Abstract
The purpose was to evaluate the possible association of serum mannose binding lectin (s-MBL) levels on type of triggering microbe, duration of diarrhoea, incidence and course of reactive arthritis (ReA) caused by Salmonella, Yersinia and Campylobacter. Sixty patients with ReA of 1–228 months duration, 173 patients with ReA or uncomplicated enterocolitis caused by Campylobacter, 226 sera from patients with elevated antibody levels against Salmonella, Yersinia or Campylobacter, and 114 blood donors were tested for s-MBL using ELISA technique, both direct mannan binding assay and sandwich ELISA. s-MBL was compared with C-reactive protein (CRP) levels and with the ability of activating complement C4. Among the 114 donors 9% had s-MBL <50 µg/l, 16% had from 50–500 µg/l and 75% had >500 µg/l. The distribution of s-MBL levels in the three-patient groups did not differ significantly from the controls. There were no indications that low s-MBL was associated with prolonged duration of arthritis, diarrhoea or individual bacterial infections. The two MBL assays were comparable with respect to serum concentrations, indicating that the actual circulating MBL was also functionally active. s-MBL exhibited acute phase reactant behaviour and correlated to CRP level, but only in patients with s-MBL concentrations exceeding 1000 µg/l. MBL in 10 randomly selected ReA sera were tested for the ability to activate complement C4. The results did not differ from those of donor controls. This study demonstrates that the distributions of s-MBL levels in serum among patients with ReA are not different from donor controls. The course, outcome or triggering bacteria are not associated with a particular level of s-MBL.
Keywords: mannose binding lectin, reactive arthritis, ELISA, C-reactive protein, complement C4
INTRODUCTION
Mannose binding lectin (MBL) is a member of a family of Ca2+-dependent collagenous lectins, most of which are components of the innate immune system [1]. It is secreted by the liver and exhibits acute phase reactant behaviour [2]. MBL binds to carbohydrate structures on the surface of microorganisms and mediates deposition of complement factors using MBL-associated serine protease 1 (MASP-1) and MASP-2 activation of the complement cascade [3]. Low concentrations of MBL cause defective opsonization and phagocytosis [4,5] and has been associated with recurrent infections in infants [6] as well as in adults [7,8]. In addition MBL deficiency has been connected to early onset of rheumatoid arthritis and to a more severe disease course [9,10]. Low s-MBL levels are strongly associated with the presence of one of three point mutations at codon 52, 54, and 57 of exon 1 in the human MBL gene, encoding three different structural variants of MBL polypeptides. Besides, large individual variation in serum MBL level can be attributed to several polymorphisms within the promotor region of the MBL gene [5].
Reactive arthritis (ReA) is triggered by certain gastrointestinal or urogenital infections that give rise to an acute oligoarticular joint inflammation sometimes associated with extra-articular phenomena [11]. The pathogenetic events leading to ReA probably take place before an adequate humoral immune response against the causative microorganism can be mounted. This prompted us to investigate whether a defect in the innate immune response in terms of MBL deficiency could be a contributing factor in the development and course of ReA.
MATERIALS AND METHODS
Patient group 1
Sera from 60 patients with a diagnosis of ReA were collected from the Department of Rheumatology, Gentofte Hospital, Copenhagen, Denmark. Disease duration ranged from 1 month up to 228 months. Male/female ratio was 42/18 with a mean age of 37 years (range 16–69 years). The triggering infection was Salmonella in 9, Yersinia in 11, Campylobacter in 8, Chlamydia trachomatis in 10, and Shigella in 1. In 21 cases no infectious agents could be found, and instead the diagnosis rested upon the development of asymmetrical oligoarthritis in combination with one or more of the following symptoms: urethritis, conjunctivitis, sacroiliac joint arthritis or keratodermia blennorrhagica. Forty-three patients (72%) were HLA-B27 positive, 6 (10%) were negative, and in 11 (18%) the B27 status was unknown.
