Abstract
Cyclin dependent kinase 5 (Cdk5) has been shown to regulate adhesion and migration of lens and corneal epithelial cells. To explore protein-protein interactions that may mediate these functions, we performed yeast two-hybrid screening on an embryonic rat lens library using Cdk5 and its regulators, p35 and p39 as baits. This screen identified an interaction between p39 and non-muscle myosin essential light chain (MLC17). GST pull-down experiments demonstrated that p39 binds directly to MLC17 through a strong binding site in the N-terminal 109 amino acids of p39. Immunoprecipitation of proteins from Cos1 cells co-transfected with GFP-MLC17 and HA-p39 confirmed that these proteins interact intracellularly. Immunofluorescence microscopy of co-transfected lens epithelial cells showed that GFP- MLC17 and HA-p39 co-localize along cytoskeletal fibrils. Moreover, endogenous rat lens p39 co-immunoprecipitated with MLC17 and myosin heavy chain II (MHC II), demonstrating that the interaction is physiological and serves to link p39 to the cytoskeleton.
Keywords: Cyclin dependent kinase 5, Cdk5, p39, myosin essential light chain, cytoskeleton, lens, epithelial cells
Introduction
Cyclin-dependent kinase 5 (Cdk5) is a unique member of the Cdk family, which plays no apparent role in cell cycle regulation and requires an activating protein (either p35 or p39) that is not a member of the cyclin family [1, 2]. Although the enzymatic activity of Cdk5 is highest in the central nervous system, where it is critical for neuronal migration and secretion [3, 4], recent reports indicate that it also has important functions in non-neuronal cells. For example, Cdk5 activity promotes glucose-dependent insulin secretion in pancreatic beta cells [5] and regulates GTP-dependent secretion from neutrophils [6]. Cdk5 retards migration of corneal epithelial cells [7], due in part to Cdk5-dependent regulation of Src activity [8]. Cdk5 also strengthens cell-matrix adhesion in lens epithelial cells [9], and is required for normal lens morphology in Xenopus embryos [10]. Thus, Cdk5 seems to be an important regulator of cytoskeletal dynamics, cell adhesion, transport, and membrane trafficking in both neuronal and non-neuronal cell types.
Our previous studies have shown that Cdk5 and both of its activators are expressed in the ocular lens [9, 11–13]. The lens is an epithelial tissue, comprising an anterior monolayer of epithelial cells overlying concentric shells of highly differentiated lens fiber cells [14]. During lens development and growth, regulated cell migration is required to establish and maintain normal lens morphology. Proliferating epithelial cells near the lens equator must migrate posteriorly to contact the vitreous humour, which contains growth factors that initiate fiber cell differentiation. Newly formed fiber cells undergo cytoskeletal reorganization [15], withdraw from the cell cycle, then simultaneously elongate and migrate along the posterior lens capsule to meet similarly elongating cells migrating from the opposite side [16]. Several observations suggest that Cdk5 may play a role in these events. It is known, for example, that Cdk5 and p35 are associated with lens fiber cell membranes, with especially high concentrations found near the posterior tips of the elongating fibers [9]. Moreover, as mentioned above, injection of a dominant negative Cdk5 construct into Xenopus embryos leads to micro-ophthalmia and abnormal lens shape [10]. Although our previous studies have established that Cdk5 promotes cell-matrix adhesion in lens epithelial cells [9], the possible role of Cdk5 in lens cell migration during differentiation has not been explored. We have searched for molecular interactions that may provide clues to functions of Cdk5 in the lens by screening an embryonic rat lens yeast two hybrid library using Cdk5, p35, and p39 as baits. Here, we report the identification of a specific interaction between p39 and non-muscle myosin essential light chain (MLC17), a component of myosin II, suggesting a possible role for Cdk5/p39 in regulating cell migration during development and differentiation of the lens.
Materials and Methods
Yeast two-hybrid library and bait construction
The entire p39 cDNA was cloned into pBD-GAL4 Cam phagemid vector (Stratagene, La Jolla, CA). The embryonic (E18) rat lens cDNA library was constructed using 5 μg of poly A+ RNA cloned into hybriZAP-2.1 vector following the manufacturer’s instruction (HybriZAP-2.1 XR library construction kit and HybriZAP-2.1 XR cDNA synthesis kit; Stratagene). The primary library contained 2 x 107 plaque-forming units with an average insert length of 1 kb. Excision and amplification of the library was performed as detailed by Stratagene.
