Abstract
The objective of the study was to investigate the relationship between various CD4+ T cell subsets and the ability of peripheral blood mononuclear cells (PBMC) to proliferate to several stimuli in vertically human immunodeficiency virus type 1 (HIV-1)-infected children. We studied 29 HIV-1-infected children on highly active antiretroviral therapy (HAART) (median duration: 12·3 months). T cell subsets were determined by flow cytometry. Plasma viral load (VL) was quantified using a standardized molecular method. Proliferative responses were evaluated by [3H]-thymidine incorporation. Decreased proliferative responses of PBMC to pokeweed mitogen (PWM) were found for HIV-1-infected children in Centers for Disease Control (CDC) clinical categories B and C when compared to the control group (P < 0·05). Similarly, children with ≤ 15% CD4+ T cells showed a decrease in proliferative responses to PWM (P < 0·01), anti-CD3 + anti-CD28 (P < 0·01) and phytohaemagglutinin (PHA) (P < 0·05) with respect to the control group and to children with CD4+ T cells ≥ 25%. Proliferative responses to PWM, anti-CD3+, anti-CD28 and PHA had a statistically significant positive correlation with CD3+/mm3, CD4+/mm3, % CD4 T cells, CD4/CD8 ratio and the percentage of naive T cell subsets (CD4+CD45RO−HLA-DR−, CD4+ CD45RA+ CD62L+, CD4+ CD45RA+), CD4+ CD62L+ and CD4+ T cells co-expressing CD38+ (CD4+ HLA-DR−CD38+, CD4+ CD38+). Moreover, we found a negative correlation between PBMC proliferative responses and % CD8 T cells, memory, memory-activated and activated CD4+ T cell subsets. Lower proliferative responses to PWM (P < 0·01) and PHA (P < 0·01) were associated with higher VL. Our data show that higher proliferative responses to PWM, anti-CD3 + anti-CD28 and PHA are associated with both non-activated and naive CD4+ T cell subsets in HIV-1-infected children on HAART.
Keywords: CD4+ T cells, children, HIV-1, proliferative response, viral load
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) causes a progressive breakdown of immunity that can be quantified by several different assays. CD4+ T cell counts, which usually determine the stage of disease, and plasma viral load (VL), which reflects active ongoing viral replication, are used to predict disease progression [1,2]. The impairment of T cell function provides a separate measurement. Impairment occurs early in HIV-1 infection, when CD4+ T cell counts are still within normal ranges, and decreases further as disease progresses [3,4]. The proliferative responses to mitogens, such as pokeweed (PWM) or phytohaemagglutinin (PHA), and to anti-CD3 plus anti-CD28 antibodies are sensitive prognostic markers for AIDS [4,5].
Activation of the immune system is another major effect of HIV-1 infection. Activation occurs early and increases throughout the course of the infection [6]. The altered expression of lymphocyte surface antigens also reflects the dynamic interaction between the immune system and HIV-1. The surface expression of HLA-DR and CD38 is significantly increased and predicts the progression to AIDS in adults [4,7,8,9,10,11]. However, in children the CD38+ marker is a maturation marker instead, because 75% of all CD4+ T cells in children normally co-express CD38 [12]. Because CD4+ T cells are central to proliferation and are a major target for HIV-1 infection, the phenotypical changes of these cells are of special interest. Furthermore, naive (CD4+ CD45RA+ CD62L+) and memory (CD4+ CD45RO+) T cells differ in several aspects, including proliferative responses, and memory T cells are more sensitive to activation induced by cell death than naive T cells [13]. In addition, memory T cells respond better to recall antigens and exhibit much higher proliferative responses to anti-CD3, whereas naive T cells respond better to mitogenic stimuli such as PWM or PHA [14]. However, proliferative responses to alloantigens are similar in both memory and naive CD4+ T cells [14].
On the other hand, highly active antiretroviral therapy (HAART) has had a tremendous impact on the course of HIV infection [15,16], resulting in important suppression of VL, reconstitution of CD4+ T cells [17,18] and a reduction in opportunistic infections [16]. However, complete recovery of CD4+ T cell counts and function is not usually achieved [19–21]. HAART in adults induces a reduction in activation markers on memory T cells [22,23], an increase in memory T cell numbers later on followed by a gradual and sustained increase in naive T cell numbers [19–21].
We have studied the relationship between various CD4+ T cell subsets and the ability of peripheral blood mononuclear cells (PBMC) to proliferate to several stimuli in vertically HIV-1-infected children.
MATERIALS AND METHODS
Patients and control subjects
Twenty-nine vertically HIV-infected infants over 5 years of age were recruited in a cross-sectional study between July 1999 and July 2000 at the Paediatric Department of the General University Hospital ‘Gregorio Marañón’ in Madrid, Spain. All infants were diagnosed after birth as HIV-infected on the basis of positive results in both DNA-polymerase chain reaction (PCR) and virus culture assays, as described previously [24]. None of these 29 children were breastfed. All children were given HAART with two inhibitors of reverse transcriptase plus an inhibitor of protease (median duration: 12·3 months; range, 6·2–24 months; Table 1). We also studied 16 age-matched HIV-negative children as a control group. The inclusion criterion of an age greater than 5 years was chosen because younger children had major fluctuations in T cell subsets that could result in misinterpretation of data [25]. Clinical classification was based on the 1994 revised guidelines of the Centers for Disease Control (CDC) [26]. It is unresolved whether the CDC immunological category (representing the nadir of an individual subject's CD4 percentage or absolute count) is of relevance to the lymphoproliferative response (LPR). We worked on the hypothesis that the ‘current immunological category’ (based on the CD4+ T cell percentage at the time of study) accounted for the LPR more than the CDC immunological category. Drugs were prescribed by the treating paediatrician according to CDC guidelines (Table 2) [27] upon obtaining written informed consent from parents or legal guardians. The study was conducted according to the Declaration of Helsinki and approved by the Ethical Committee of our hospital.
