Abstract
Thl and Thl-inducing cytokines and T cell responses were investigated in human salmonellosis. Serum IFN-γ, IL-12 and IL-18 levels were increased significantly in patients with salmonellosis. The increase in serum IL-15 and IL-18 levels was more significant and prolonged in patients with the systemic form of salmonellosis than in those with the gastroenteric form. The serum IFN-γ level was correlated significantly with IL-12 and IL18 levels, and the IL-15 level was correlated significantly with IL-18. Upon stimulation with Salmonella in vitro, mononuclear cells from salmonellosis patients produced significantly higher amounts of IFN-γ and IL-12 compared with those from healthy controls. Anti-IL-12 moAb or anti-IL18 MoAb significantly inhibited Salmonella-induced IFN-γ production in vitro. γδ T cells expressed significantly higher levels of IFN-γ mRNA in salmonellosis patients than in healthy controls. The results suggest that Th1-inducing cytokines appear to be involved in the in vivo response against Salmonella infection, promoting IFN-γ production by αβ and γδ T cells which plays a protective role against Salmonella.
Keywords: γδ T cell, Th1-cytokine, Salmonellosis
INTRODUCTION
Salmonella species are the common cause of enteric infections in humans and are associated with significant mortality in the world. Therefore, knowledge of the host immune response against Salmonella, an intracellular pathogen, is essential for the understanding of its pathophysiology, prophylaxis and treatment.
The immune response to Salmonella infection has been studied extensively in mice [1–3]. IFN(interferon)-γ and IFN-γ-inducing cytokines such as IL-12, IL-18 and IL-15 have been shown to play principal roles in the defence against murine Salmonella infection by studies using gene knockouts and cytokine neutralization [1–7]. IL-12 induces Th1 differentiation and IFN-γ-production from Th1 cells, while IL-18 and IL-15 play synergistic roles with IL-12 as co-stimulants for optimal IFN-γ-production.
In humans, there have been only a limited number of in vivo studies on the defence mechanism against Salmonella infection. We have reported previously a critical role for γδ T cells in salmonellosis [8]. Recently, deficiencies of the IFN-γ receptor 1 or 2, the IL-12 receptor and IL-12 have been identified to be associated with increased risks for severe and recurrent intracellular infections in humans [1,9–11].
In the present study, the levels of Th1 and Th1-inducing cytokines such as IFN-γ, IL-12, IL-15 and IL-18 were serially determined and their correlations with total, αβ and γδ T cell responses and clinical symptoms/signs in salmonellosis were investigated.
MATERIALS AND METHODS
Patients
Thirty-six patients (16 male and 20 female) with Salmonella infection were included in the present study. All were immunocompetent and were treated with antibiotics. The median age was 9·8 years (range 1·2–57 years). The diagnosis of Salmonella infection was made by positive stool culture and, in seven cases, in combination with blood culture. Five patients were found to be infected with Salmonella typhi, one with S. enterica serovar Paratyphi, 15 with S. enterica serovar Enteritidis, two with S. enterica serovar Typhimurium, six with S. enterica serovar Oranienburg, one with S. enterica serovar Panama, one with S. enterica serovar Saintpaul, one with S. enterica serovar Braenderup, one with S. enterica serovar Chester, one with S. enterica serovar Newport and two with others (Salmonella O4). The symptoms of Salmonella infections include gastroenteritis, bacteraemia and enteric fever [12]. In the present study, the 36 patients with Salmonella infections were classified into two groups: 10 patients with the systemic form characterized by the dominant systemic symptoms (fever of over 10 days' duration, malaise, lethargy) and 26 patients with gastroenteritis (gastroenteric form). The systemic group included five patients with S. typhi, one with S. serovar paratyhpi and four with S. serovar Oranienburg. The acute phase was defined as the period with symptoms such as fever or diarrhoea, and ranged from 11 to 21 days for the systemic form and from 2 to 8 days for the gastroenteric form. Age-matched normal individuals were used as controls for flow cytometric analysis. Blood samples were obtained after informed consent was received and this study was approved by the ethics committee of the Fukuoka Children's Hospital and Medical Center for Infectious Diseases.