Yeast two-hybrid screening
YRG2 competent yeast cells were transfected with the p39 bait plasmid to create a stable cell line. The YRG2/p39 yeast cells were transfected with 40 μg of library cDNA and positive clones were selected on His−/Leu−/Trp− SD plates (2.67% Difco™ yeast nitrogen base without amino acids {BD Biosciences, Franklin Lakes, NJ}, 1 M sorbitol, 2% agar). All positive clones were further screened for lac Z expression by filter lift assay. Briefly, colonies were transferred from His−/Leu−/Trp− SD plates to nitrocellulose filters, lysed by repetitive freeze-thaw cycles, and incubated with the beta-galactosidase substrate, X-gal (5-bromo-4-chloro-3-indolyl-beta-D-galactopyranoside) according to manufacturer’s protocol (Stratagene).
To isolate plasmids from positive colonies for bacterial transformation, a single yeast colony was grown overnight in 2 ml of 2% Difco™ peptone (BD Biosciences), 1% yeast extract, 0.1 mM adenine sulfate, pH 5.8, at 30°C. The yeast were collected by centrifugation and lysed in 0.2 ml of 10 mM Tris-HCl (pH 8.0), 0.1 M NaCl, 1 mM EDTA, 2% Triton X-100, 1% SDS, and 0.2 ml phenol-chloroform-isoamyl alcohol (25:24:1 (v/v/v)), with 0.3 g of acid-washed glass beads. The plasmid DNA was precipitated with 0.3 M sodium acetate and 100% ethanol and used for transformation of XLI-Blue MRF’ competent cells. Target or bait plasmids were selected on LB-ampicillin or LB-chloramphenicol agar plates, respectively. The resulting bacterial clones were screened by restriction digestion analysis and inserts were sequenced by dideoxy chain termination using the CEQ-DTCS Quick start kit (Beckman Coulter).
Cell culture and transfection
Cells were cultured at 37°C in a humidified atmosphere of 95% air and 5% CO2.The rabbit lens epithelial cell line (N/N1003A) was grown in Dulbecco’s minimum essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% rabbit serum, 50 μg/ml gentamicin; monkey kidney epithelial cells (Cos1) were grown in 10% fetal calf serum (Invitrogen), 100 μg/ml penicillin/streptomycin (Invitrogen). Cos1 cells were transiently transfected with the indicated plasmids using FuGENE 6 (Roche Diagnostics; Basel, Switzerland); N/N1003A cells were transfected with lipofectamine (Invitrogen). Transfected cells were harvested after 48 hr.
Extraction and RT-PCR of rat lens RNA
Lenses were isolated from 2-day-old newborn rat lenses by microdissection and immediately frozen on dry ice. Frozen tissues were thawed in Triazol (Invitrogen) for isolation of total RNA, according to the manufacturer’s instructions. RNA was digested with DNase I (amplification grade; Invitrogen), 1 unit/μg RNA for 15 min at room temperature and heat inactivated for 10 min at 65°C. RT-PCR was performed in a two-step procedure. 1 μg of total RNA was reverse transcribed (Superscript II; Invitrogen) with random hexamers (Perkin Elmer Life Sciences, Boston, MA; Promega) and the resulting cDNA was used for PCR reaction.
Generation and cloning of PCR products
Primers used for PCR were as follows:
For MLC17
PCR primers for cloning into pET-28a (Novagen) and pGEX4T-1 (Amersham Biosciences):
Forward: 5′CCCGGGGGATCCATGTGTGACTTCACCGAGGACCAG-3′
Reverse: 5′CGCCGCGCGGCCGCTCAGCCATTCAGCACCATCCG-3′
PCR primers for cloning into pCMV-HA (Clontech Laboratories, Inc.):
Forward: 5′CCCGGGGAGATCTCTATGTGTGACTTCACCGAGGACCAG-3′
Reverse: 5′CGCCGCGCGGCCGCTCAGCCATTCAGCACCATCCG-3′
PCR primers for cloning into pEGFP-C1 (Clontech Laboratories, Inc.):
Forward: 5′CCCGGGAGATCTATGTGTGACTTCACCGAGGACCAG-3′
Reverse: 5′CGCCGCGTCGACTCAGCCATTCAGCACCATCCG-3′
For p39
Construction of p39 PCR products was described previously [13].