Table 1.
Summary of antiretroviral treatment of all HIV-infected children
| CDC clinical category | Current immunological category* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time on HAART | Basal VL | ΔVLa | A | B | C | >25% | 15–25% | <15% | |
| 2NRTI+Amprenavir | 21.5 | 4.25 | −0.25 | 1 | 1 | 1 | 1 | ||
| 2NRTI+Efavirenz | 8.1 | 4.97 | −2.67 | 1 | 1 | ||||
| 2NRTI+Indinavir | 11.6 | 2.66 | −0.27 | 1 | 3 | 1 | 2 | 1 | |
| 2NRTI+Nelfinavir | 5.1 | 4.43 | −0.61 | 2 | 6 | 3 | 4 | 4 | 3 |
| 1NRTI+Nelfinavir+ Efavirenz | 10.2 | 4.54 | 0.69 | 1 | 1 | ||||
| 1NRTI+Nelfinavir+ Nevirapine | 12.7 | 3.93 | −0.11 | 1 | 4 | 4 | 1 | ||
| 2NRTI+Ritonavir | 20 | 2.48 | −0.18 | 1 | 1 | ||||
| 2NRTI+Saquinavir+ Indinavir | 22.4 | 4.74 | −2.14 | 3 | 2 | 1 | |||
| 2NRTI+Saquinavir+ Nelfinavir | 11.9 | 5.73 | −0.46 | 1 | 1 | ||||
(a) Change in log viral load on HAART. VL: viral load. CDC: Centers for Disease Control. NRTI: nucleoside analogue HIV-1 reverse transcriptase inhibitor. HAART: highly active antiretroviral therapy.
CD4+ T cell percentages.
Table 2.
Summary of the characteristics of HIV-infected children and the healthy non-HIV control group at the time of the cross-sectional study.
| Characteristics | Control group | HIV |
|---|---|---|
| No. HIV-1 children | 13 | 29 |
| Age (years)a | 10.6 (5.3–18) | 9.7 (5.3; 17.6) |
| CDC clinical category | ||
| ″A | – | 8 (27.6%) |
| ″B | – | 8 (27.6%) |
| ″C or AIDS | – | 13 (44.8%) |
| Current immunological category | ||
| ″>25% CD4+ | – | 13 (44.8%) |
| ″15–25% CD4+ | – | 9 (31.0%) |
| ″<15% CD4+ | – | 7 (24.1%) |
| Lymphocyte subsetsa | ||
| ″% CD4+ | 40.5 (29.5; 55.4) | 23.6 (2; 46.2) |
| ″% CD8+ | 22.1 (13.7; 28.1) | 46.6 (32; 86) |
| ″CD4+/mm3 | 1183 (588; 3123) | 558 (86; 2510) |
| ″CD8+/mm3 | 654 (304; 1641) | 1112 (339; 4276) |
| Virological characteristicsa | ||
| ″Log10 VL (copies/ml) | – | 3.82 (1.3; 5.80) |
VL = viral load; AIDS = Acquired immunodeficiency syndrome
Values are expressed as median. (min, max). CDC: Centers for Disease Control.
Quantification of T cell subsets in peripheral blood
Total counts and percentages of CD4+ and CD8+ T cells were analysed by trucount™ (Becton-Dickinson Immunocytometry Systems, San José, CA, USA) using whole blood, whereby cells were selected by means of an SSC gate against anti-CD45 [28] following the manufacturer's instructions. The acquisition was carried out in a FACSCalibur cytometer (Becton-Dickinson) using the CELLQuest (Becton-Dickinson) acquisition program immediately after cell staining. trucount™ Control Beads were used routinely as a quality control.