Cytokine assays
Serum IFN-γ, IL-12p70, IL-15 and IL-18 levels were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturers' instructions. IFN-γ and IL-15 ELISA kits were purchased from BioSource International, Inc. (Camarillo, CA, USA), the IL-12 ELISA kit from Genzyme Diagnostics, (Cambridge, MA, USA) and the IL-18 ELISA kit from MBL Medical and Biological Laboratories Co. Ltd (Nagoya, Japan). The detection limits of the ELISA kits for IFN-γ, IL-12, IL-15 and IL-18 were 4 pg/ml, 0·5 pg/ml, 11 pg/ml and 12·5 pg/ml, respectively. IFN-γ and IL-12p70 ELISA kits for in vitro cytokine production were from Amersham Pharmacia Biotech (Uppsala, Sweden) and R&D Systems, Inc. (Minneapolis, MN, USA), respectively.
Cytokine production in vitro
Mononuclear cells (MNC) were isolated from whole blood by density-gradient centrifugation. MNC from healthy controls or three patients were suspended at a concentration of 5 × 105/ml in antibiotic-free RPMI containing 10% fetal calf serum. The MNC were incubated for 4 h in the presence or absence of live S. enterica serovar typhimurium (5 × 106 CFU/ml) or live Escherichia coli (5 × 106 CFU/ml) in a 5% CO2 incubator at 37°C. Then, the MNC were washed twice with the above medium containing ABPC (aminobenzyl penicillin, 100 µg/ml) and SM (streptomycin, 100 µg/ml), resuspended at a concentration of 5 × 105/ml and cultured for 3 days. Culture supernatants were collected at days 1 and 3 for cytokine assays.
The effects of cytokine-neutralizing antibodies on IFN-γ production were assayed with S. enterica serovar Typhimurium-stimulated MNC (5 × 105 cells/ml) from healthy controls in the presence or absence of anti-IL-12 MoAb (10 µg/ml), anti-IL-15 MoAb (5 µg/ml), anti-IL-18 MoAb (1 µg/ml) or mouse IgG1. Culture supernatants on day 3 were collected for IFN-γ assay by ELISA. Anti-IL-12 and IL-15-neutralizing monoclonal antibodies were purchased from Genzyme and anti-IL-18 MoAb from MBL. The mouse IgG1 control antibody was purchased from Coulter-Immunotech Co. (Miami, FL, USA).
Flow cytometric analysis
Anti-human CD3-fluorescein isothiocyanate (FITC), HLA-DR- phycoerythrin (PE), CD69-PE, CD4-phycoerythrin–cyanin 5·1 (PC5), panγδ -PC5 and CD8-PC5 MoAbs were purchased from Coulter-Immunotech Co. Three-colour flow cytometric analysis was performed as described previously [13], using an EPICS XL, Beckman-Coulter (Hialeah, FL, USA).
Cell sorting
MNC were incubated with PE-anti-CD3 MoAb and PC5-anti-γδ or αβ TCR MoAb (Coulter-Immunotech Co.) for 30 min at 4°C, washed twice, resuspended in PBS and subjected to sorting using a cell sorter (Epics Altra, Coulter). γδ T cells and αβ T cells were sorted as CD3-positive/γδTCR-negative and CD3-γδTCR-negative fractions, respectively. The purities of the γδ T cells and αβ T cells were 96·9 ± 1·9% and 99·6 ± 0·4%, respectively.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) by Taq Man method
Total RNA was isolated from frozen MNC pellets using an RNA extraction kit using Isogen (Nippon Gene, Osaka, Japan). The high molecular weight carrier ethachinmate (Nippon Gene) was added to the RNA solution at the isopropyl alcohol precipitation step to increase the recovery of RNA. cDNA synthesis was performed using a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech) with random hexamers.