PCR reactions were run for 5 min at 95°C, followed by 35 cycles of 1 min at 95°C, 1 min at 55°C, 1 min at 72°C, and a final extension of 10 min at 72°C.
GST-fusion proteins and affinity purification pull-down assay
The GST-MLC17 fusion protein was expressed from the pGEX-4T-1 MLC17 clone and bound to glutathione-coupled beads according to manufacturer’s protocol (Amersham Biosciences, Buckinghamshire, England). One μg of GST-MLC17 was used per experiment. S35-labeled proteins were generated by in vitro transcription and translation of the appropriate plasmid DNA using the TNTR Quick Coupled Transcription/Translation System (Promega Life Science, Madison, WI). The GST-MLC17 and S35-labeled proteins were incubated overnight in immunoprecipitation buffer (50mM Tris, pH7.5, 15 mM EGTA, 100 mM NaCl, 0.1% Triton X-100, 1 mM dithiothreitol, 1 mM phenylmethyl sulfonyl fluoride, and Complete™ protease inhibitor (Roche Applied Science), containing 0.5 mg BL21 soluble extract), rinsed repeatedly in the same buffer, and stripped with LDS loading buffer (Invitrogen) for electrophoresis on NuPage 4-12% Bis Tris gels (Invitrogen). The gels were stained with Coomassie blue, destained, soaked in Amplify™ (Amersham Biosciences, Buckinghamshire, England), dried and autoradiagraphed.
Antibodies
Living Color full-length anti-GFP polyclonal antibody was purchased from Clontech Laboratory Inc.; anti-hemagglutinin (HA) antibody; anti-MLC17 mouse monoclonal antibody was purchased from Abcam Inc., Cambridge; anti-MHC II-B polyclonal antibody was procure from Covance Inc.; anti-Cdk5 (C-8) as well as horseradish peroxidase-linked anti-rabbit IgG or anti-mouse IgG secondary antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Goat anti-mouse Alexa568-conjugated secondary antibody was purchased from Molecular Probes, Carlsbad, CA. Antibody to an N-terminal peptide of p39 (KGRRPGGLPEE) was raised in rabbits and affinity purified against the antigenic peptide by Abcam Inc, Cambridge, MA.
Protein extraction, immunoprecipitation, and immunoblotting
Cells and tissues were lysed in PBSTDS buffer (1X phosphate buffered saline, 1%Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS containing Complete™ protease inhibitor) or in immunoprecipitation buffer (50 mM Tris; pH 7.5,15mM EGTA, 100mM NaCl, 0.1%TritonX-100, 1 mM dithiothretol, 1 mM phenylmethyl sulphonyl fluoride and Complete™ protease inhibitor). Lysates were pre-cleared with protein G agarose beads. Immunoprecipitation and immunoblotting were carried out as described previously [13] using 2 μg of antibody for 200 μg protein in a volume of 300 μl for each sample.
Immunofluorescence and fluorescence microscopy
For fluorescence microscopy of HA-p39 and GFP-MLC17, transiently transfected Cos1 cells were fixed with 4% paraformaldehyde for 5 minutes, rinsed with phosphate buffered saline (PBS), blocked with 5% normal goat serum for 1 hour, incubated with anti-HA monoclonal antibody for 1 hour, rinsed with PBS and then incubated with goat anti-mouse Alexa568-conjugated secondary antibody, rinsed and cover slipped. Alexa568 (excitation 568) and EGFP (excitation 488) were detected using a Leica TCS-SP2 laser scanning confocal microscope (Leica Microsystems, Germany).
Results and Discussion
Identification of MLC17 cDNA by yeast two-hybrid screening
To identify lens proteins that interact with p39 we screened a yeast two-hybrid library of E18 embryonic rat lens cDNA using the GAL-4 DNA binding domain/p39 (pBD-p39) fusion plasmid as bait. Several prey sequences that supported growth on medium lacking histidine, leucine, and tryptophan, and had significant β-galactosidase activity were isolated and sequenced. Sequence analysis of two such plasmids after rescue in E. coli revealed the partial coding sequences (the last 70 amino acids) for the Rattus norvegicus myosin essential light chain (GenBank accession no: XM_343144), which has a predicted protein size of ~17 kDa (MLC17).