The monoclonal antibodies used for the analysis of CD4+ T cell subsets were conjugated with fluorescein-isothyocianate (FITC) (anti-IgG1, anti-HLA-DR, anti-CD45RA, anti-CD38), phycoerithrin (PE) (anti-IgG1, anti-CD45RO, anti-CD62L, anti-HLA-DR) and peridinin chlorophyl protein (PerCP) (anti-CD4). The monoclonal antibodies were obtained from Becton-Dickinson (Becton-Dickinson), except for anti-CD38 which was from Immunotech (Marseille, France). Three-colour phenotypical characterizations of lymphocytes were performed by flow cytometry in whole, lysed and washed blood [29]. Naive CD4+ T cells were defined as CD62L+ and CD45RA+ bright T cells (CD4+ CD45RAhi/CD62L+). Memory cells were defined as CD4+ CD45RO+ or CD4+ CD45RA−CD62L+ T cells. Activated T cells were defined as CD4+ HLA-DR+ and CD4+ HLA-DR+ CD38+ 0. Memory-activated CD4+ were defined as CD4+ CD45RO+ HLA-DR+. CD4+ CD38+ are mainly naive CD4+ T cells [12]. Acquisition was performed in a FACScan (Becton-Dickinson) cytometer using the Lysis II acquisition program (Becton-Dickinson) within 2 h of cell staining. The optimal parameters for acquisition (detector sensitivity, detector amplification and compensation) were determined using Calibrate as a reagent (Becton-Dickinson) and the AutoComp (Becton-Dickinson) program periodically. Five thousand events were compiled using a collection gate for CD4+ T cells. The gate was defined using the low side scatter (SSC) and high expression of CD4 [28,30]. Data were analysed using the Lysis II analysis program (Becton-Dickinson). Appropriate isotypic controls (IgG1-FITC; IgG1-PE) were used to evaluate the non-specific staining, which was deducted from the remaining results. The absolute figures of the different analysed subsets were calculated by multiplying the percentage value by the total number of CD4+ T cells.
Proliferative response of peripheral blood mononuclear cells
PBMC were isolated from blood by Ficoll-Paque density gradient centrifugation (Pharmacia, Uppsala, Sweden). Total PBMC were seeded in 96-well flat-bottom microtitre plates (2 × 105/100 µl per well). PBMC were stimulated with either 1 µg/ml of anti-CD3 (SPV3Tb, kindly provided by Dr J.E. de Vries, DNAX, Palo Alto, CA, USA) plus 1 µg/ml anti-CD28 (Becton-Dickinson), 1 µg/ml of phytohaemagglutinin (PHA) (Murex Biotech Limited, Dartford, UK) or 4 µg/ml of pokeweed (PWM) (Sigma Chemical Co., St Louis, MI, USA). PBMC were cultured for 72 h at 37°C in an atmosphere containing 5% CO2, after which 1 mCi of [3H]-thymidine was added and after a further 6 h the culture was stopped. The proliferative response was evaluated by incorporation of [3H]-thymidine and cells were harvested in glass fibre filters using an automatic cell harvester (Skatron, Norway). Radioactive incorporation was measured in a liquid scintillation spectrometer (1450 Microbeta Trilux, Wallac, Turku, Finland). The assay was carried out in quadruplicate cultures.
Quantitative viral load assay
Blood samples were collected in EDTA tubes, the plasma was separated within 4 h and stored at −70°C. Plasma viral load was measured in 200 µl plasma using a quantitative reverse transcriptase PCR (RT-PCR) assay (Amplicor monitor, Roche Diagnostic Systems) [31].
Statistics
CD4+ and CD8+ counts are expressed as percentages. In all analyses, VL was transformed to log10-scale in order to normalize distribution. The relationship between variables was investigated using a Spearman correlation coefficient. Values range between − 1 (a perfect negative relationship) and + 1 (a perfect positive relationship), 0 indicates no relationship. The Mann–Whitney U-test, a non-parametric analogue of the variance analysis of one factor, was used for comparisons between groups.
Proliferation of PBMC is expressed as stimulation index (SI):
This was chosen to normalize assays. No statistically significant differences of the spontaneous proliferative responses between HIV-1-infected children and healthy controls were found. These SI of HIV-infected children were standardized with respect to a control group of healthy children of similar age range. HIV-infected children were divided into three groups: (a) P1: children with a proliferative response to PWM similar to that of the control group (within 1 standard deviation (s.d.)); (b) P2: children with a lower proliferative response to PWM (lower than 1 s.d.) but similar cell proliferation to PHA than the control group (within 1 s.d.); (c) P3: children with lower proliferative responses to both PWM and PHA than the control group (lower than 1 s.d.). HIV-1-infected children were grouped in this way because it has been reported that there is a progressive loss of lymphoproliferative responses during HIV-1 infection, first PWM and later PHA [3]. Our goal was to describe the values of the different T cell subsets according to lymphoproliferative responses.
According to immunological classification based on the 1994 revised guidelines of the CDC, there was one child in immunological category 1 (IC-1), six children in IC-2 and 22 children in IC-3. Because this classification did not provide much information we divided the children into current immunological categories according to the CD4+ T cell percentage at the time of study.