Patient group 2
One hundred and seventy-three sera from patients with Campylobacter infection verified by stool culture and followed for 2 years were included [12]. All patients had participated in a study to evaluate the incidence of post-infectious joint symptoms after Campylobacter infection. They had responded to a questionnaire inquiring about duration of diarrhoea, other gastrointestinal symptoms and onset of pain in a previously healthy joint within 4 weeks following the infection. They were further asked to mark on a drawing (38 joint score figure) the location of either painful or swollen joints. An equal number of patients with enterotoxigenic Escherichia coli enterocolitis received the same questionnaire and served as disease controls (Locht H et al. in press). Twenty-seven Campylobacter patients (16%) were diagnosed with probable reactive arthritis/arthralgia and 146 had uncomplicated enterocolitis.
Patient group 3
Two hundred and twenty-six sera were collected from the Department of Gastrointestinal Infections, Statens Serum Institut, Copenhagen. All serum samples were submitted from family physicians or hospital doctors and taken as part of routine investigations from patients with either gastrointestinal or articular complaints, or both. The physicians responsible for sera that were found positive (i.e. exceeding the cut-offs set by the laboratory) for antibodies against Salmonella, Yersinia or Campylobacter were mailed a questionnaire within 2 months asking about the diagnosis of the patient, gastrointestinal symptoms, painful or swollen joints and extra-articular manifestations. A total of 316 questionnaires were returned and in 226 a diagnosis of either uncomplicated enterocolitis or ReA was made. Ninety sera were excluded because of inaccurate information or because the clinical circumstances were irrelevant to the present study. The ratio of uncomplicated enterocolitis/ReA was: Salmonella, 47/42; Yersinia, 35/54; Campylobacter, 24/24.
Sera from 114 randomly selected blood donors were obtained from the Blood Bank at Rigshospitalet, Copenhagen, Denmark and served as controls.
Laboratory tests
MBL sandwich ELISA assay was performed essentially as described [13]. Briefly MBL was quantified by a semiautomated time-resolved sandwich immunofluorescence assay using the monoclonal antibody HYB 131–1 [Statens Serum Institut (SSI), Copenhagen] as catcher and biotinylated HYB 131–1 as detector antibody and steptavidin-Eu-chelate for quantification of bound second antibody. The assay was run on the AutoDelfia platform (Perkin Elmer, Shelton, CT, USA) using buffers provided by the manufacturer (Perkin Elmer). As standard a serum pool was used calibrated against highly purified MBL. The intra- and inter-assay co-efficients of variation were <5%. The assay range was 0·95 µg/l−60·9 µg/l. All samples were analysed in duplicate. The stability of MBL was assessed by subjecting a serum pool to 37°C, 4°C and repeated freeze/thaw cycles followed by quantifications using HYB 131–1. MBL was stable for 28 days at 4°C and 3 days at 37°C. Seven freeze/thaw cycles did not influence the MBL quantification.
ELISA for quantification of ligand-binding activity of MBL
The functional activity of MBL in patient samples was determined as the mannan-binding activity in an ELISA. The assay included a plasma standard – with a concentration measured by MBL sandwich ELISA – defined as having 100% mannan-binding activity, one negative and two positive controls. Samples were analysed in two dilutions in duplicate.
Between each step the ELISA plates were washed with 10 mm Tris (Sigma, Vallensbeck Strand, Denmark)/0·5 m NaCl (Unikem, Copenhagen, Denmark)/0·5% Tween 80 (Merck, Altertslund, Denmark)/5 mm CaCl2 (Merck), pH 7·4.
(i) Standard, controls and samples diluted in incubation buffer (10 mm Tris/0·5 m NaCl/0·05% Tween 80/5 mm CaCl2/0·1% HSA (SSI), pH 7·4) were applied to Microwell plates (Nunc, Naperville, IL, USA) coated with mannan [Sigma M 3640, 12·5 µg/ml in 50 mm NaHCO3 (Merck), pH 9·6, overnight at room temperature] and incubated for 16–24 h at 4°C. (ii) Biotinylated monoclonal anti-MBL (HYB 131–1, SSI) in incubation buffer was applied to the wells, incubated 1·5 h at room temperature. (iii) HRP-streptavidin (Zymed Laboratories Inc., South San Francisco, CA, USA) in incubation buffer was added the wells, incubated 1 h at room temperature. (iv) Staining with ortho-phenylene-diamine (Kem-en-Tec, Copenhagen, Denmark)/0·012% H2O2 in 35 mm citric acid (Merck)/67 mm Na2HPO4, pH 5, for 30 min at room temperature. Reactions were terminated by 1 m H2SO4 (Merck).