The forward (G1) and reverse (G2) primers of the IFN-γ gene were designed to be located on exons 3 and 4, between which a 2424-bp intron exists, to avoid the amplification of genomic DNA. The sequences of the PCR primers and the TaqMan probe for the IFN-γ gene were as follows: G1; ACGAGATGACTTCGAAAA GCTG, G2; TTTAGCTGCTGGCGACAGTTC, TaqMan probe; CGGTAACTGACTTGAATGTCCA ACGCAA. IFN-ƒÁ TaqMan probe (TG) was labelled at the 5′ end with the reporter dye molecule, FAM (6-carboxyfluorescein: emission I, 538 nm). A TaqMan ribosomal RNA control reagent kit was used to study an internal control utilizing an rRNA TaqMan probe (TR) labelled with JOE (6-carboxy-4,5-dichloro-2,7-dimethoxyfluorescein; emission l 546 nm). Both TaqMan probes were labelled with the quencher fluor TAMRA (6-carboxytetramethylrodamine; emission l 582 nm) at the 3′ end via a linker arm nucleotide (LAN).
IFN-γ/ ribosomal (r)RNA expression was analysed by an ABI PRISM 7700 Sequence Detector (Perkin Elmer, Foster City, CA, USA). In brief, a master mixture containing all reagents for PCR was prepared at a final concentration of 1 × TaqMan Universal PCR Master Mix. The PCR primer set and the TaqMan probe for IFN-γ were at the final concentrations of 200 nM and 100 nM, respectively, and those for rRNA at the final concentration of 50 nM in a final volume of 25 µl.
The PCR conditions were as follows: 50°C for 2 min and 95°C for 10 min, followed by 50 (IFN-γ) or 35 (rRNA) cycles of amplification at 94°C for 15 s and 60°C for 1 min. During each cycle of the PCR, the 5′−3′ exonuclease activity of Taq DNA polymerase cleaves the TaqMan probe, thereby increasing the fluorescence of the reporter dye at the appropriate wavelength. The increase in fluorescence (ΔRn) was proportional to the concentration of template in the PCR mixture. The PCR cycle number at the threshold line is represented as Ct. The expression level of IFN-γ was corrected by that of the internal control, rRNA. The IFN-γ gene expression level was defined as a ratio to that (1·00) of PHA-stimulated MNC. Five patients, one patient with the systemic form and four with gastroenteric form, and four healthy controls were involved in this study.
Statistical analysis
The Kruskal–Wallis test was performed to analyse the significance of the different values between the three groups. The Bonferroni test was further applied to investigate the significance of the differences between the individual groups. Student's t-test was used to compare values between the groups in the in vitro cytokine assay. Spearman's correlation coefficient by rank test was used to analyse the correlation between serum cytokines levels, or numbers of certain T cell subsets in peripheral blood of patients with salmonellosis. The cytokine levels under detection limits were calculated as 0. Mann–Whitney's U-test was used to analyse the significance of differences between the two groups. Differences were considered to be significant when the P-value was less than 0·05.
RESULTS
IFN-γ is one of the representative Th1 cytokines involved in the clearance of intracellular pathogens. IL-12 is a Th1-inducing cytokine; IL-12, in concert with IL-18, strongly induces IFN-γ production. IL-15 shares many of the biological properties of IL-2 and plays a critical role in the host defence against intracellular pathogens through T cell activation. We measured these cytokines to investigate their role in the pathophysiolosy of salmonellosis in humans. Serum IFN-γ, IL-12, IL-15 and IL-18 levels during the acute phase in patients with salmonellosis were significantly higher than those in controls (Fig. 1). In addition, serum levels of IL-15 and IL-18 in patients with the systemic form of salmonellosis were significantly higher than those in patients with the gastroenteric form (Fig. 1).