RESULTS
Proliferative responses of PBMC according to CDC clinical categories and viro-immunological markers
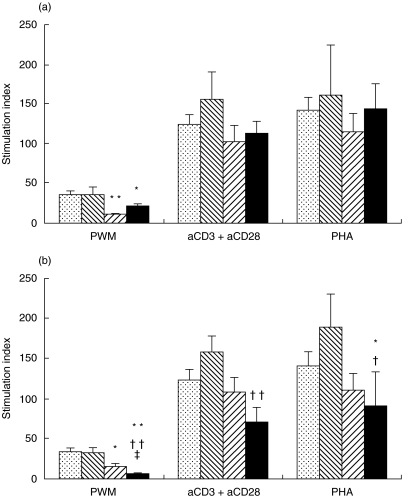
We performed a cross-sectional study in 29 vertically HIV-infected children (mean age 10·4 ± 0·6 years, range 5·2–17·6). Their clinical, immunological and virological characteristics at the time of the cross-sectional study are shown in Table 2. When we stratified these children according to clinical categories, we found a significant loss of PBMC proliferative responses to PWM in clinical categories B and C compared to the control group, but we did not find any differences in the proliferative responses to anti-CD3 + anti-CD28 or PHA (Fig. 1). We used anti-CD3 + anti-CD28 because it is a better stimulus than anti-CD3 alone. CD28 delivers a second signal to T cells that considerably enhances T cell reactivity in vitro (approximately eight- to 10-fold). Also, we showed lower variation coefficients for the double stimulation than reactivity to CD3 monoclonal antibody alone (median 5 versus 20, respectively). When we stratified children by current immunological category, children with CD4+ T cells ≤ 15% showed significant decreases in proliferative responses to PWM, anti-CD3+ anti-CD28 and PHA with respect to both the control group and children with CD4+ T cells ≥ 25% (proliferative responses to PWM, PHA and anti-CD3 plus anti-CD28 in HIV-1 children and healthy controls). Moreover, children with CD4+ T cells 15–25% showed significantly lower proliferative responses than the control group, and significantly higher responses than the children with CD4+ T cells ≤ 15% (Fig. 1). On the other hand, HIV-children with CD4+ T cells ≤ 15% or clinical categories C showed higher values of VL than other HIV children (P < 0·05).
Fig. 1.
Proliferative responses to PWM, PHA and anti-CD3 plus anti-CD28 in HIV-1 children and healthy controls. Values expressed as mean ± s.e.m. (min; max) and absolute number (%); CDC: Centers for Disease Control; CIC = current immunological category. aCD3 = anti-CD3; aCD28 = anti-CD28. Statistical differences from the healthy control group (*P < 0·05; **P < 0·01). Statistical differences with HIV-infected children in clinical category B or immunological category 2 (†P < 0·05; ††P < 0·01). Statistical differences with HIV-infected children in clinical category C or immunological category 3 (‡P < 0·05; ‡‡P < 0·01).(a)  , Control group;
, Control group;  , CDC-A;
, CDC-A;  , CDC-B; ▪, CDC-C. (b)
, CDC-B; ▪, CDC-C. (b)  , Control group;
, Control group;  , CIC-1 (≥ 25% CD4+);
, CIC-1 (≥ 25% CD4+);  , CIC-2 (15–25% CD4+); ▪, CIC-3 (≤ 15% CD4+).
, CIC-2 (15–25% CD4+); ▪, CIC-3 (≤ 15% CD4+).
A positive correlation between PBMC proliferative responses to PWM, anti-CD3 + anti-CD28 and PHA with CD3+ T cells/mm3, CD4+ T cells/mm3, % CD4+ and CD4/CD8 ratio was found (Table 3). However, a negative correlation between % CD8+ T cells and PBMC proliferative responses to PWM, anti-CD3 + anti-CD28 and PHA was found (Spearman's correlation analysis of CD3+, CD4+ and CD8+ T cell percentages, and viral load with proliferative response). A negative correlation between VL and PBMC proliferative responses to PWM and PHA was also found (Table 3).
Table 3.
Spearman's correlation analysis of CD3+, CD4+ and CD8+ T cell percentages and viral load with proliferative response of stimulated PBMC in all HIV-infected children on HAART
| PWM | aCD3 + aCD28 | PHA | |
|---|---|---|---|
| CD3+/mm3 | 0.38* | 0.45* | 0.47** |
| CD4+/mm3 | 0.78** | 0.68** | 0.69** |
| CD8+/mm3 | 0.03 | 0.15 | 0.26 |
| % CD3 | −0.12 | −0.14 | −0.08 |
| % CD4 | 0.72** | 0.57** | 0.53** |
| % CD8 | −0.69** | −0.48** | −0.38* |
| CD4/CD8 | 0.70** | 0.56** | 0.47* |
| Log10 VL (copies/ml) | −0.42* | −0.25 | −0.40** |
PHA= phytohaemagglutinin; PWM= pokeweed; aCD3= anti-CD3; aCD28= anti-CD28; VL= viral load.
Level of significance (P < 0.05)
Level of significance (P < 0.01).
Correlation of PBMC proliferative responses with CD4+ T cell subsets and plasma viral load
A positive correlation between PBMC proliferative responses to PWM, anti-CD3 + anti-CD28 and PHA and % CD4+ T cells with naive CD4+ T cells (CD4+ CD45RO−HLA-DR−, CD4+ CD45RA+ CD62L+, CD4+ CD45RA+), CD4+ CD62L+ was observed. The same trend was observed for the same subsets of cells co-expressing CD38+ (CD4+ HLA-DR−CD38+, CD4+ CD38+) (Table 4). Therefore, the higher the proliferative response, the higher the proportion of naive T cells.
Table 4.