The colour intensity was measured in an ELISA-reader at 490 nm. The ligand-binding activity was estimated from the standard curve.
The assay range was 12·1–90·7 ng/ml. The interassay coefficient of variation was <10%.
Assay for the complement activating capacity of the MBL-MASP complex
The complement-activating ability of MBL through the MBL pathway was measured in patient samples as the C4-depositing capacity of the mannan-bound MBL complex in a specific sandwich ELISA. The assay was performed essentially as described by Petersen et al. in [14].
As a standard, a normal human plasma with a C4-depositing capacity of 1·23 units/ml, calibrated against the standard from [14], and a concentration of 2 µg MBL/ml was used, giving a specific activity of 0·62 units/µg MBL. A highly purified MBL preparation served as control. Serum samples were diluted and set up in duplicates. The results were estimated as the mean of at least three sample dilutions, calculated from the standard curve in units/ml, and the specific activity in units/µg, calculated by dividing with the s-MBL concentrations.
The assay range was 4·9–49 units/ml. The assay has been performed with defined specifications on a routine basis in our laboratory for 4 years. The interassay co-efficient of variation was <12%.
Ten randomly chosen sera from group 1 with s-MBL ranging from 1745 to 5876 µg/l were tested.
C-reactive protein assay
CRP was quantified by a sandwich ELISA assay using rabbit polyclonal anti-CRP (DAKO A073, DAKO, Glostrup, Denmark) as catcher and horseradish peroxidase conjugated rabbit polyclonal anti-CRP (DAKO P227) as detector. Calibrators were calibrated against Human C-Reactive Protein Kalibrator (DAKO X923). Assay range was 0·32 µg/l−5·1 µg/l. All samples were analysed in duplicate.
Ethics
The Copenhagen Regional Ethical Committee has approved the project (KF 01–078/99).
Statistics
The non-parametric Spearman rank correlation test was used to compare s-MBL levels in different assays and to test for correlation with duration of diarrhoea and arthritis. The chi square test was used to compare differences between patient groups.
RESULTS
Comparison of MBL assays
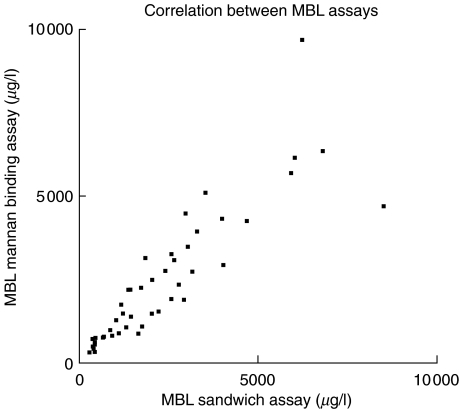
In order to evaluate the functional mannan binding activity, 49 sera from patient group 1 were tested in parallel in the MBL sandwich assay and MBL mannan binding ELISA. There was a good overall correlation (r = 0·95; 95% CI 0·91–0·97; P < 0·0001). When values obtained in the sandwich assay were set to 100%, the mean difference between individual measurements in the direct ELISA was 117·6% ± 37% (range 55%–232%) (Fig. 1).
Fig. 1.
Sera from 49 patients with a diagnosis of ReA were tested in a direct mannan binding ELISA (y-axis) and a sandwich ELISA (x-axis) for determination of serum MBL concentration. Only sera with MBL concentration above 250 µg/l were tested.