Fig. 1.
Serum Th1 and Th1-inducing cytokine levels in the acute phase. S: systemic form, G: gastroenteric form, C: controls. IL-15 and IL-18 levels in the systemic form are increased significantly compared with those in the gastroenteric form or in the controls. The error bars show the medians, 10th and 90th percentiles. Significant differences by Kruskal–Wallis or Bonferroni test are indicated as single (*P < 0·05) or double (**P < 0·01) asterisks. The detection limits of the ELISA kits for IFN-γ, IL-12, IL-15 and IL-18 were 4 pg/ml, 0·5 pg/ml, 11 pg/ml and 12·5 pg/ml, respectively. N = total number of samples analysed; U = number of samples below the detection limit.
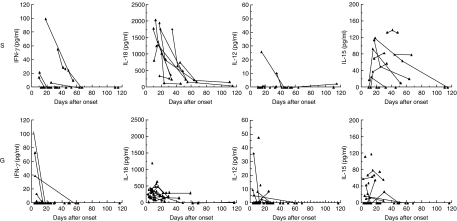
By serial determination of serum cytokine levels in salmonellosis, serum IL-15 and IL-18 levels were shown to have prolonged elevation in the systemic form of salmonellosis compared with the gastroenteric form (Fig. 2). The serum IL-15 and IL-18 levels in the systemic form were significantly higher than those in the gastroenteric form at days 11–20 (P = 0·05 and P < 0·01, respectively, data not shown).
Fig. 2.
Serial determination of Th1 and Th1-inducing cytokines in systemic and gastroenteric forms. By serial determination of serum cytokine levels in salmonellosis, serum IL-15 and IL-18 levels were shown to have prolonged elevation in the systemic form of salmonellosis compared with the gastroenteric form. The detection limits of the ELISA kit of IFN-γ, IL-12. IL-15 and IL-18 were 4 pg/ml, 0·5 pg/ml, 11 pg/ml, and 12·5 pg/ml, respectively. S: systemic form, G: gastroenteric form.
Correlations between the maximum levels of cytokines in each salmonellosis patient were studied, including both the systemic and the gastroenteric forms. As shown in Table 1, the IFN-γ level correlated significantly with IL-12 (P < 0·05) and IL-18 (P < 0·01) levels. In addition, the IL-15 level significantly correlated with the IL-18 level (P < 0·01).
Table 1.
Correlation between cytokines
| ρ | P | |
|---|---|---|
| IFN-γ-IL-12 | 0·532 | 0·013 |
| IFN-γ-IL-15 | 0·313 | 0·063 |
| IFN-γ-IL-18 | 0·455 | 0·0039 |
| IL-12-IL-15 | 0·337 | 0·091 |
| IL-12-IL-18 | 0·0258 | 0·94 |
| IL-15-IL-18 | 0·632 | 0·00044 |
ρ: Spearman's correlation coefficient by rank test.
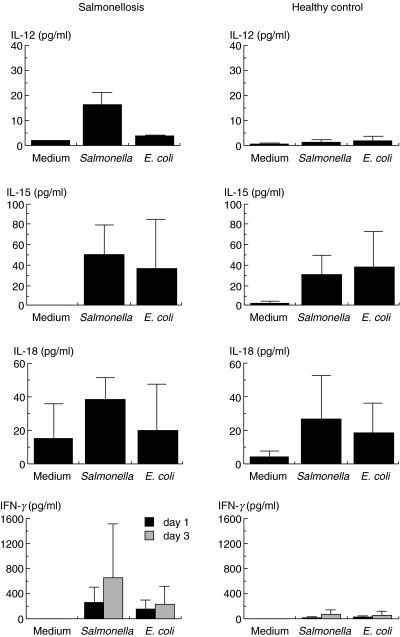
We next investigated whether these Th1 and Th1-inducing cytokines were released upon stimulation with Salmonella in vitro. As shown in Fig. 3, significant amounts of IL-15 and IL-18 were produced by MNC from both salmonellosis patients and healthy controls following either Salmonella- or E. coli-stimulation. On the other hand, significant amounts of IL-12 and IFN-γ, in addition to IL-15 and IL-18, were produced only upon Salmonella stimulation of MNC from salmonellosis patients. IFN-γ production by MNC following stimulation with Salmonella was significantly inhibited by anti-IL-12 MoAb (P < 0·01) or anti-IL-18 MoAb (P < 0·05), but not by anti-IL-15 MoAb (Fig. 4).