Spearman correlation analysis of CD4+ T cell percentages, viral load and proliferative responses with the different CD4+ T cell subset percentages in all HIV-infected children on HAART included in the study
| PWM | aCD3 +aCD28 | PHA | %TCD4+ | Log10 VL | |
|---|---|---|---|---|---|
| CD4+CD45RO+DR+ | −0.84** | −0.70** | −0.72** | −0.73** | 0.53** |
| CD4+CD45RO−DR+ | 0.13 | 0.32 | −0.04 | 0.12 | 0.32 |
| CD4+CD45RO+DR− | −0.64** | −0.57** | −0.45* | −0.57** | −0.05 |
| CD4+CD45RO−DR− | 0.77** | 0.65** | 0.62** | 0.70** | −0.22 |
| CD4+CD45RO+ | −0.76** | −0.66** | −0.61** | −0.68** | 0.19 |
| CD4+CD45RA+CD62L+ | 0.83** | 0.77** | 0.70** | 0.85** | −0.25 |
| CD4+CD45RA−CD62L+ | −0.68** | −0.84** | −0.74** | −0.59** | 0.28 |
| CD4+CD45RA+CD62L− | 0.11 | 0.45* | 0.06 | 0.07 | 0.21 |
| CD4+CD45RA−CD62L− | −0.70** | −0.57** | −0.46* | −0.82** | 0.18 |
| CD4+CD62L+ | 0.68** | 0.51** | 0.43* | 0.79** | −0.20 |
| CD4+CD45RA+ | 0.82** | 0.77** | 0.69** | 0.85** | −0.25 |
| CD4+DR+CD38+ | −0.68** | −0.50** | −0.66** | −0.50** | 0.60** |
| CD4+DR−CD38+ | 0.70** | 0.59** | 0.66** | 0.86** | −0.31 |
| CD4+DR+CD38− | −0.70** | −0.59** | −0.62** | −0.79** | 0.37 |
| CD4+DR−CD38− | −0.46* | −0.43* | −0.41* | −0.67** | −0.16 |
| CD4+DR+ | −0.79** | −0.62** | −0.71** | −0.67** | 0.54** |
| CD4+CD38+ | 0.52** | 0.49** | 0.48** | 0.73** | 0.10 |
PHA= phytohaemagglutinin; PWM= pokeweed; aCD3= anti-CD3; aCD28= anti-CD28; VL= viral load.
Level of significance (P < 0.05
Level of significance (P < 0.01).
Conversely, a negative correlation between PBMC proliferative responses to PWM, anti-CD3 + anti-CD28 and PHA and % CD4+ T cells with memory (CD4+ CD45RO+, CD4+ CD45RO+ HLA-DR−, CD4+ CD45RA−CD62L+, CD4+ CD45RA−CD62L−), memory-activated (CD4+ CD45RO+ HLA-DR+) and activated (CD4+ HLA-DR+ CD38+, CD4+ HLA-DR+ CD38−, CD4+ HLA-DR+) CD4+ T cells was found (Table 4). A positive correlation between VL and activated (CD4+ HLA-DR+ CD38+ and CD4+ HLA-DR+) and memory-activated (CD4+ CD45RO+ HLA-DR+) CD4+ T cells was also found (Table 4).
Correlation between PBMC proliferative responses to PWM and PHA and CD4+ T cell subsets numbers
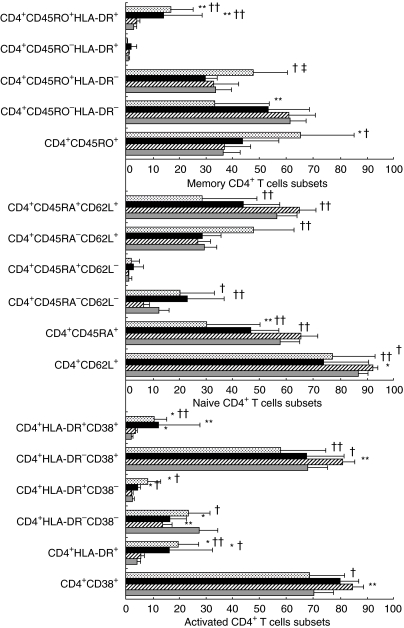
We rated the 29 HIV-infected children based on their proliferative responses to PWM and PHA into three groups (see Statistics). Differences among the three groups of HIV-infected children are shown in Fig. 2. A progressive increase of memory (CD4+ CD45RO+, CD4+ CD45RA−CD62L+, CD4+ CD45RA−CD62L−), memory-activated and activated CD4+ T cells (CD4+ HLA-DR+, CD4+ HLA-DR+ CD38+, CD4+ HLA-DR+ CD38−) was associated with the loss of PBMC proliferative responses to PWM and to PHA (Fig. 2). A progressive and parallel decrease in naive CD4+ T cells (CD4+ CD45RA+ CD62L+), CD4+ CD45RA+, CD4+ CD62L+, CD4+ CD38+ and CD4+ HLA-DR−CD38+ T cells with the loss of proliferative responses to PWM and PHA in the P3 group was also found (Fig. 2).
Fig. 2.
Summary of the mean percentage values of activated, memory and naive CD4+ T cell subsets stratified according to proliferative responses (see Statistics): (a) P1: children with a normal proliferative response to PWM; (b) P2: children with a lower proliferative response to PWM but normal cell proliferation to PHA; (c) P3: children with lower proliferative responses to both PWM and PHA. (*) Differences with control group (*P < 0·05; **P < 0·01). Differences with P1 (†P < 0·05; ††P < 0·01). Differences with P2 (‡‡P < 0·01).  , P3 = PWM and PHA lower than control; ▪, P2 = PWM lower than, and PHA similar to control;
, P3 = PWM and PHA lower than control; ▪, P2 = PWM lower than, and PHA similar to control;  , P1 = PWM similar to control;
, P1 = PWM similar to control;  , control non-HIV.