MBL correlation to disease manifestations, age, and triggering bacteria
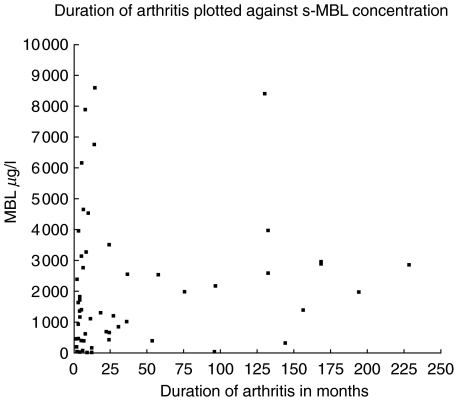
The distribution of s-MBL levels among the 60 ReA patients from group 1 were: <50 µg/l, 7 (12%); 50–500 µg/l, 13 (21%);>500 µg/l, 40 (67%). When MBL concentrations were compared with disease duration, there was no indication that low s-MBL led to a prolonged period of arthritis. On the contrary, a slightly positive correlation was observed between disease duration and MBL concentration (r = 0·26; 95% CI 0·001–0·49; P = 0·043) (Fig. 2). This, however, was not due to patients with chronic arthritis having higher inflammatory activity, because CRP levels were negatively correlated to duration of arthritis (r = −0·28; 95% CI −0·51 to −0·02; P = 0·032).
Fig. 2.
MBL concentration in sera detected by the sandwich ELISA from 60 patients with ReA plotted against duration of arthritis ranging from 1 month up to 228 months.
Age of onset of arthritis was 39·7 years in group 1 for those with s-MBL < 50 µg/l and 40·3 years for patients with s-MBL > 500 µg/l. The same figures for patients in group 3 were 41·4 and 41·3 years, respectively.
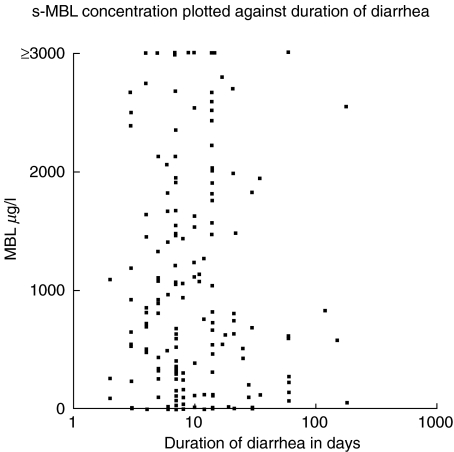
In a previous study of patients from group 2, we found that Campylobacter ReA patients had significantly longer durations of diarrhoea than those with uncomplicated enterocolitis, median 13 versus 7 days (Locht H et al. in press). But when the number of days with diarrhoea was compared with s-MBL levels there was no indication that low s-MBL was associated with a longer duration of gastroenteritis. This was true for the whole group and when ReA and non-ReA patients were analysed separately (Fig. 3).
Fig. 3.
Duration of diarrhoea in 173 patients with Campylobacter infection verified by stool culture plotted against serum MBL concentration measured by sandwich ELISA technique.
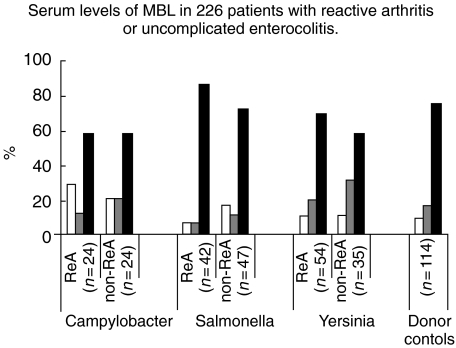
The MBL concentrations detected in the 226 sera from patient group 3 were divided into three levels: <50 µg/l, 50–500 µg/l, and> 500 µg/l. Among the Salmonella patients, the distribution was 3/8, 3/5 and 36/34 for ReA versus non-ReA; 6/4, 11/11, and 37/20 for Yersinia; and 7/5, 3/5, and 14/14 for Campylobacter, respectively (Fig. 4). Neither between individual bacterial species nor between ReA/non-ReA patients were there any statistically significant deviations from the donor population.
Fig. 4.