Fig. 3.
In vitro cytokine production upon bacterial stimulation in patients with salmonellosis and healthy controls.MNC were incubated with Salmonella, E. coli or medium alone for 4 h, washed and cultured in the presence of antibiotics for 1 day for IL-12, IL-15 and IL-18 determination and for 1 or 3 day(s) for IFN-γ. Culture supernatants were assayed by ELISA. IL-15 and IL-18 were produced following Salmonella or E. coli stimulation in both salmonellosis patients and controls. On the other hand, IL-12 and IFN-γ were preferentially produced upon stimulation with Salmonella in salmonellosis patients. ▪, Day 1;  , day 3.
, day 3.
Fig. 4.
Effects of anticytokine monoclonal antibodies on Salmonella-induced IFN-γ production in controls.The effects of cytokine-neutralizing antibodies on IFN-γ production were assayed with S. enterica serovar typhimurium-stimulated MNC (5 × 105 cells/ml) from healthy controls in the presence or absence (medium alone) of anti-IL-12 MoAb (10 µg/ml), anti-IL-15 MoAb (5 µg/ml) or anti-IL-18 MoAb (1 µg/ml). Culture supernatants at day 3 were collected for IFN-γ assay with ELISA. Anti-IL-12 and anti-IL-18 monoclonal antibodies significantly inhibited Salmonella-induced IFN-γ production, while the anti-IL-15 monoclonal antibody did not. No significant inhibition was observed with an isotype-matched antibody control (IgG1). Significant differences by Student's t-test are indicated as single (*P < 0·05) or double (**P < 0·005) asterisks.
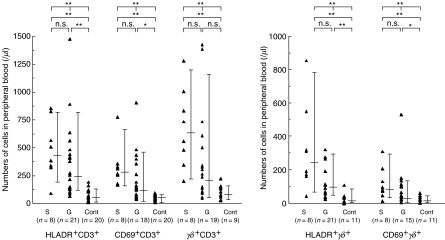
To investigate the T cell response in salmonellosis, absolute cell numbers of T cell subsets were calculated from the percentage determined by flow cytometry. As shown in Fig. 5, γδ T cell and HLA-DR +γδ T cell numbers were significantly elevated in salmonellosis, consistent with our previous study [8]. An activation ratio (HLA-DR +γδ or αβ T cells/total γδ or αβ T cells) in γδ T cells (mean ± s.d.: 0·40 ± 0·22) was also significantly higher than that in αβ T cells (0·22 ± 0·12) (P < 0·01), data not shown.
Fig. 5.
Surface marker analysis of T cells in systemic and gastroenteric forms of Salmonella infection.The absolute numbers of the indicated cells in peripheral blood, calculated from the cell number of lymphocytes in peripheral blood and the percentage of each lymphocyte subset as measured by flow cytometric analysis, are shown. Absolute counts of γδ T cells and HLA-DR +γδ T cells were elevated significantly in the systemic compared to the gastroenteric form. An activation ratio in γδT cells was higher than that in αβ T cells. The error bars show the values of median, 90th percentile and 10th percentile. Significant differences by Kruskal–Wallis test or Bonferroni test are indicated as single asterisks (*P < 0·05).