, control non-HIV.
DISCUSSION
Although several immune alterations have been identified during HIV-1 infection, only a few studies have examined their interrelationship in HIV-1-infected children on HAART. The impairment of the lymphoproliferative function in HIV-1 infection is particularly interesting because it may be observed earlier during infection than the overt decline of CD4+ T cells and because such changes are reported to be related to disease progression. Furthermore, proliferative responses return to normal levels after the initiation of antiretroviral therapy [4], indicating that those proliferative alterations are induced by HIV-1 infection. In accordance with this, we found that HIV-children with CD4+ T cells ≤ 15% or clinical category C had higher values of VL than other HIV children, due possibly to a poorer response to HAART. However, analyses of the proliferative capacity of T cells are rarely used for determining the HIV-1 disease stage or monitoring therapy, in part because these assays are more laborious than serological or flow cytometric ones, and must be performed with freshly obtained blood samples. In this study, we found that PWM appears to be a more sensitive stimulus for detecting the impairment of the lymphoproliferative response in HIV-1 infected children on HAART in the B and C clinical categories, in agreement with previous results in adults [4]. This may be explained, at least partially, because PHA acts via both CD2- and TCR/CD3-dependent pathways, while PWM involves primarily TCR/CD3-induced responses. Thus, our results may indicate that the proliferative defect associated with HIV-1 infection mainly alters the TCR/CD3-induced response.
HIV infection also induces a decrease in CD4+ T cell numbers and concomitantly activates the immune system [9,32–34]. This chronic activation of the immune system has been associated with an increase in expression of activation markers on T cells [9,33,34], i.e. CD38 and HLA-DR, in adults [35]. In our study, we observed a strong positive correlation between activated CD4+ T cells and VL, and a negative correlation between activated T cells (CD4+ CD38+ HLA-DR+, and CD4+ HLA-DR+) and % CD4+ T cells and PBMC proliferation to PWM, anti-CD3 + anti-CD28 and PHA. These data suggest that in children, activated T cells (CD4+ CD38+ HLA-DR+ and CD4+ HLA-DR+) are associated directly with VL [19]. The relationship between CD38 expression in CD4+ T cells and VL showed a different pattern compared with that between HLA-DR and VL, due probably to the influence of age and cell type on the expression of CD38 [8,36–39]. Contrary to adults, CD4+ CD38+ is not a good disease progression marker in HIV-infected children [11,40–42]. Although we selected children over 5 years of age to minimize the effect of age on CD38 expression, and despite the fact that we found a positive correlation between CD4+ CD38+, % CD4+ T cells and PBMC proliferative responses, we did not find any correlation between CD4+ CD38+ and VL. Therefore, our data indicate that CD4+ CD38+ T cells should not be used as a progression marker in HIV-infected children. However, the expression of CD38 on CD4+ T cells seems to correlate with a more preserved immune system, as shown by our data rating HIV-infected children according to their loss of PBMC proliferative responses to PWM and PHA.
The loss of reactivity to mitogenic stimuli occurs with the progression of disease [3]. Naive and memory T cells differ in several aspects including cellular proliferative responses. Memory T cells respond better to recall antigens and present much higher proliferative responses to anti-CD3, whereas naive T cells respond better to mitogenic stimuli such as PWM or PHA [14]. We have found that the loss of PBMC proliferative responses to PWM, anti-CD3, anti-CD28 and PHA inversely correlated with memory and memory-activated T cells. Indeed, the P3 group (with diminished responses to both PWM and PHA) showed higher values of memory and memory/activated CD4+ T cells than the P1 and P2 groups.
HAART produces an important suppression of VL and an increase in CD4 T cell numbers [17,18], drastically reducing opportunistic infections [16]. However, the complete recovery of CD4+ T cell counts and function is not usually achieved [19–21]. The reduction of VL in HIV-infected subjects undergoing HAART therapy is accompanied by an increase of PBMC proliferative responses in vitro that is due mainly to CD4+ T cells [21]. In agreement with the above results, we found a strong positive correlation between proliferative responses and CD4+ cell numbers. We also found qualitative differences between PWM, anti-CD3, anti-CD28 and PHA-induced proliferation. Although this is a cross-sectional study, the recovery of the proliferative responses to PHA, but not to PWM, may be ascribed to HAART, due probably to its dependence of antigen presenting cells, suggesting that proliferative response to PWM is a more sensitive marker of immunological deterioration.
Naive T cells are crucial for the immune response and their loss have a severe impact in HIV infection [16]. We have observed a positive correlation between naive CD4+ T cells and immunological markers (PBMC proliferation and % CD4+) and a negative correlation with VL. Altogether, this indicates that a preserved immune system, with a high level of naive CD4+ T cells, maintains its capacity to respond to antigens and control viral replication. In agreement with this, the P3 group showed lower levels of naive CD4+ T cells than the P2 and P1 groups. By contrast, activated CD4+ T cells (high HLA-DR expression, co-expression of CD45RO and HLA-DR or CD38 and HLA-DR) are strong and earlier markers of immunological deterioration and viral replication.