Sera from 226 patients with ReA or uncomplicated enterocolitis caused by Salmonella, Yersinia or Campylobacter and 114 blood donor control were tested for MBL concentration. There were no statistical differences between individual patient groups or between patients and donors. White columns (MBL < 50 µg/l), grey columns (MBL 50–500 µg/l), black columns (MBL > 500 µg/l).
CRP versus MBL concentrations
CRP was measured in 103 randomly selected samples. When sera with MBL levels> 1000 µg/l were compared with CRP there was a fairly good correlation (r = 0·33; 95% CI 0·08–0·54; P = 0·0097) indicating that MBL acts as an acute phase protein at higher concentrations. However, when sera with MBL levels <1000 µg/l were analysed there was no correlation to CRP concentrations (r = −0·08; 95% CI –0·39–0·24; P = 0·61).
Complement C4-activating capacity
Ten patient samples were randomly chosen among the high-level s-MBL group 1 patients for analysing the C4-depositing capacity and for calculation of the specific activities. The results ranged from 0·44 to 0·76 units/µg MBL and did not differ significantly from normal human serum [14], indicating that lack of capacity to bind MASP and/or activate MASP, leading to a functional MBL deficiency, was apparently not a part of the disease pathogenesis.
DISCUSSION
All sera were measured for s-MBL concentration in a sandwich ELISA with the same monoclonal antibody used as catcher and detector. Practically the same concentrations were, however, obtained when an alternative MBL ELISA assay was used where MBL was allowed to bind directly to its ligand, thus indicating that the amount measured was also functionally active. Individuals homozygous for the MBL gene point mutations giving rise to low or immeasurable s-MBL levels have been shown, by immune electrophoresis of whole serum, to possess MBL of a low molecular weight and with low binding affinity [15].
We found no indications that low s-MBL levels represented a risk factor for contracting ReA. This applied to all ReA patients irrespective of the triggering micro-organism. Neither was the course of disease influenced in terms of chronicity, duration of diarrhoea, age of onset of arthritis, or extra-articular symptoms (data not shown). The distribution of s-MBL levels among the various groups did not differ significantly from the donor controls. Quite heterogeneous groups of patients with, e.g. cystic fibrosis [16], HIV [17], and individuals undergoing immunosuppression during cancer chemotherapy [18] have been shown to be very susceptible to infections when they possess very low levels of s-MBL. However, some studies have failed to show an association between s-MBL and upper airways infections in children [19,20]. Certain of the former studies from highly specialized referral centres may have been biased in assessing patients who may suffer from additional immunological defects and thus may overestimate the importance of MBL. In one comprehensive study from a population-based prospective cohort in Greenland [6], the risk of acute respiratory tract infections was correlated to MBL insufficiency only in infants between the ages of 6 through 17 months. The risk in children below that age or older was less or none. ReA is not a common disease in childhood and the patient populations here studied are adults. This may also contribute to the lack of association.
S-MBL covaries with CRP, and thus functions as an acute phase protein [2], but this only applied to patients with serum levels exceeding 1000 µg/l of MBL. Lower s-MBL levels did not show any correlation to inflammatory activity as measured by CRP. In the group with MBL concentration in the range from 0 to 1000 µg/l, one will probably find individuals that are homo- or heterozygous for the MBL gene point mutations or promoter gene polymorphisms and therefore might respond inadequately to increased inflammatory activity.
Apart from infectious diseases, MBL deficiency has been associated with several immuno-inflammatory conditions, particularly SLE [21] and rheumatoid arthritis (RA). RA patients with low s-MBL levels were characterized by a younger age at disease onset, a higher joint swelling score [22], faster progression of radiographic joint destruction, and a higher erythrocyte sedimentation rate [10]. Although we examined three different patient populations, such an association could not be demonstrated between ReA and s-MBL and thus it seems that ReA, although being at the interface of infection and arthritis, probably is unaffected by the circulating s-MBL level.