GST pull-down Assays
To determine whether MLC17 and p39 interact directly, a full length rat MLC17 cDNA was generated by RT-PCR of rat lens RNA and cloned into the pGEX4T-1 vector. The GST-MLC17 fusion protein expressed from this clone was immobilized on a glutathione-agarose matrix and incubated with in vitro translated S35-labeled, p39, p35, or Cdk5 (Fig. 1). The proteins retained on the matrix were eluted, separated by gel electrophoresis, and autoradiographed. The GST-MLC17 fusion protein showed a strong interaction with p39, but did not bind to p35 or Cdk5, demonstrating the specificity of the interaction. Although the Cdk5 activators, p35 and p39, are 57% identical at the protein level [2, 17] and largely compensate for each other in mice with homozyogous deletions of one or the other gene [18], several lines of evidence suggest that p39 may have specific functions under normal, physiological conditions. For example, in pancreatic beta cells, insulin-containing secretory vesicles are associated exclusively with p39 [5]. In the developing brain, p35 and p39 have distinct temporal and spatial expression patterns [19–21]. Cdk5/p39 phosphorylates the microtubule associated protein, tau, more efficiently than does Cdk5/p35 [20], and finally, p39 seems to be responsible for localization of the Kelch-domain protein, muskelin, in actin-rich regions at the cell periphery [13]. The present finding provides additional evidence that these two activators of Cdk5 may have distinct cellular functions.
Figure 1. GST pull-down assays to confirm binding and map the binding site.
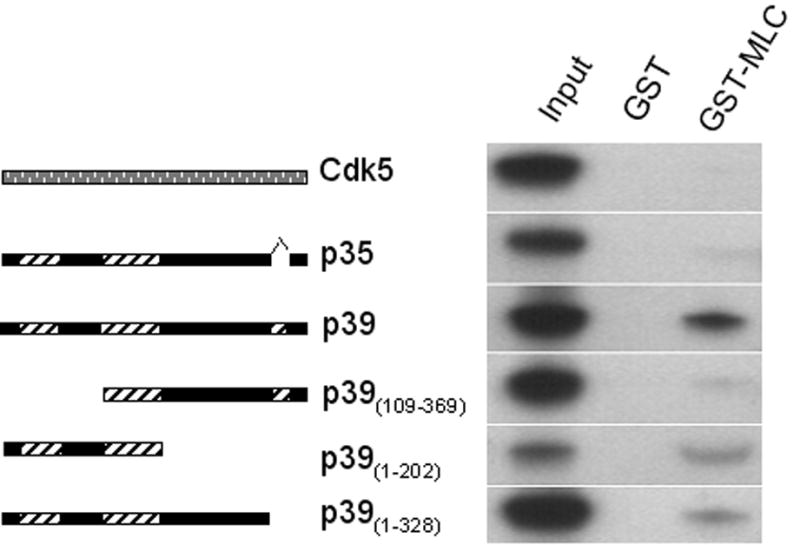
cDNA clones of p39, p35, Cdk5, and three deletion constructs of p39 were in vitro translated and used for GST pull-down assays with GST- MLC17 bound to glutathione beads. The schematic diagrams to the left indicate regions of structural similarity in p35 and p39 and illustrate the deletion constructs used for mapping the binding site. Solid bars represent regions of identity or near identity in p35 and p39, while diagonal hatches indicate regions with little or no similarity. Full length Cdk5 is depicted as a stippled bar. Autoradiograms of eluted proteins shown on the right indicate that GST-MLC17 bound to S35-labeled, full-length p39, but not to full-length p35 or Cdk5; deletion of 108 amino acids at the N-terminus in the p39(109–369) construct almost obliterated binding, while binding to the isolated N-terminal region (p39(1–202)) was stronger than that of the full length (as compared to input). Deletion of 41 amino acids at the C-terminus slightly reduced binding, suggesting that this region may stabilize binding in the full length protein.