In summary, our data suggest that the loss of proliferative responses to PWM is a good and reliable marker of clinical progression during HIV infection. Additionally, naive, memory and activated CD4+ T cell subset responses reflect the status of the immune system, as these subsets correlate differently with CD4+ T cell counts and VL. Moreover, memory and activated CD4+ T cells were associated with the loss of cell proliferation to mitogenic stimuli. Longitudinal studies should be carried out to highlight these correlations between the functional capacity of the immune system and the variations in the different T-cell subsets.
Acknowledgments
We thank Dr Dolores Gurbindo (Sección de Inmunopaediatría, Hospital Gregorio Marañón, Madrid, Spain) for her participation in the selection and control of patients. We would also like to thank Dolores García Alonso and Consuelo Muñoz for their excellent technical assistance. This work was funded by grants from Fundación para la Investigación y Prevención del SIDA en España (FIPSE 3008/99), Programa Nacional de Salud (SAF 99–0022), Fondo de Investigación Sanitaria (00/0207), Comunidad de Madrid (08·5/0034/2001) and Bristol-Myers, S.A. (Grupo Bristol-Myers Squibb).
References
- 1.Resino S, Gurbindo MD, Bellón JM, Sanchez-Ramón S, Muñoz-Fernández MA. Predictive markers of clinical outcome in vertically HIV-1 infected infants. A prospective longitudinal study. Pediatr Res. 2000;47:509–15. doi: 10.1203/00006450-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Gurbindo D, Resino S, Sanchez-Ramon S, León JA, Munoz-Fernández MA. Correlation of viral load and CD8 T-lymphocytes with development of neurological manifestations in vertically HIV-1-infected infants. A prospective longitudinal study. Neuropediatrics. 1999;30:197–204. doi: 10.1055/s-2007-973490. [DOI] [PubMed] [Google Scholar]
- 3.Clerici M, Stocks NI, Zajac RA, et al. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass HZ, Fahey JL, Nishanian P, et al. Relation of impaired lymphocyte proliferative function to other major human immunodeficiency virus type 1-induced immunological changes. Clin Diagn Lab Immunol. 1997;4:64–9. doi: 10.1128/cdli.4.1.64-69.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins MK. The ups and downs of T cell costimulation. Immunity. 1994;1:443–6. doi: 10.1016/1074-7613(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 6.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–63. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Bass HZ, Nishanian P, Hardy WD, et al. Immune changes in HIV-1 infection: significant correlations and differences in serum markers and lymphoid phenotypic antigens. Clin Immunol Immunopathol. 1992;64:63–70. doi: 10.1016/0090-1229(92)90060-2. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Bofill M, Lipman M, et al. CD8+,CD38+ lymphocyte percent: a useful immunological marker for monitoring HIV-1-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:158–62. doi: 10.1097/00042560-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. Cell Immunol. 1993;150:72–80. [PubMed] [Google Scholar]
- 10.Kestens L, Vanham G, Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–41. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Martino M, Rossi ME, Azzari C, Gelli MG, Galli L, Vierucci A. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–8. doi: 10.1203/00006450-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Jackson DG, Bell JI. Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J Immunol. 1990;144:2811–5. [PubMed] [Google Scholar]
- 13.Janossy G, Borthwick N, Lomnitzer R, et al. Lymphocyte activation in HIV-1 infection. I. Predominant proliferative defects among CD45R0+ cells of the CD4 and CD8 lineages. AIDS. 1993;7:625–31. doi: 10.1097/00002030-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Akbar AN, Salmon M, Janossy G. The synergy between naive and memory T cells during activation. Immunol Today. 1991;12:184–8. doi: 10.1016/0167-5699(91)90050-4. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society–USA panel. JAMA. 1997;277:1962–9. [PubMed] [Google Scholar]
- 16.Powderly WG, Landay A, Lederman MM. Recovery of the immune system with antiretroviral therapy. the end of opportunism? JAMA. 1998;280:72–7. doi: 10.1001/jama.280.1.72. [DOI] [PubMed] [Google Scholar]
- 17.van Rossum AM, Niesters HG, Geelen SP, et al. Clinical and virologic response to combination treatment with indinavir, zidovudine, and lamivudine in children with human immunodeficiency virus-1 infection: a multicenter study in the Netherlands. On behalf of the Dutch Study Group for Children with HIV-1 infections. J Pediatr. 2000;136:780–8. doi: 10.1067/mpd.2000.106234. [DOI] [PubMed] [Google Scholar]
- 18.Sleasman JW, Nelson RP, Goodenow MM, et al. Immunoreconstitution after ritonavir therapy in children with human immunodeficiency virus infection involves multiple lymphocyte lineages. J Pediatr. 1999;134:597–606. doi: 10.1016/s0022-3476(99)70247-7. [DOI] [PubMed] [Google Scholar]