Finally, IFN-γ mRNA was measured quantitatively using an ABI PRISM 7700 Sequence Detector αβ T cell- and γδ T cell-sorting. As shown in Fig. 6, sorted γδ T cells from salmonellosis patients contained significantly higher levels of IFN-γ mRNA compared with those from healthy controls. In a patient with the systemic form of the disease, the highest levels of IFN-γ mRNA in γδ (IFN-γ/rRNA: 0·52) and αβ (0·26) T cells during the acute phase were observed. These levels declined during the convalescent phase (γδT cells: 0·11, αβ T cells: 0·06).
Fig. 6.
TaqMan quantitative PCR analysis of IFN-γmRNA from sorted γδ cells and αβ T cells from patients with salmonellosis and healthy controls. Sorted γδ T cells from patients with salmonellosis contained significantly higher levels of IFN-γ mRNA than those from healthy controls. In the systemic form, much higher levels of IFN-γ mRNA in γδ (IFN-γ/rRNA: 0·52) and αβ (0·26) T cells were observed. The significant difference by Mann–Whitney's U-test is indicated as a single asterisk (P < 0·05).
DISCUSSION
The present study has demonstrated differences in the degree and duration of cytokine responses between the gastroenteric and systemic forms of Salmonella infection by serial determination of IFN-γ and IFN-γ -inducing cytokines in human Salmonella infection (Figs 1 and 2). IL-15 and IL-18 responses in the systemic form returned to normal levels much later than those in the gastroenteric form. These results indicated a stronger IFN-γ and IFN-γ-inducing cytokine response in the systemic form. As it takes around 6 weeks to eliminate even an attenuated virulent strain of Salmonella in mice [3], dissemination of Salmonella in systemic sites might result in prolonged survival of the bacteria and characteristic features of cytokine and cellular immune responses in the patients with systemic infection [12,14,15].
IL-12 plays a pivotal role in promoting type 1 cytokine responses and cell-mediated immunity against intracellular microbial pathogens [1,4,15–20]. In addition, deficiency or blocking of the IL-12/IL-12 receptor pathway causes an increased susceptibility to Salmonella infection in mice and humans [1,9–11]. Possible explanations for the only slight elevation of IL-12p70 levels even in the systemic form of salmonellosis in vivo in spite of an elevation in in vitro studies include that (1) maximal levels of IL-12 were produced at earlier time points (Fig. 3), (2) IL-12 was sufficient in only small quantities because neutralization of a small amount of IL-12 resulted in strong inhibition of IFN-γ production (Fig. 4) or (3) IL-12 was secreted locally at mucosal sites or within lymph nodes [16,21–26].
IL-18 was originally designated as an IFN-γ -inducing factor and is produced by monocytes/macrophages [24,27]. IL-18 exerts its action fully in synergy with IL-12 in the induction of IFN-γ by T cells [24,25]. IL-18 has been shown to play a crucial role in the control of Salmonella infection in mice, as well as in control of intracellular infection with Mycobacterium leprae in humans [28]. In addition, IL-18 and IL-12 are potent inducers of IFN-γ by Salmonella stimulation in vitro[22,29]. Consistent with the above results, the IFN-γ level correlated significantly with the IL-12 or IL-18 level in our experiments, suggesting a possible involvement of IL-12 and IL-18 in IFN-γ induction against human Salmonella infection in vivo. Higher levels of production of IL-12, IL-15, IL-18 and IFN-γ in in vitro stimulation with Salmonella in patients with salmonellosis than controls might reflect the in vivo activation of these cytokine producing cells (Fig. 3).
IL-15 is a cytokine that shares many biological activities with IL-2 [7,21]. IL-15 has recently been reported to play a role in protection against Salmonella infection through activation of NK cells and γδ T cells in mice [5,30,31]. In this study, the IL-15 level significantly correlated with the IL-18 level, possibly because both are produced by activated macrophages/monocytes [7,26].
