Abstract
Background
Cognitive impairment in general is known to predict functional disability, but it is not clear whether performance on specific cognitive domains predicts future disability trends among non-demented elderly.
Method
In a representative elderly community-based cohort over up to 10 years of follow-up, we examined predictors of longitudinal trajectories in ability to perform Instrumental Activities of Daily Living (IADL) among non-demented elderly. We used trajectory analyses to identify homogeneous groups with respect to trends over time in the numbers of IADL disabilities, and their association with baseline demographics, social engagement, depression, physical well-being, general and domain-specific cognitive functions. We excluded from these analyses those found to have dementia at baseline or at any time during follow-up.
Results
Trajectory analysis revealed 3 homogeneous latent groups which we characterized as No Decline (no decline in abilities to perform IADL tasks over the course of study), Moderate Decline (some functional decline) and Sharp Decline (steep functional decline followed by death). Compared to the Sharp Decline group, the No Decline group was associated with higher baseline functions in all cognitive domains, and the Moderate Decline group was associated with higher baseline functions in all cognitive domains except psychomotor speed and naming. The Moderate and No Decline Groups did not differ on any cognitive measure.
Conclusion
Among community dwelling elderly who remained free from dementia throughout the study, poorer scores in all cognitive domains predicted sharp functional decline followed by death.
Introduction
Cognitive impairment and dementia are strong predictors of incident disability (e.g., 1-3). It is unclear however, which, if any, specific domains of cognitive functions are particularly important in predicting future disability trends. Thus far, studies focusing on multiple domains of cognition and disabilities in non-clinical samples have been cross-sectional in design (4-6), while longitudinal studies have been limited to the examination of a single cognitive domain (executive function) (7-8). Our main aim in this longitudinal study is to examine whether specific cognitive domains predict trajectories of disability in instrumental activities of daily living (IADL) among non-demented elderly, after controlling for known confounders including physical and psychological well-being, social engagement, and demographic factors.
We identified latent groups (9) whose 8-year longitudinal trajectories varied in total numbers of IADL disabilities, and examined multiple cognitive functions as predictive factors for these trajectories.
Methods
The data reported here were collected as part of the Monongahela Valley Independent Elders Survey (MoVIES Project), a prospective epidemiological study of dementia in a largely rural, blue-collar community in Southwestern Pennsylvania. The background, cohort, and methods of the study have been reported in greater detail earlier (10, 11). Briefly, 1422 individuals aged 65 years or older living in the community were recruited by age-stratified random sampling of voter registration and other lists for the selected area, with a further 259 volunteers included from the same area at study entry (1987-1989). Surviving and consenting subjects were re-assessed in a series of biennial “waves” until 2002. The most frequent reason for attrition between successive waves was death (9%-14%), with less for other reasons such as dropout and relocation (average 2.8%).
Data on several variables including IADL were first collected at Wave 2 (1989-1991), which therefore served as the baseline for the current analyses. The cohort at Wave 2 (baseline) consisted of 1341 adults, aged 66+ years (mean 74.9, SD (5.5)). We excluded 122 prevalent cases of dementia (defined later) at baseline. To minimize the potential for undetected subclinical dementia to influence the results, we also excluded 253 incident cases identified during followup. After excluding 13 subjects (1.4%) with missing data, the remaining 953 subjects served as the basis of the current report. Informed consent was obtained according to procedures approved annually by the University of Pittsburgh Institutional Review Board.
Dementia assessment
Diagnosis of dementia was based on a multi-stage case-ascertainment process. At baseline and each follow-up, all participants were screened with a cognitive test battery described later, incorporating the neuropsychological panel of the Consortium to Establish a Registry for Alzheimer Disease (CERAD) (12). Clinical (diagnostic) assessment, blind to the screening data, was offered to all subjects who met operational definitions for “cognitive impairment” at any wave, and for “cognitive decline” between waves, and also to a matched sample of “unimpaired” controls selected at baseline (11, 13). Clinical assessment followed a standardized protocol to determine the presence or absence of dementia, according to the DSM-III-R (14), stage of dementia, according to the Clinical Dementia Rating (CDR) (15) on which a rating of 0 indicates no dementia and ratings of 0.5 and higher reflect increasing severity of dementia. For the present analyses, prevalent and incident dementia cases (CDR≥0.5) any time during follow-up were excluded.
Outcome variables
IADL measurement: IADL was assessed using the Older Americans Resources and Services questionnaire (16) , which asks about ability to perform 7 activities: using the telephone, getting to places out of walking distance, shopping for groceries (assuming subject has transportation), preparing meals, doing housework, taking medications, and handling money. On each IADL item participants were regarded as having disability if they were reported as requiring help or being completely unable to perform the task independently.