To identify the region of p39 responsible for binding MLC17, we performed GST pull-down experiments using three truncated fragments of p39: GST-p39(109–369) , lacking the first 108 amino acids; GST-p39(1–202), lacking 167 amino acids at the C-terminus; and GST-p39(1–328), lacking only the last 41 amino acids at the C-terminus, 32 of which constitute a unique insertion. While both C-terminal truncations retained the ability to bind MLC17, deletion of 108 amino acids at the p39 N-terminus almost abolished binding (Fig 1). Thus, the specific binding site for MLC17 is present in the N-terminal region of p39. This binding site for MLC17 in the p39 N-terminus does not overlap regions of p39 that are needed for binding and activation of Cdk5 [22]. Indeed, the three dimensional structure of Cdk5/p25 [22] suggests that the binding sites for Cdk5 and MLC17 will be situated on opposite sides of the p39 protein, enabling p39 to bind both proteins simultaneously.
Interaction of p39 with MLC17 and MHC II-B in rat tissues
We next tested the physiological relevance of the interaction between p39 and MLC17 by determining whether the endogenous proteins interact in rat tissues. Since the MLC17 antibody was originally raised against ventricular essential light chain, we first determined whether it could be used to detect non-muscle MLC17 by testing its ability to recognize the GST- MLC17 (non-muscle essential light chain) fusion protein (Fig. 2A, left panel). The antibody detected no immunoreactive bands from bacteria transformed with the parental GST vector, pGEX, either before or after affinity purification on glutathione-coupled beads, although control immunoblots using anti-GST antibody showed strong expression of GST (not shown). In contrast, the antibody reacted strongly with the GST- MLC17 fusion protein, confirming that it does, indeed, recognize this protein. Moreover, when tested against lens cell extracts, the anti-MLC17 antibody recognized a single band near the expected 17kDa molecular weight of non-muscle essential light chain (Fig. 2B). The only additional cross-reacting proteins were approximately 35kDa and 55kDa. Since the antibody reacts strongly with recombinant GST-MLC17 and expression of MLC17 in lens has been demonstrated by the production of the full-length clone by RT-PCR of lens mRNA, the immunoreactive band near the 17kDa marker was identified as MLC17.
Figure 2. (A) Recognition of non-muscle myosin essential light chain.
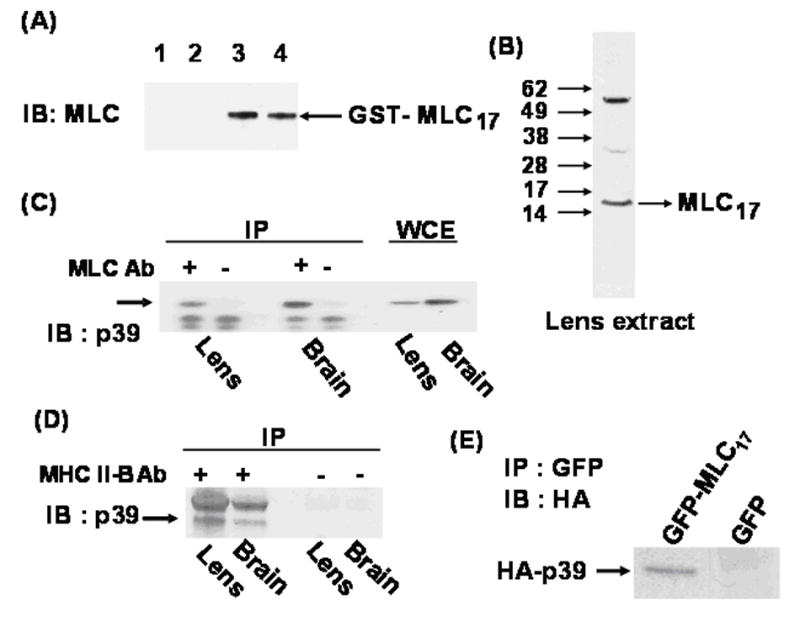
Left panel: MLC antibody raised against ventricular essential light chain was used for immunoblots of bacteria expressing GST-MLC17 or GST. Lanes 1–4 as follows: 1) Extract from GST transformed cells after purification on GSH-beads. 2) Same as lane 1, before purification. 3) Extract from GST-MLC17 transformed cells after purification on GSH-beads. 4) Same as lane 3, before purification. (B) Immunoblot of rat lens extract using anti-MLC antibody. (C) Co-immunoprecipitation of endogenous p39 with MLC17. Extracts from 2-day-old rat lens and brain were incubated with (+) or without (−) anti-MLC antibody (Ab). Immunoprecipitated proteins and whole cell extracts (WCE) were immunoblotted (IB) with anti-p39 antibody. (D) Co-immunoprecipitation of endogenous p39 with MHC IIB. Similar immunoprecipitations were performed with (+) or without (−) anti-MHC II-B antibody (Ab) and immunoblotted with anti-p39 antibody. Upper band in (+) lanes is rabbit IgG. (E) Intracellular association of GFP-MLC17 and HA-p39 fusion proteins. HA-p39 was co-transfected into Cos1 cells with GFP-MLC17 or GFP only (as control). After 48 hour whole cell extracts (WCE) were immunoprecipitated with GFP antibody and immunoblotted with HA antibody to detect co-immunoprecipitated HA-p39.