References
- 1.Holmskov UL. Collectins and collectin receptors in innate immunity. APMIS. 2000;100(Suppl):1–59. [PubMed] [Google Scholar]
- 2.Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner MW, Hamvas RMJ. Mannose-binding lectin: structure, function, genetics and disease associations. Rev Immunogenet. 2000;2:305–22. [PubMed] [Google Scholar]
- 4.Kuhlman M, Joiner K, Ezekowitz RA. The human mannose-binding protein functions as an opsonin. J Exp Med. 1989;169:1733–45. doi: 10.1084/jem.169.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack DL, Klein NJ, Turner MW. Mannose-binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180:86–99. doi: 10.1034/j.1600-065x.2001.1800108.x. [DOI] [PubMed] [Google Scholar]
- 6.Koch A, Melbye M, Sorensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–21. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 7.Kakkanaiah VN, Shen GQ, Ojo-Amaize EA, Peter JB. Association of low concentrations of serum mannose-binding protein with recurrent infections in adults. Clin Diagn Laboratory Immunol. 1998;5:319–21. doi: 10.1128/cdli.5.3.319-321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Summerfield JA, Ryder S, Sumiya M, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–9. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 9.Garred P, Madsen HO, Marquart H, et al. Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol. 2000;27:26–34. [PubMed] [Google Scholar]
- 10.Graudal NA, Madsen HO, Tarp U, et al. The association of variant mannose-binding lectin genotypes with radiographic outcome in rheumatoid arthritis. Arthritis Rheum. 2000;43:515–21. doi: 10.1002/1529-0131(200003)43:3<515::AID-ANR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Sieper J, Braun J, Kingsley GH. Report on the fourth international workshop on reactive arthritis. Arthritis Rheum. 2000;43:720–34. doi: 10.1002/1529-0131(200004)43:4<720::AID-ANR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Strid MA, Engberg J, Larsen LB, Begtrup K, Molbak K, Krogfelt KA. Antibody responses to campylobacter infections determined by an enzyme-linked immunosorbent assay: 2-year follow-up study of 210 patients. Clin Diagn Laboratory Immunol. 2001;8:314–9. doi: 10.1128/CDLI.8.2.314-319.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipscombe RJ, Beatty DW, Ganczakowski M, et al. Mutations in the human mannan binding lectine gene: frequencies in several population groups. Eur J Hum Genet. 1996;4:13–9. doi: 10.1159/000472164. [DOI] [PubMed] [Google Scholar]
- 14.Petersen SV, Thiel S, Jensen L, Steffensen R, Jensenius JC. An assay for the mannan-binding lectin pathway of complement activation. J Immunol Meth. 2001;257:107–16. doi: 10.1016/s0022-1759(01)00453-7. [DOI] [PubMed] [Google Scholar]
- 15.Lipscombe RJ, Sumiya M, Summerfield JA, Turner MW. Distinct physicochemical characteristics of human mannose binding protein expressed by individuals of differing genotype. Immunology. 1995;85:660–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Garred P, Pressler T, Madsen HO, et al. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–7. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garred P, Madsen HO, Balslev U, et al. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–40. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 18.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 19.Garred P, Brygge K, Sorensen CH, Madsen HO, Thiel S, Svejgaard A. Mannan-binding protein – levels in plasma and upper-airways secretions and frequency of genotypes in children with recurrence of otitis media. Clin Exp Immunol. 1993;94:99–104. doi: 10.1111/j.1365-2249.1993.tb05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homoe P, Madsen HO, Sandvej K, Koch A, Garred P. Lack of association between mannose-binding lectin, acute otitis media and early Epstein-Barr virus infection among children in Greenland. ScandJ Infect Dis. 1999;31:363–6. doi: 10.1080/00365549950163798. [DOI] [PubMed] [Google Scholar]
- 21.Garred P, Madsen HO, Halberg P, et al. Mannose-binding lectin polymorphisms and susceptibility to infection in systemic lupus erythematosus. Arthritis Rheum. 1999;42:2145–52. doi: 10.1002/1529-0131(199910)42:10<2145::AID-ANR15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Graudal NA, Homann C, Madsen HO, et al. Mannan binding lectin in rheumatoid arthritis. A longitudinal study. J Rheumatol. 1998;25:629–35. [PubMed] [Google Scholar]