Information was obtained by self-report from study participants except when the respondents could not answer the questions or could not understand the questions. Under this circumstance, informants were asked about the participants' ability. In the present cohort, restricted to subjects free from dementia throughout the study, only 8 subjects out of 953 subjects had at one or more IADL questions answered by their informants throughout the entire study follow-up. We reran models excluding these 8 subjects and obtained virtually identical results. Therefore, we report the results including these 8 subjects.
We summed the IADL disability items for which subjects needed either partial or complete help, yielding a scoring range of 0 (can do all tasks independently) to 7 (disabled in all tasks). We added a score of 8 to represent mortality during follow-up, thus extending the IADL scoring range from 0 through 8. Since we know functional disability is the most powerful predictor of mortality besides age (e.g., 17) we conceptualized disability as being on a continuum that ends with death. Rather than exclude subjects who died during followup, which would have skewed the sample towards the less disabled, we treated mortality as if it were an additional, most severe level of disability. Alternatively, to examine the influence of death on disability trajectories and their associations with covariates, we also ran models excluding those who died during follow-up.
Explanatory Variables
Demographic variables: age at baseline, sex, education (less than high school education vs. high school and over) and recruitment status (random vs. volunteer sample).
Cognitive function: Tests included the Mini-Mental State Exam (MMSE) (18), Trailmaking Tests A and B (19), CERAD 10-word Word List Learning and Delayed Recall (13), Story Immediate Retell and Delayed Recall (20), Initial Letter (P and S) and Category (Fruits and Animals) Fluency (21), 15-item CERAD version of the Boston Naming Test (12, 22), CERAD Constructional Praxis (23), and Clock Drawing (24).
Some cognitive domains were assessed using composite scores, grouping selected tests together on conceptual grounds as well as previous factor analysis (25), other domains were assessed by a single test. Composites were created by first z-transforming each individual test score based on the distribution at baseline (wave 2), and then combining and averaging z-transformed tests. In addition to global cognitive function (MMSE), the domains examined in this study were:
(1) Learning (composite of Word List Learning test and Story Immediate Retell), (2) Recall (composite of Word List Delayed Recall and Story Delayed Recall), (3) Visuospatial (composite of Clock Drawing and CERAD Constructional Praxis), (4) Fluency (composite of Verbal Fluency for categories and initial letters), (5) Psychomotor Speed (Trail Making A Test alone; correct connections per second), (6) Executive function (Trail Making B Test alone; correct connections per second), (7) Naming (Boston Naming Test alone).
Social engagement: Social engagement was assessed by response to a question asking how often the subject attended meetings or activities related to churches, lodges, societies, volunteer groups, etc. The answers were coded as 0=did not belong to any organizations, 1=<1/month, 2=1/month, 3=2-4/month, 4=2-6 days/week, and 5=daily.
Depression: Depression was examined using the modified Center for Epidemiologic Studies-Depression Scale (mCES-D) (26, 27) in which higher scores reflect more depressive symptoms. As previously reported (26), we used a threshold of 5 or more symptoms (capturing the most depressed 10% of the sample at baseline) to indicate depression.
Physical well-being: the total number of prescription medications which the participant reported taking regularly was used as an objective measure of overall morbidity/medical burden (28). Baseline disability (the IADL score 0-7) at baseline was also included as a covariate to adjust for physical well-being.
Statistical Methods
Trajectory modeling is a latent class analysis which identifies homogeneous groups within a population assumed to contain different trajectories. To examine patterns (trajectories) of the numbers of IADL disabilities over time and death, the SAS procedure PROC TRAJ (9) (refer to http://www.andrew.cmu.edu/user/bjones) was used. This procedure basically combines two separate statistical models and estimates their parameters simultaneously using Maximum Likelihood Estimates. The first model builds trajectories for the different latent groups as a function of time from baseline. The second model builds a multinomial regression model that examines the associations of covariates with the probability of membership in the homogeneous latent groups. Here, the trajectory of the total number of IADL disabilities and death over time, reported at Waves 2 through 6, was modeled by a censored normal distribution. Since the models use data collected over varying lengths of follow-up, participants who drop out over the course of follow-up do not need to be excluded. Covariates included in the models were described earlier.
The Bayesian Information Criteria (BIC) (29) were used to identify the optimal number of homogenous groups. Domain-specific cognitive scores were each included in a separate model along with the above-mentioned covariates.