- 19.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. J Med Chem. 1997;40:2164–76. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 20.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 21.Kelleher AD, Sewell WA, Cooper DA. Effect of protease therapy on cytokine secretion by peripheral blood mononuclear cells (PBMC) from HIV-infected subjects. Clin Exp Immunol. 1999;115:147–52. doi: 10.1046/j.1365-2249.1999.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelleher AD, Carr A, Zaunders J, Cooper DA. Alterations in the immune response of human immunodeficiency virus (HIV)-infected subjects treated with an HIV-specific protease inhibitor, ritonavir. J Infect Dis. 1996;173:321–9. doi: 10.1093/infdis/173.2.321. [DOI] [PubMed] [Google Scholar]
- 23.Bohler T, Walcher J, Holzl-Wenig G, et al. Early effects of antiretroviral combination therapy on activation, apoptosis and regeneration of T cells in HIV-1-infected children and adolescents. Am J Public Health. 1999;89:947–8. doi: 10.1097/00002030-199905070-00006. [DOI] [PubMed] [Google Scholar]
- 24.Munoz-Fernandez MA, Obregon E, Navarro J, et al. Relationship of virologic, immunologic, and clinical parameters in infants with vertically acquired human immunodeficiency virus type 1 infection. Pediatr Res. 1996;40:597–602. doi: 10.1203/00006450-199610000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Denny T, Yogev R, Gelman R, et al. Lymphocyte subsets in healthy children during the first 5 years of life. JAMA. 1992;267:1484–8. [PubMed] [Google Scholar]
- 26.Centers for Diseases Control Prevention (CDCP) Revised classification system for HIV-1 infection in children less than 13 years of age. MMWR. 1994;43:1–13. [Google Scholar]
- 27.Centers for Diseases Control Prevention (CDCP) Guidelines for use of antiretroviral agents in pediatric HIV infection. MMWR. 1998;47:RR–4. [PubMed] [Google Scholar]
- 28.Nicholson JK, Hubbard M, Jones BM. Use of CD45 fluorescence and side-scatter characteristics for gating lymphocytes when using the whole blood lysis procedure and flow cytometry. Cytometry. 1996;26:16–21. doi: 10.1002/(SICI)1097-0320(19960315)26:1<16::AID-CYTO3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson J, Kidd P, Mandy F, Livnat D, Kagan J. Three-color supplement to the NIAID DAIDS guideline for flow cytometric immunophenotyping. Cytometry. 1996;26:227–30. doi: 10.1002/(SICI)1097-0320(19960915)26:3<227::AID-CYTO8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.Resino S, Navarro J, Bellón JM, et al. Naïve and memory CD4+ T cells and T cell activation markers in HIV-1 infected children on HAART. Clin Exp Immunol. 2001;125:266–73. doi: 10.1046/j.1365-2249.2001.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muñoz-Fernández MA, Navarro J, Obregon E, et al. Immunological and virological markers of disease progression in HIV-1 infected children. Acta Paediatr Suppl. 1997;421:56–9. doi: 10.1111/j.1651-2227.1997.tb18319.x. [DOI] [PubMed] [Google Scholar]
- 32.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–8. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 33.Levacher M, Hulstaert F, Tallet S, Ullery S, Pocidalo JJ, Bach BA. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–82. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips AN, Sabin CA, Elford J, Bofill M, Lee CA, Janossy G. CD8 lymphocyte counts and serum immunoglobulin A levels early in HIV infection as predictors of CD4 lymphocyte depletion during 8 years of follow-up. AIDS. 1993;7:975–80. doi: 10.1097/00002030-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Altman A, Coggeshall KM, Mustelin T. Molecular events mediating T cell activation. Adv Immunol. 1990;48:227–360. doi: 10.1016/s0065-2776(08)60756-7. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger M, Chu FN, Badamchian M, et al. A distinctive form of soluble CD8 is secreted by stimulated CD8+ cells in HIV-1-infected and high-risk individuals. Clin Immunol Immunopathol. 1994;73:252–60. doi: 10.1006/clin.1994.1195. [DOI] [PubMed] [Google Scholar]
- 37.Plaeger-Marshall S, Isacescu V, O'Rourke S, Bertolli J, Bryson YJ, Stiehm ER. T cell activation in pediatric AIDS pathogenesis: three-color immunophenotyping. Clin Immunol Immunopathol. 1994;71:27–32. doi: 10.1006/clin.1994.1046. [DOI] [PubMed] [Google Scholar]
- 38.Ibegbu C, Spira TJ, Nesheim S, et al. Subpopulations of T and B cells in perinatally HIV-infected and noninfected age-matched children compared with those in adults. J Acquir Immune Defic Syndr. 1994;7:340–8. doi: 10.1006/clin.1994.1047. [DOI] [PubMed] [Google Scholar]
- 39.Schlesinger M, Peters V, Jiang JD, Roboz JP, Bekesi JG. Increased expression of activation markers on CD8 lymphocytes in children with human immunodeficiency virus-1 infection. Pediatr Res. 1995;38:390–6. doi: 10.1203/00006450-199509000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Hultin LE, Cumberland WG, et al. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection: results of 6 years of follow-up. Cytometry. 1996;26:1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Navarro J, Resino S, Bellón JM, et al. Association of CD8+ T lymphocyte subsets with the most commonly used markers to monitor HIV-1 infection in children treated with highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:525–32. doi: 10.1089/08892220151126607. [DOI] [PubMed] [Google Scholar]
- 42.Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T cell activation or an active player in virus/host interactions? Aids. 2000;14:1079–89. doi: 10.1097/00002030-200006160-00004. [DOI] [PubMed] [Google Scholar]