αβ T cells are important for protective immunity against Salmonella in mice and humans. On the other hand, murine γδ T cells also appear to play a supportive role in resistance [3,31,32] and produce a significant level of IFN-γ in response to a Salmonella-infected murine monocyte/macrophage cell line [33]. Although we showed that γδ T cells were activated preferentially and expanded in human Salmonella infection [8], it remains to be elucidated whether human γδT cells play a role in resistance. Next, correlations between cell numbers and cytokine levels were investigated. Activated total T cell numbers tended to be increased in accordance with the higher levels of IFN-γ, IL-15 and IL-18, although there were no significant correlations (P = 0·067, 0·076, 0·079, respectively, data not shown). On the other hand, activated γδ T cell numbers had such a tendency only with serum IL-15 levels (P = 0·080, data not shown), supporting the idea that IL-15 is one of the γδ T cell-expanding cytokines [33,34]. Finally, the relative contribution of αβ and γδ T cells to IFN-γ production was studied by αβT cell- and γδ T cell-sorting, followed by TaqMan quantitative PCR analysis of IFN-γ mRNA. γδ T cells from patients with salmonellosis were found to contain significantly higher levels IFN-γ mRNA than those from healthy controls, which indicated at least a certain contribution of IFN-γ-producing γδ T cells to the protection against human Salmonella infection in vivo.
In summary, IL-12, IL-18 and IL-15, with complex mutual interactions, appear to be involved in total T cell activation, as well as γδ T cell expansion, resulting in IFN-γ production in human Salmonella infection.
Acknowledgments
This work was supported in part by a Grant-In-Aid for Scientific Research (B) to Hara T from the Ministry of Education, Science, Sports and Culture of Japan.
References
- 1.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 2.Lalmanach AC, Lantier F. Host cytokine response and resistance to Salmonella infection. Microb Infect. 1999;1:719–26. doi: 10.1016/s1286-4579(99)80073-2. [DOI] [PubMed] [Google Scholar]
- 3.Mittrucker HW, Kaufmann HE. Immune response to infection with Salmonella typhimurium in mice. J Leuk Biol. 2000;62:457–63. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 4.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 5.Hirose K, Nishimura H, Matsuguchi T, Yoshikai Y. Endogenous IL-15 might be responsible for early protection by natural killer cells against infection with an avirulent strain of Salmonella choleraesuis in mice. J Leuk Biol. 1999;66:382–90. doi: 10.1002/jlb.66.3.382. [DOI] [PubMed] [Google Scholar]
- 6.Mastroeni P, Clare S, Khan S, et al. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–83. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Hara T, Mizuno Y, Takagi K, et al. Predominant activation and expansion of Vγ9-bearing γδ T cells in salmonella infection. J Clin Invest. 1992;90:204–10. doi: 10.1172/JCI115837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altare F, Lammas D, Revy P, et al. Inherited interleukin 12 deficiency in a child with Bacille–Calmette–Guerin and salmonella enteritidis disseminated infection. J Clin Invest. 1998;102:2035–40. doi: 10.1172/JCI4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dejong R, Altare F, Haagen IA, et al. Severe mycobacterial and salmonella infection in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–8. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 11.Ottenhoff THM, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-1 cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–4. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 12.Gomez HF, Salmonella Cleary TG. Textbook of pediatric infectious diseases. In: Feigin RD, Cherry JD, editors. 4. Vol. 1. Philadelphia: WB Saunders Co.; 1998. pp. 1321–34. [Google Scholar]
- 13.Honda K, Takada H, Nagatoshi Y, et al. Thymus-independent expansion of T lymphocytes in children after allogeneic bone marrow transplantation. Bone Marrow Trans. 2000;25:647–52. doi: 10.1038/sj.bmt.1702198. [DOI] [PubMed] [Google Scholar]