Results
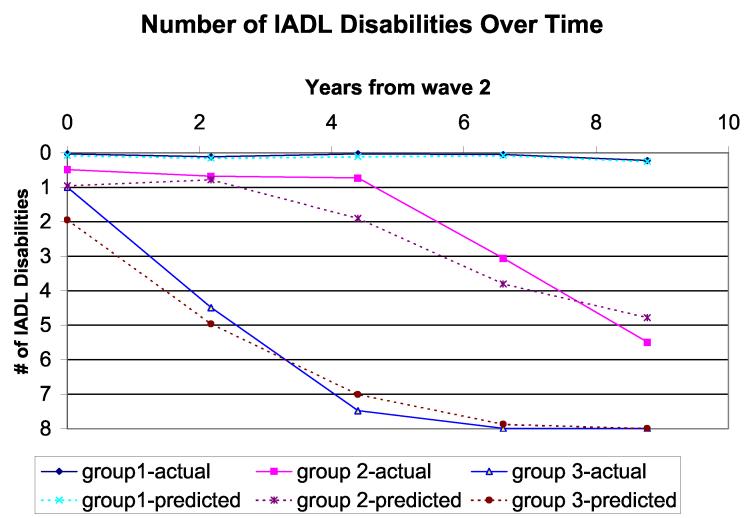
The trajectory analysis identified 3 homogeneous groups as the best model based on the BIC (Figure 1). The procedure calculates the probability of each subject belonging to each trajectory and identifies a subject as belonging to one trajectory based on the largest probability. Figure 1 shows both actual trajectory (solid line; using exact probabilities of belonging to each trajectory for each subject) and estimated trajectory (dotted line; using the model-assigned group identification for each subject) including all covariates except domain-specific cognitive scores. Although the BIC varied slightly depending on the specific cognitive measure included in the model, the 3-trajectory model always provided the best fit to the data, with trajectories virtually identical to those in Figure 1. Based on the shapes of IADL trajectories, we named them No Decline (group 1: stable at disability state), Moderate Decline (group 2: numbers of IADL disabilities increased somewhat over time), and Sharp Decline (group 3: number of IADL disabilities increased sharply followed by death).
Figure 1.
Trajectories of total numbers of IADL disabilities over time
In this community-dwelling cohort of 953 adults free of dementia, the majority entered the study with either no IADL disabilities (78.2%) or only one disability (14.1%). Table 1 shows the baseline characteristics of the overall cohort and of the 3 latent trajectory-defined groups described above.
Table 1.
Descriptive Statistics
| Overall N=953 | Subgroups based on IADL trajectories (see Fig 1) | |||
|---|---|---|---|---|
| Group1 (No Decline) N=502 | Group2 (Moderate Decline) N=255 | Group3 (Sharp Decline) N=196 | ||
| Numbers of IADL disabilities at baseline (% distribution) | ||||
| 0 (%) | 78.2 | 96.6 | 63.1 | 51.0 |
| 1 | 14.1 | 3.4 | 28.2 | 23.0 |
| 2 | 4.1 | 0 | 5.5 | 12.8 |
| 3 | 1.3 | 0 | 1.6 | 4.1 |
| 4 | 1.7 | 0 | 1.6 | 6.1 |
| 5 | 0.3 | 0 | 0 | 1.5 |
| 6 | 0.5 | 0 | 0 | 1.5 |
| 7 | 0 | 0 | 0 | 0 |
| Mean (SD) | 0.38 (0.90) | 0.05 (0.23) | 0.51 (0.90) | 0.92 (1.39) |
| Age : Mean(SD) | 73.29 (5.06 ) | 71.29 (3.46 ) | 74.63 (4.80 ) | 76.67(6.33 ) |
| Sex: % Female | 60.76% | 61.55% | 66.27% | 51.53% |
| Education: % High School or Over | 65.37% | 70.52% | 61.59% | 57.14% |
| Recruitment status: % Volunteer | 19.73% | 22.71% | 19.61% | 12.24% |
| Total Numbers of Rx meds: Mean (SD) | 1.88 (1.99 ) | 1.20 (1.47 ) | 2.31(2.05 ) | 3.05 (2.35 ) |
| Depression: % with >=5 depressive symptoms | 8.60% | 6.77% | 7.06% | 15.31% |
| Social Engagement Frequency Score: Mean (SD) | 3.70 (1.53 ) | 3.96 (1.36 ) | 3.74 (1.53 ) | 2.99 (1.72 ) |
Table 2 shows the association of the baseline cognitive score and other covariates with the three trajectories, using the Sharp Decline trajectory as a reference group. Each column of Table 2 represents the result of a model with all covariates including MMSE. The basic model (Model 1) includes no specific cognitive domains; Models 2 through 8 each includes one of seven cognitive domains. As an example, a one standard deviation increase in the Learning composite at baseline score is associated with a 95% increase in odds of being in the No Decline group (i.e., OR=1.95) and a 71% increase in odds of being in the Moderate Decline group (i.e., OR=1.71), compared with the Sharp Decline group.