We next immunoprecipitated protein extracts of rat lens and brain using this antibody and immunoblotted for p39. The results showed that MLC17 forms an endogenous protein complex containing p39 in both tissues (Fig. 2C). Since we expected MLC17 to be associated with MHC II-B, the heavy chain component of non-muscle myosin, we also performed the immunoprecipitation using anti-MHC II-B antibody (Fig. 2D). The results showed that p39 is also associated with MHC II-B and, presumably, with the non-muscle myosin II hexamer. We were not able to detect significant co-immunoprecipitation of Cdk5 with either MLC17 or MHC II-B (not shown), raising the possibility that Cdk5 may associate only transiently with the myosin-p39 complex. As further confirmation that MLC17 and p39 interact intracellularly, Cos1 cells were transiently transfected with HA-tagged p39 and GFP-tagged MLC17. After 48 hours cell extracts were immunoprecipitated with GFP antibody and immunoblotted with HA antibody. The results showed that HA-p39 bound strongly to GFP-MLC17 but not to the GFP only (Fig. 2E)
Subcellular localization of p39 and MLC17
To determine the location of the p39-MLC17 interaction, N/N1003A lens epithelial cells were transiently transfected with HA-p39 and GFP-MLC17, and examined by fluorescence microscopy. Cells transfected with HA-p39 alone showed diffuse cytoplasmic staining, with intense punctuate staining, especially near the cell periphery (Fig. 3a), in agreement with previous findings [13, 23], while cells expressing very high levels of HA-p39 showed intense nuclear and perinuclear staining, as previously described [13] (Fig. 3b). Cells transfected with GFP-MLC17 alone showed the expected cytoskeletal localization of myosin, along cytoplasmic fibrils and along the cell periphery (Fig. 3c). This localization was not changed by co-transfection with HA-p39, except that GFP-MLC17 also accumulated in the nucleus (Fig. 3d). The localization of HA-p39, however, was markedly affected in the co-transfected cells, with HA-p39 co-localizing with GFP-MLC17 along cytoskeletal fibrils and along the cell periphery (Figs.3e,f). Thus, the interaction between p39 and MLC17 provides a novel mechanism for linking p39 to the cytoskeleton in the lens. Although MLC17 itself does not contain a potential site for phosphorylation by Cdk5, the binding of p39 to myosin may position Cdk5 to phosphorylate other cytoskeletal substrates, providing a possible mechanism for Cdk5-dependent cytoskeletal regulation during fiber cell differentiation and migration. Direct binding of p39 to MLC17 provides a potential explanation for the observed cytoskeletal localization of p39 in brain [13, 23], and for Cdk5-dependent cytoskeletal effects in other tissues that express both p39 and MLC17, such as pancreas [5]. Further exploration of this interaction and its biological consequences provides a new means of examining the role of Cdk5 in cell adhesion and migration.
Figure 3. Localization of GFP-MLC17 and HA-p39 in transfected cells.