- 14.Lee VT, Schneewind O. Type lll secretion machines and the pathogenesis of enteric infections caused by Yersinia and Salmonella spp. Immunol Rev. 1999;168:241–55. doi: 10.1111/j.1600-065x.1999.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 15.Schaible UE, Collins HL, Kaufmann SHE. Confrontation between intracellular bacteria and the immune system. Adv Immunol. 1999;71:267–75. doi: 10.1016/s0065-2776(08)60405-8. [DOI] [PubMed] [Google Scholar]
- 16.Bohn E, Sing A, Zumbihl R, et al. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of bacterial infection in mice. J Immunol. 1998;160:299–307. [PubMed] [Google Scholar]
- 17.Bost KL, Clements JD. Intracellular Salmonella dublin induces substantial secretion of the 40-kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect Immun. 1997;65:3186–92. doi: 10.1128/iai.65.8.3186-3192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naiki Y, Nishimura H, Kawano T, et al. Regulatory role of peritoneal NK1.1 (+) alpha beta T cells in infection. J Immunol. 1999;163:2057–63. [PubMed] [Google Scholar]
- 19.Mosser DM, Karp CL. Receptor mediated subversion of macrophage cytokine production by intracellular pathogens. Curr Opin Immunol. 1999;11:406–11. doi: 10.1016/s0952-7915(99)80068-5. [DOI] [PubMed] [Google Scholar]
- 20.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 21.Doherty TM, Seder RA, Sher A. Induction and regulation of IL-15 expression in murine macrophages. J Immunol. 1996;156:735–41. [PubMed] [Google Scholar]
- 22.Dybing JK, Walters N, Pascual DW. Role of endogenous interleukin-18 in resolving wild-type and attenuated Salmonella typhimurium infections. Infect Immun. 1999;67:6242–8. doi: 10.1128/iai.67.12.6242-6248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell MA, Luo Y, Chen X, Szilvasi A, Hunter SE, Clinton SK. Role of IL-12 in the induction and potentiation of IFN-γ in response to Bacillus–Calmette–Guérin. J Immunol. 1999;163:4246–52. [PubMed] [Google Scholar]
- 24.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-γ production by T ceIIs. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 25.Okamura H, Kashiwamura S, Tsutsuit H, Yoshimoto T, Nakanishi K. Regulation of interferon-γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 26.Yoshimoto T, Takeda H, Tanaka Y, et al. IL-12 upregulate IL-18 R expression on T cells, Th1 cells and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;151:3400–7. [PubMed] [Google Scholar]
- 27.Dinarello CA. IL-18: a Th1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 28.Garcia VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–21. [PubMed] [Google Scholar]
- 29.Elhofy A, Bost KL. Limited interleukin-18 response in Salmonella-infected murine macrophages and in Salmonella-infected mice. Infect Immun. 1999;67:5021–6. doi: 10.1128/iai.67.10.5021-5026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagaki-Ohara K, Nishimural H, Mitani A, Yoshikai Y. Interleukin-15 preferentially promotes the growth of intestinal intraepithelial lymphocytes bearing γδ T cell receptor in mice. Eur J Immunol. 1997;27:2885–91. doi: 10.1002/eji.1830271121. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura H, Hjromatsu K, Kobayashi N, et al. IL-15 is a novel growth factor for murine γδT cells induced by Salmonella infection. J Immunol. 1996;156:663–9. [PubMed] [Google Scholar]
- 32.Nishimura H, Washizu J, Naiki J, et al. MHC class II-dependent NK1.1+γδT cells are induced in mice by Salmonella infection. J Immunol. 1999;162:1573–81. [PubMed] [Google Scholar]
- 33.Garcia VE, Jullien DS, Uyemura K, Shuai K, Morita CT, Modlin RL. IL-15 enhances the response of human T cells to nonpeptide microbial antigens. J Immunol. 1998;160:4322–29. [PubMed] [Google Scholar]
- 34.Boullier S, Poquet Y, Debord T, Fournie JJ, Gougeon ML. Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vγ9Vδ2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur J Immunol. 1999;29:90–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<90::AID-IMMU90>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]