Table 2.
Results of Trajectory Model, adjusting for age, sex, education, recruitment status and numbers of IADLdisabilities at baseline.
| No Decliner (Group 1) compared to Sharp Decliner (Group 3) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Baseline characteristics | Model 1 Odds Ratio 95% C.I. | Model 2 Odds Ratio 95% C.I. | Model 3 Odds Ratio 95% C.I. | Model 4 Odds Ratio 95% C.I. | Model 5 Odds Ratio 95% C.I. | Model 6 Odds Ratio 95% C.I. | Model 7 Odds Ratio 95% C.I. | Model 8 Odds Ratio 95% C.I. |
| Total numbers of Rx meds | 0.67*** (0.60-0.75) | 0.67*** (0.60-0.75) | 0.67*** (0.59-0.75) | 0.67*** (0.59-0.75) | 0.67*** (0.60-0.75) | 0.67*** (0.60-0.76) | 0.68*** (0.60-0.77) | 0.67*** (0.59-0.76) |
| Depression | 0.8 (0.35-1.83) | 0.90 (0.39-2.09) | 0.93 (0.40-2.17) | 0.80 (0.35-1.87) | 0.83 (0.36-1.92) | 0.85 (0.36-1.97) | 0.89 (0.34-2.11) | 0.87 (0.37-2.05) |
| Frequency of Social engagement | 1.26** (1.09-1.46) | 1.26** (1.09-1.46) | 1.24** (1.07-1.44) | 1.26** (1.08-1.47) | 1.25** (1.07-1.45) | 1.24** (1.07-1.44) | 1.22** (1.04-1.42) | 1.28** (1.10-1.48) |
| MMSE | 1.44 (0.99-2.09) | 1.28 (0.87-1.89) | 1.20 (0.81-1.78) | 1.24 (0.84-1.83) | 1.31 (0.88-1.93) | 1.43 (0.97-2.11) | 1.32 (0.89-1.96) | 1.35 (0.92-1.98) |
| Learning/Immediate Recall Composite | 1.95*** (1.34-2.84) | --- | --- | -- | -- | -- | -- | |
| Memory/Delayed Recall Composite | 1.59* (1.10-2.32) | --- | -- | -- | -- | -- | ||
| Verbal Fluency Composite | 1.70** (1.22-2.35) | -- | -- | -- | -- | |||
| Visuo-spatial Composite | 1.65** (1.15-2.38) | -- | -- | -- | ||||
| Motor speed | 1.46** (1.11-1.92) | -- | -- | |||||
| Executive function | 1.53** (1.13-2.08) | -- | ||||||
| Naming | 1.50* (1.08-2.07) | |||||||
| Moderate Decliner (Group 2) compared to Sharp Decliner (Group 3) | ||||||||
| Baseline characteristics | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. | Odds Ratio 95% C.I. |
| Total numbers of Rx meds | 0.90* (0.81-0.99) | 0.90* (0.81-0.99) | 0.89* (0.81-0.99) | 0.89* (0.81-0.99) | 0.89* (0.81-0.99) | 0.89* (0.81-0.99) | 0.89* (0.81-0.99) | 0.89* (0.81-0.99) |
| depression | 0.56 (0.27-1.17) | 0.64 (0.30-1.37) | 0.66 (1.02-1.37) | 0.58 (0.27-1.25) | 0.62 (0.29-1.29) | 0.62 (0.29-1.32) | 0.67 (0.31-1.43) | 0.63 (0.3-1.33) |
| Frequency of Social engagement | 1.19* (1.03-1.37) | 1.20* (1.04-1.40) | 1.18* (1.02-1.37) | 1.20* (1.04-1.39) | 1.18* (1.02-1.37) | 1.20* (1.03-1.39) | 1.18* (1.02-1.37) | 1.21** (1.05-1.4) |
| MMSE | 1.17 (0.84-1.63) | 1.02 (0.72-1.46) | 0.98 (0.69-1.4) | 1.00 (0.71-1.42 | 1.08 (0.76-1.54) | 1.20 (0.84-1.7) | 1.12 (0.79-1.62) | 1.11 (0.79-1.57) |
| Learning/Immediate Recall Composite | 1.71** (1.19-2.47) | --- | --- | -- | -- | -- | -- | |
| Memory/Delayed Recall Composite | 1.88*** (1.31-2.73) | --- | -- | -- | -- | -- | ||
| Verbal Fluency Composite | 1.68** (1.23-2.31) | -- | -- | -- | -- | |||
| Visuo-spatial Composite | 1.42* (1.02-2.0) | -- | -- | -- | ||||
| Motor speed | 1.27 (0.97-1.66) | -- | -- | |||||
| Executive function | 1.37* (1.02-1.85) | -- | ||||||
| Naming | 1.22 (0.94-1.58) | |||||||
| BIC | −4357 | −4274 | −4271 | −4328 | −4291 | −4254 | −4220 | −4306 |
p<0.05
p<0.01
p<0.001.