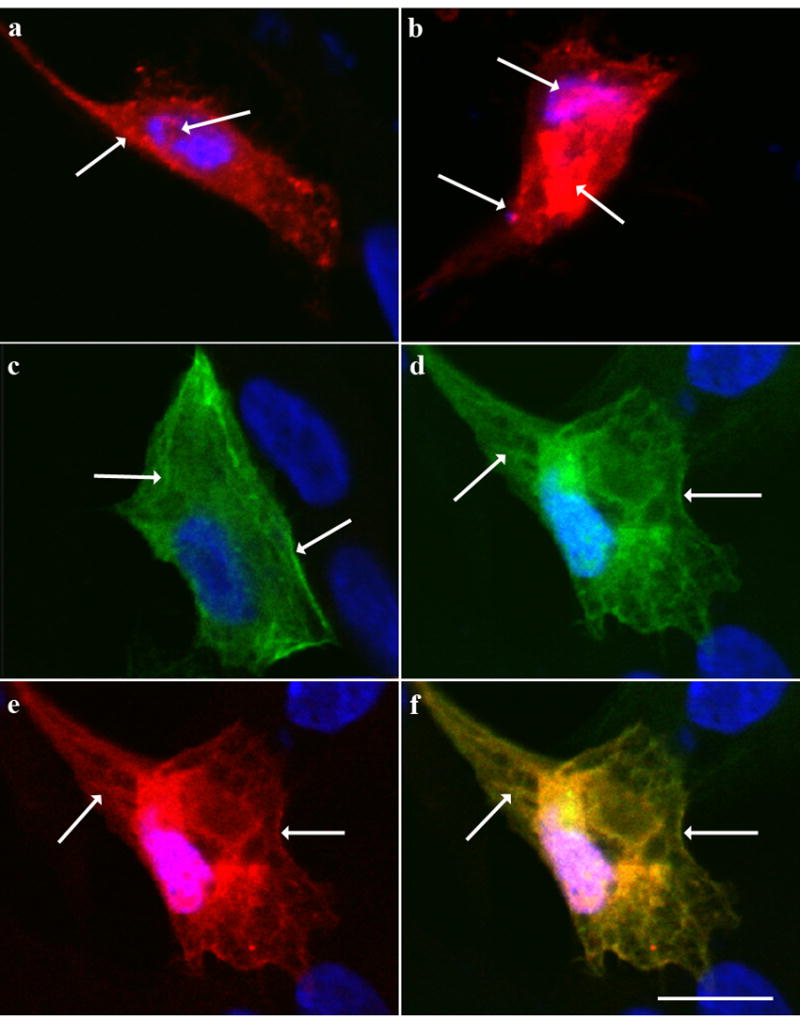
N/N1003A cells transfected with HA-p39 and/or GFP-MLC17, were immunostained with anti-HA antibody, and counterstained with DAPI (blue) to show nuclei.(a) Cell expressing HA-p39 (red) at low levels, showing diffuse staining throughout the cytoplasm with numerous puncta, especially along the periphery (left arrow). Occasional puncta were observed in the nuclei (right arrow) (b) Cell expressing HA-p39 at high levels, showing accumulation in the nucleus (upper left arrow) and cytoplasm (right arrow) as well as in puncta along the periphery (lower right arrow). (c) Cell expressing GFP-MLC17 (green) showing numerous cytoplasmic filaments (left arrow), often along the periphery (right arrow). (d) GFP-MLC17 (green) in cell expressing both GFP-MLC17 and HA-p39. Left arrow, cytoplasmic fibrils; right arrow, staining along cell periphery. Note accumulation in the nucleus. (e) HA-p39 (red) in cell expressing both GFP-MLC17 and HA-p39. Left arrow shows cytoplasmic fibrils; right arrow, staining along cell periphery. (f) Merged image of D and E, showing extensive co-localization of GFP-MLC17 and HA-p39. Scale bar = 5 microns
Acknowledgments
We are grateful to Dr. Chun Gao for advice and helpful discussions, to Dr. Marsha Rosner for providing a p39 cDNA clone and to the NEI Biological Imaging Core for confocal microscopy. This study was funded by the NEI Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lew J, Beaudette K, Litwin CM, Wang JH. Purification and characterization of a novel proline-directed protein kinase from bovine brain. J Biol Chem. 1992;267:13383–13390. [PubMed] [Google Scholar]
- 2.Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- 3.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 4.Smith DS, Greer PL, Tsai LH. Cdk5 on the brain. Cell Growth Differ. 2001;12:277–283. [PubMed] [Google Scholar]
- 5.Lilja L, Johansson JU, Gromada J, Mandic SA, Fried G, Berggren PO, Bark C. Cyclin-dependent kinase 5 associated with p39 promotes Munc18-1 phosphorylation and Ca(2+)-dependent exocytosis. J Biol Chem. 2004;279:29534–29541. doi: 10.1074/jbc.M312711200. [DOI] [PubMed] [Google Scholar]
- 6.Rosales JL, Ernst JD, Hallows J, Lee KY. GTP-dependent secretion from neutrophils is regulated by Cdk5. J Biol Chem. 2004;279:53932–53936. doi: 10.1074/jbc.M408467200. [DOI] [PubMed] [Google Scholar]
- 7.Gao C, Negash S, Guo HT, Ledee D, Wang HS, Zelenka P. CDK5 regulates cell adhesion and migration in corneal epithelial cells. Mol Cancer Res. 2002;1:12–24. [PubMed] [Google Scholar]
- 8.Gao CY, Stepp MA, Fariss R, Zelenka P. Cdk5 regulates activation and localization of Src during corneal epithelial wound closure. J Cell Sci. 2004;117:4089–4098. doi: 10.1242/jcs.01271. [DOI] [PubMed] [Google Scholar]
