. Fourth, the frequency of mEPSCs recorded from CA1 pyramidal cells in slices treated with CPP increased 3-fold, consistent with an increase in the number of functional synapses. However, alternative explanations such as an increase in the probability of release or the activation of silent synapses are also possible. Given our immunohistochemical and Western blot results, we suggest that the increase in mEPSC frequency is most likely caused by an increase in the number of functional synaptic connections formed by newly sprouted axons with target cells in area CA1.
The mechanism by which pyramidal cells detect and react to a change in NMDA receptor activation is unknown. Because there appear to be no presynaptic NMDA receptors on axons of CA3 pyramidal cells (25), it is likely that the induction of sprouting by MK-801 reflects changes in the activation of NMDA receptors in their somatodendritic membrane. There is evidence that the expression of brain-derived neurotrophic factor by hippocampal cells is affected by NMDA receptor antagonists (28–30). One possibility, therefore, is that MK-801 results in a change in the level of one or more neurotrophins, and that this then initiates axonal sprouting. We are currently testing this hypothesis. Furthermore, it is interesting to note that CA1 cells sprout in response to MK-801 treatment but not after Schaffer collateral transection. A general inability of CA1 cells to sprout thus cannot account for the failure of deafferented CA1 cells to generate new axons.
In conclusion, the balance between NMDA and non-NMDA receptor activation plays a critical role in determining the extent of axonal sprouting by hippocampal pyramidal cells in organotypic slice cultures. Non-NMDA receptor antagonists, and by inference insufficient non-NMDA receptor activation, prevent lesion-induced axonal sprouting by CA3 pyramidal cells. In contrast, NMDA receptor antagonists, and by inference insufficient NMDA receptor activation, induce axonal sprouting. We therefore suggest that NMDA receptor activation normally suppresses axonal outgrowth and the formation of new synaptic connections by hippocampal pyramidal cells.
Acknowledgments
We thank L. Heeb, L. Rietschin B. Niederöst, R. Kagi, H. Kasper, and R. Schöb for excellent technical assistance, Dr. K. F. Meiri for providing the anti-GAP-43 antibody-producing cells, Dr. P. Vincent for the mEPSC analysis program, and Prof. M. E. Schwab and Drs. J.-C. Poncer and M. Scanziani for helpful discussions and for critically reading the manuscript. This work was supported by the Dr. Eric Slack-Gyr and Swiss National Science (31–41829.94) Foundations and the Epilepsy Foundation of America.
ABBREVIATIONS
- CPP
3-(RS)-2-carboxypiperazine-4-yl)-propyl-1-phosphonic acid
- EPSP
excitatory postsynaptic potential
- GAP-43
growth associated protein of 43 kDa
- IPSP
inhibitory postsynaptic potential
- mEPSC
miniature excitatory postsynaptic current
- MK-801
methyl-10,11-dihydro-5-H-dibenzocyclohepten-5,10-imine
- NBQX
6-nitro-7-sulfamoylbenzoquinoxaline-2,3-dione
- NMDA
N-methyl-d-aspartate
- GFAP
glial fibrillary acidic protein
References
- 1.Raisman G. Brain Res. 1969;14:25–48. doi: 10.1016/0006-8993(69)90029-8. [DOI] [PubMed] [Google Scholar]
- 2.David S, Aguayo A J. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 3.Vidal-Sanz M, Bray G M, Villegas-Pérez M P, Thanos S, Aguayo A J. J Neurosci. 1987;7:2894–2907. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnell L, Schwab M E. Nature (London) 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 5.Schwab M E, Kapfhammer J P, Bandtlow C E. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- 6.Schnell L, Schneider R, Kolbeck R, Barde Y-A, Schwab M E. Nature (London) 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- 7.Sawai H, Clarke D B, Kittlerova P, Bray G M, Aguayo A J. J Neurosci. 1996;16:3887–3894. doi: 10.1523/JNEUROSCI.16-12-03887.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguayo A J, Bray G M, Rasminsky M, Zwimper T, Carter D, Vidal-Sanz M. J Exp Biol. 1990;153:199–224. doi: 10.1242/jeb.153.1.199. [DOI] [PubMed] [Google Scholar]
- 9.Cotman C W, Nadler J V. In: Neuronal Plasticity. Cotman C W, editor. New York: Raven; 1978. pp. 227–269. [Google Scholar]
- 10.Cotman C W, Nieto-Sampedro M, Harris E W. Physiol Rev. 1981;61:684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- 11.Steward O. In: The Hippocampus. Isaacson R L, Pribran K H, editors. New York: Plenum; 1986. pp. 65–111. [Google Scholar]
- 12.Laurberg S, Zimmer J. J Comp Neurol. 1981;200:433–459. doi: 10.1002/cne.902000310. [DOI] [PubMed] [Google Scholar]
- 13.Wuarin J-P, Dudek F E. J Neurosci. 1996;16:4438–4448. doi: 10.1523/JNEUROSCI.16-14-04438.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoppini L, Buchs P-A, Muller D. Neuroscience. 1993;57:985–994. doi: 10.1016/0306-4522(93)90043-f. [DOI] [PubMed] [Google Scholar]
- 15.Perez Y, Morin F, Beaulieu C, Lacaille J-C. Eur J Neurosci. 1996;8:736–748. doi: 10.1111/j.1460-9568.1996.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 16.McKinney R A, Debanne D, Gähwiler B H, Thompson S M. Nat Med. 1997;3:990–996. doi: 10.1038/nm0997-990. [DOI] [PubMed] [Google Scholar]
- 17.Meiri K F, Pfenninger K H, Willard M B. Proc Natl Acad Sci USA. 1986;83:3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cline H, Debski E A, Constantin-Paton M. Proc Natl Acad Sci USA. 1987;84:4342–4345. doi: 10.1073/pnas.84.12.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bear M F, Kleinschmidt A, Gu Q, Singer W. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutula T, Koch J, Golarai G, Watanabe Y, McNamara J O. J Neurosci. 1996;16:7398–7406. doi: 10.1523/JNEUROSCI.16-22-07398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gähwiler B H. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 22.Gähwiler B H, Thompson S M, McKinney R A, Debanne D, Robertson R T. Culturing Nerve Cells. 2nd Ed. Cambridge, MA: MIT Press; 1998. pp. 461–498. [Google Scholar]
- 23.Morrisey J A. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 24.Vincent P, Marty A. Neuron. 1993;11:885–893. doi: 10.1016/0896-6273(93)90118-b. [DOI] [PubMed] [Google Scholar]
- 25.Capogna M, Gähwiler B H, Thompson S M. J Neurophysiol. 1996;76:3149–3158. doi: 10.1152/jn.1996.76.5.3149. [DOI] [PubMed] [Google Scholar]
- 26.Bisby M A, Tetzlaff W. Mol Neurosci. 1992;6:107–123. doi: 10.1007/BF02780547. [DOI] [PubMed] [Google Scholar]
- 27.Zafra F, Hengerer B, Leibrock J, Lindholm D, Thoenen H. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zafra F, Castrén E, Thoenen H, Lindholm D. Proc Natl Acad Sci USA. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castrén E, da Penha Berzaghi M, Lindholm D, Thoenen H. Exp Neurol. 1993;122:244–252. doi: 10.1006/exnr.1993.1124. [DOI] [PubMed] [Google Scholar]
- 30.Hughes P, Dragunow M, Beilharz E, Lawlor P, Gluckman P. NeuroReport. 1993;4:183–186. doi: 10.1097/00001756-199302000-00017. [DOI] [PubMed] [Google Scholar]