Compared with the Sharp Decline group, the No Decline group was associated with higher baseline scores on all cognitive domains as well as fewer total prescription medications, and higher frequency of social engagement, but not with depression. MMSE was not significant in any models.
Compared with the Sharp Decline group, the Moderate Decline group was associated with higher baseline scores on all cognitive domains except psychomotor speed and naming. Total prescription medications, and higher frequency of social engagement were also significantly associated. Neither depression nor MMSE was significant.
We also examined the difference between the Moderate and the No Decline groups by making the reference group the No Decline Group. None of the cognitive functions distinguished the two trajectory groups, but number of prescription drugs was significantly associated with the Moderate Decline group.
As a reference for interested clinician readers, Table 3 shows the actual mean (SD) baseline cognitive test scores for each of 3 trajectory groups and the overall sample.
Table 3.
Raw cognitive test scores at baseline for each trajectory group
| Composite | Overall Sample Mean(SD) | No Decline Group Mean(SD) | Moderate Decline Group Mean(SD) | Sharp Decline Group Mean(SD) | |
|---|---|---|---|---|---|
| MMSE | 27.51 (1.86) | 27.86 (1.65) | 27.36 (1.79) | 26.78 (2.20) | |
| Learning | Word List Immediate Recall | 20.08 (3.46 ) | 20.67 (3.25 ) | 19.94 (3.48 ) | 18.74 (3.58 ) |
| Story Immediate Retell | 6.83 (2.81 ) | 7.12 (2.73 ) | 7.19 (2.72 ) | 5.63 (2.82 ) | |
| Memory | Word List Delayed Recall | 6.88 (1.70 ) | 7.13 (1.61 ) | 6.85 (1.65 ) | 6.27 (1.85 ) |
| Story Delayed Retell | 6.20 (2.91 ) | 6.56 (2.81 ) | 6.52 (2.81 ) | 4.86 (2.90 ) | |
| Verbal Fluency | Verval Fluency Letters | 23.01 (7.35 ) | 23.83 (7.13 ) | 23.02 (7.19 ) | 20.87 (7.69 ) |
| Verbal Fluency Categories | 27.60 (7.35 ) | 28.69 (5.73 ) | 27.67 (5.63 ) | 24.68 (5.75 ) | |
| Visuo-spatial | Clock Drawing | 7.26 (0.88 ) | 7.39 (0.74 ) | 7.25 (0.85 ) | 6.93 (1.14 ) |
| Constructional Praxis | 9.57 (1.33 ) | 9.78 (1.22 ) | 9.54 (1.40 ) | 9.09 (1.39 ) | |
| Psychomotor speed | Trail A (connections / second) | 0.57 (0.19 ) | 0.62 (0.18 ) | 0.55 (0.18 ) | 0.47 (0.18 ) |
| Executive function | Trail B (connections / second) | 0.22 (0.09 ) | 0.25 (0.09 ) | 0.21 (0.09 ) | 0.16 (0.08) |
| Naming | Boston Naming | 14.31 (0.09 ) | 14.47 (0.88 ) | 14.23 (0.95 ) | 13.98 (1.34 ) |
The effect of death on the association between trajectories and covariates
Table 4 shows the proportion of those who died during the follow-up among each of the three trajectories and follow-up waves during which the death occurred. Only one subject died among the No Decline group. Death occurred during later waves (3rd and 4th follow-up) among the Moderate Decline group, while it occurred during earlier waves (1st through 3rd follow-up) among the Sharp Decline Group.
Table 4.