- 9.Negash S, Wang HS, Gao C, Ledee D, Zelenka P. Cdk5 regulates cell-matrix and cell-cell adhesion in lens epithelial cells. J Cell Sci. 2002;115:2109–2117. doi: 10.1242/jcs.115.10.2109. [DOI] [PubMed] [Google Scholar]
- 10.Philpott A, Tsai L, Kirschner MW. Neuronal differentiation and patterning in Xenopus: the role of cdk5 and a novel activator xp35.2. Dev Biol. 1999;207:119–132. doi: 10.1006/dbio.1998.9146. [DOI] [PubMed] [Google Scholar]
- 11.Gao CY, Bassnett S, Zelenka PS. Cyclin B, p34cdc2, and H1-kinase activity in terminally differentiating lens fiber cells. Dev Biol. 1995;169:185–194. doi: 10.1006/dbio.1995.1136. [DOI] [PubMed] [Google Scholar]
- 12.Gao CY, Zakeri Z, Zhu Y, He H, Zelenka PS. Expression of Cdk5, p35, and Cdk5-associated kinase activity in the developing rat lens. Dev Genet. 1997;20:267–275. doi: 10.1002/(SICI)1520-6408(1997)20:3<267::AID-DVG9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Ledee DR, Gao CY, Seth R, Fariss RN, Tripathi BK, Zelenka PS. A specific interaction between muskelin and the cyclin-dependent kinase 5 activator p39 promotes peripheral localization of muskelin. J Biol Chem. 2005;280:21376–21383. doi: 10.1074/jbc.M501215200. [DOI] [PubMed] [Google Scholar]
- 14.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Weber GF, Menko AS. Actin filament organization regulates the induction of lens cell differentiation and survival. Dev Biol. 2006;295:714–729. doi: 10.1016/j.ydbio.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 16.Zelenka PS. Regulation of cell adhesion and migration in lens development. Int J Dev Biol. 2004;48:857–865. doi: 10.1387/ijdb.041871pz. [DOI] [PubMed] [Google Scholar]
- 17.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 18.Ko J, Humbert S, Bronson RT, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong YG, Rosales JL, Marzban H, Sillitoe RV, Park DG, Hawkes R, Lee KY. The cyclin-dependent kinase 5 activator, p39, is expressed in stripes in the mouse cerebellum. Neuroscience. 2003;118:323–334. doi: 10.1016/s0306-4522(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Saito T, Hisanaga S, Pant HC, Kulkarni AB. Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J Biol Chem. 2003;278:10506–10515. doi: 10.1074/jbc.M211964200. [DOI] [PubMed] [Google Scholar]
- 21.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Tarricone C, Dhavan R, Peng J, Areces LB, Tsai LH, Musacchio A. Structure and regulation of the CDK5-p25(nck5a) complex. Mol Cell. 2001;8:657–669. doi: 10.1016/s1097-2765(01)00343-4. [DOI] [PubMed] [Google Scholar]
- 23.Humbert S, Dhavan R, Tsai L. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci. 2000;113:975–983. doi: 10.1242/jcs.113.6.975. Pt 6. [DOI] [PubMed] [Google Scholar]