Proportion of those who died during follow-up among each trajectory group and the waves where death occurred
| Trajectory Groups | % of those who died N (%) | Number of deaths during each follow-up wave | Among those who died: Mean duration to death | |||
|---|---|---|---|---|---|---|
| 1st Follow-up N (%) |
2nd follow-up N (%) |
3rd follow-up N (%) |
4th follow-up N (%) |
Years (std) | ||
| No Decline | 1 (0.31%) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 1 (100%) | 8.22 (NA) |
| Moderate Decline | 135 (41.8%) | 0 (0 %) | 0 (0 %) | 57 (42.2 %) | 78 (57.8%) | 7.69 (1.20) |
| Sharp Decline | 187 (57.9%) | 85 (45.5 %) | 81 (43.3 %) | 21 (11.2 %) | 0 (0%) | 3.53 (1.58) |
NA: Not applicable
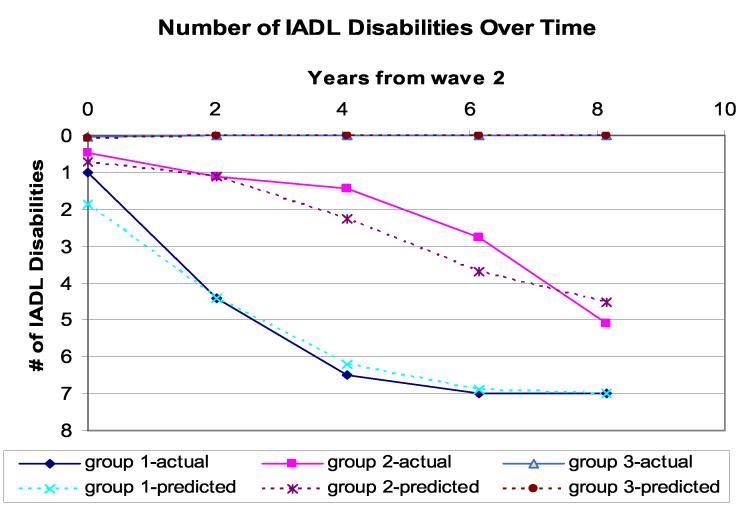
Figure 2 shows the IADL trajectories after excluding those who died during follow-up from the sample. The three-trajectory model was still found to be the best model. However, none of the cognitive functions distinguished the trajectory groups; the total numbers of prescription medications and social engagement remained significant as previously found.
Figure 2.
Trajectories of total numbers of IADL disabilities over time excluding those who died during follow-up
In post hoc analyses we repeated the analyses limiting them to participants free from any disabilities at baseline. The results remained the same comparing the No Decline and Sharp Decline groups, except social engagement was no longer significant. In the comparison of the Moderate Decline and the Sharp Decline groups, visuo-spatial composite, psychomotor speed, executive function, and naming were all nonsignificant. The total number of prescription medications remained significant, but, as with the No Decline group, social engagement no longer distinguished the Moderate Decline group from the Sharp Decline group.
In post hoc cross sectional analysis, executive function, indicated by Trails B, was the only significant variable (OR=0.28, 95%CI: 0.10-0.79) in the model where the outcome was disability in 3 or more IADL tasks. However, in the model where the outcome was disability in 2 or more IADL tasks, none of the cognitive domains was significant.
Discussion
In this 10-year study of a community-based aging cohort free from dementia, all cognitive domains assessed at baseline predicted subsequent trajectories of functional decline. Previous cross-sectional studies of cognitive and functional ability have shown strong associations between executive function and ability to perform IADLs (4-6, 30, 31), as did our own post-hoc cross-sectional analysis restricting the definition of disability to 3 or more IADLs. Our longitudinal analyses, however, showed that the group that experienced virtually no functional decline over the next 8 years had subtle yet significantly higher functioning in all cognitive domains at baseline, even after controlling for demographics, baseline IADL status, depression, general morbidity, and social engagement. The group which declined moderately over 8 years also had higher baseline cognitive functions, compared with the sharply declining group, in all domains except psychomotor speed and naming. However, these significant associations between cognitive functions and IADL trajectories disappeared after excluding those who died during follow-up, suggesting that the IADL decline associated with cognition largely represented the declines experienced by those who died during follow-up. Further, among Moderate Decliners, death occurred mostly during later follow-up waves, while among the Sharp Decliners, death occurred during earlier follow-up waves. Thus, potentially, longer follow-up of the Moderate Decline group might reveal an eventual steep decline similar to that reported here for the Sharp Decline group. This finding is in line with the bulk of literature on the terminal decline showing that a decline in cognitive function occurs 6 years (32) or even more than a decade preceding death (33) It is remarkable that even among non-demented elderly, disability trajectories differed in relation to distance to death, and that baseline cognitive functions were so significant in distinguishing the disability trajectories. Since our cohort was restricted to elders who remained free from dementia throughout the study's 8-year duration, it seems unlikely that the link between cognition and disability trajectory was mediated by a dementing disorder. However, it is possible that some subjects had sub-clinical dementia for longer than our study duration.
According to Salthouse's processing-speed theory (34), cognitive performance is degraded when processing is slow because the products of early processing may no longer be available when later processing is complete (i.e., relevant operations cannot be successfully executed). Thus, variability in processing speed leads to age-related variance observed across various cognitive domains. Our aim in this study was to find the cognitive domains predictive of future disability trajectories, rather than to identify a hierarchy of declines among cognitive domains. Possibly, the Moderate Decline group at baseline was already starting to show evidence of “normal aging” to be followed by gradual IADL decline until death, with psychomotor speed and word retrieval being the domains to deteriorate the earliest.
Additionally, past research has shown that gait velocity is a strong predictor of adverse events (35) and slowing gait is an indicator of subclinical diseases and frailty (36, 37). Although gait velocity involves more than psychomotor speed, the slowing in fine motor movements required for Trails A in our study might be a harbinger of slowing of gross motor movements and gait, which in turn might suggest the presence of disease, gradual disability and death.
Social engagement, the extent to which individuals engage with their social environments, has previously been found to be associated with better physical health (38), and to predict lesser disabilities (39) and less cognitive decline (40) over time. Although our measurement of social engagement was limited in nature, it significantly distinguished the No Decline and Moderate Decline groups from the Sharp Decline group. However, it lost its ability to predict disability once we restricted the sample to those with no baseline IADL disabilities. Our measure of social engagement might be as much a consequence of existing disability as it was a predictor of future disability.
Our data are based on the relatively rural and largely white communities of Southwestern Pennsylvania and may not generalize to other populations. Using the total number of IADL tasks for which subjects cannot perform by themselves ignores the qualitative differences involved in IADL tasks. Despite our relatively large sample size, we could not disaggregate subjects by specific combinations of disabled IADL.
Conclusion
We have found that, among the elderly free from dementia throughout the study, specific (but not general) cognitive domains were important in predicting future disability pathways followed by death. Future studies should tease out the mechanisms underlying the observed associations of cognitive domains, disabilities and death. In the meantime, clinicians may find cognitive assessments useful in anticipating their patients' functional declines over time.
Acknowledgement
This work was supported by K01AG023014, R01AG07562, and K24AG023014 from National Institute on Aging. We thank Dr. Gerda Fillenbaum at Duke University and Dr. Bobby Jones at Carnegie Mellon University for their helpful suggestions. This study was approved by the Oregon State University Institutional Review Board.
REFERENCES
- 1.Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, von Strauss E, Winblad B. Dementia is the major cause of functional dependence in the elderly: 3-year follow-up data from a population-based study. Am J Public Health. 1998;88:1452–1456. doi: 10.2105/ajph.88.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaum CS, Ofstedal MB, Liang J. Low cognitive performance, comorbid disease, and task-specific disability: findings from a nationally representative survey. J Gerontol Med Sci. 2002;57:M523–M531. doi: 10.1093/gerona/57.8.m523. [DOI] [PubMed] [Google Scholar]
- 3.Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H. Cognitive impairment as a strong predictor of incident disability in specific ADL-IADL tasks among community-dwelling elders: the Azuchi Study. Gerontologist. 2005;45:222–230. doi: 10.1093/geront/45.2.222. [DOI] [PubMed] [Google Scholar]
- 4.Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clin Neuropsychol. 2000;14:187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- 5.Carlson MC, Fried LP, Xue QL, Bandeen-Roche K, Zeger SL, Brandt J. Association between executive attention and physical functional performance in community-dwelling older women. J Gerontol Soc Sci. 1999;54:S262–S270. doi: 10.1093/geronb/54b.5.s262. [DOI] [PubMed] [Google Scholar]
- 6.Owsley C, Sloane M, McGwin G, Jr, Ball K. Timed instrumental activities of daily living tasks: relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- 7.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 8.Lavery LL, Starenchak SM, Flynn WB, Stoeff MA, Schaffner R, Newman AB. The clock drawing test is an independent predictor of incident use of 24-hour care in a retirement community. J Gerontol Med Sci. 2005;60:M928–M932. doi: 10.1093/gerona/60.7.928. [DOI] [PubMed] [Google Scholar]
- 9.Jones BL, Nagin DS, Roeder K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 10.Ganguli M, Ratcliff G, Belle S, Huff FJ, Kancel MJ, Fischer L, Kuller LH. Effects of age, gender, and education on cognitive tests in an elderly rural community sample: norms from the Monongahela Valley Independent Elders Survey (MoVIES) Neuroepidemiology. 1991;10:42–52. doi: 10.1159/000110246. [DOI] [PubMed] [Google Scholar]
- 11.Ganguli M, Dodge HH, Chen P, Belle S, DeKosky ST. Ten-year incidence of dementia in a rural elderly US community population: the MoVIES Project. Neurology. 2000;54:1109–1116. doi: 10.1212/wnl.54.5.1109. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC, Heyman A, Mohs RC, Hughes JP, Belle Gv, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I: Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 13.Ganguli M, Belle S, Ratcliff G, Seaberg E, Huff FJ, Porten K, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: The MoVIES project. J Gerontol Med Sci. 1993;48:M152–M161. doi: 10.1093/geronj/48.4.m152. [DOI] [PubMed] [Google Scholar]
- 14.APA . Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition American Psychiatric Association; Washington, DC: 1987. [Google Scholar]
- 15.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 16.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 17.van den Brink CL, Tijhuis M, van den Bos GA, Giampaoli S, Nissinen A, Kromhout D. The contribution of self-rated health and depressive symptoms to disability severity as a predictor of 10-year mortality in European elderly men. Am J Public Health. 2005;95:2029–2034. doi: 10.2105/AJPH.2004.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Neuropsychology Press; Tempe: 1985. [Google Scholar]
- 20.Becker JT, Boller F, Saxton J, McGonigle-Gibson KL. Normal rates of forgetting of verbal and non-verbal material in Alzheimer's disease. Cortex. 1987;23:59–72. doi: 10.1016/s0010-9452(87)80019-9. [DOI] [PubMed] [Google Scholar]
- 21.Lezak MD. Neuropsychologial Assessment. 34th ed. Oxford University Press; New York: 1995. [Google Scholar]
- 22.Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 23.Rosen WG, Mohs RC, David KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 24.Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis D. Clock drawing: A neuropsychological analysis. Oxford University Press, Inc.; New York: 1994. [Google Scholar]
- 25.Ratcliff G, Dodge H, Birzescu M, Ganguli M. Tracking cognitive functioning over time: ten-year longitudinal data from a community-based study. Appl Neuropsychol. 2003;10:76–88. doi: 10.1207/S15324826AN1002_03. [DOI] [PubMed] [Google Scholar]
- 26.Ganguli M, Gilby J, Seaberg E, Belle S. Depressive symptoms and associated factors in a rural elderly population: the MoVIES project. Am J Geriatr Psychiatry. 1995;3:144–160. doi: 10.1097/00019442-199500320-00006. [DOI] [PubMed] [Google Scholar]
- 27.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.Mulsant BH, Ganguli M, Seaberg EC. The relationship between self-rated health and depressive symptoms in an epidemiological sample of community-dwelling older adults. J Am Geriatr Soc. 1997;45:954–958. doi: 10.1111/j.1532-5415.1997.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz G. Estimating the dimension of a model. Ann of Stat. 1978;6:416–464. [Google Scholar]
- 30.Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- 31.Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46:590–596. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 32.Hassing LB, Johansson B, Berg S, Nilsson SE, Pedersen NL, Hofer SM, McClearn G. Terminal decline and markers of cerebro- and cardiovascular disease: findings from a longitudinal study of the oldest old. J Gerontol Psychol Sci. 2002;57:P268–276. doi: 10.1093/geronb/57.3.p268. [DOI] [PubMed] [Google Scholar]
- 33.Bosworth HB, Schaie KW. Survival effects in cognitive function, cognitive style, and sociodemographic variables in the Seattle Longitudinal Study. Exp Aging Res. 1999;25:121–139. doi: 10.1080/036107399244057. [DOI] [PubMed] [Google Scholar]
- 34.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 35.Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, Mayorga LM. Gait Velocity as a Single Predictor of Adverse Events in Healthy Seniors Aged 75 Years and Older. J Gerontol Med Sci. 2005;60:M1304–M1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 36.Bloem BR, Gussekloo J, Lagaay AM, Remarque EJ, Haan J, Westendorp RG. Idiopathic senile gait disorders are signs of subclinical disease. J Am Geriatr Soc. 2000;48:1098–1101. doi: 10.1111/j.1532-5415.2000.tb04786.x. [DOI] [PubMed] [Google Scholar]
- 37.Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 38.Everard KM, Lach HW, Fisher EB, Baum MC. Relationship of activity and social support to the functional health of older adults. J Gerontol Sci Soc. 2000;55:S208–S212. doi: 10.1093/geronb/55.4.s208. [DOI] [PubMed] [Google Scholar]
- 39.Mendes de Leon CF, Glass TA, Berkman LF. Social engagement and disability in a community population of older adults: the New Haven EPESE. Am J Epidemiol. 2003;157:633–642. doi: 10.1093/aje/kwg028. [DOI] [PubMed] [Google Scholar]
- 40.Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131:165–173. doi: 10.7326/0003-4819-131-3-199908030-00002. [DOI] [PubMed] [Google Scholar]