Abstract
HLA class II-restricted proliferative and cytotoxic T cell (CTL) responses to B cell chronic lymphocytic leukaemia (B-CLL) can be generated using autologous dendritic cells (DCs) pulsed with tumour cell lysate. In this study a number of different approaches were used to optimize further the in vitro system. First, the effects of a variety of maturation agents were studied. The addition of TNF-α, polyriboinosinic polyribocytidylic acid (Poly(I:C)) and LPS to autologous DCs resulted in the emergence of only a small percentage of CD83+ DCs, IFN-α having no demonstrable effect. Only the addition of Poly(I:C) to DCs resulted in modestly increased specific cytotoxicity to B-CLL targets, IFN-α and LPS having no effect. Secondly, T cells were pretreated with IL-15, prior to culturing with lysate-pulsed autologous DCs. A significant increase in T cell activation (P = 0·038), IFN-γ secretion (P = 0·030) and specific cytotoxicity to B-CLL targets (P = 0·006) was demonstrated compared to untreated T cells. Thirdly, monocyte derived DCs electrofused with B-CLL B cells were compared with lysate-pulsed DCs. T cells stimulated by fused DCs generated higher levels of specific cytotoxicity to autologous B-CLL B cell targets than those stimulated by lysate pulsed DCs (P = 0·013). Blocking studies demonstrated inhibition of this cytotoxicity by both anti-CD4 (P = 0·062) and anti-CD8 monoclonal antibodies (P = 0·018), suggesting the generation of both HLA class I- and HLA class II-restricted CTL responses. In summary, in vitro B-CLL-specific T cell responses can be enhanced further by preincubating T cells with IL-15 and using autologous fused DC–B-CLL hybrids instead of autologous lysate-pulsed DCs. These preliminary data require confirmation with larger numbers of patients. Such an approach, however, may eventually provide effective immunotherapy for treatment of B-CLL.
Keywords: B cell chronic lymphocytic leukaemia, dendritic cells, fusion, IL-15, tumour
INTRODUCTION
HLA class II-restricted proliferative and cytotoxic T cell responses to B cell chronic lymphocytic leukaemia (B-CLL) can be generated by pulsing autologous dendritic cells (DCs) with tumour cell lysate [1]. However, the levels of specific antitumour cytotoxicity detected in this B-CLL system were not as high as those reported for other tumours [2–6]. There are a number of potential explanations for this. First, there are inherent differences in the immunogenicity of B-CLL compared to other tumours. Secondly, immunomodulation of the DCs, secondary to immunosuppressive factors secreted by the B-CLL cells, may impair antigen processing and presentation. Thirdly, dysregulation of the T cell repertoire in B-CLL has been demonstrated [7]. Fourthly, the in vitro system itself may be technically suboptimal. In this study we attempted to optimize the B-CLL system taking into account the above factors.
In order for DCs to present antigen optimally to T cells, they need to reach a stage of maturation, phenotypically characterized as CD83+ [8]. The monocyte-derived DCs in our system were relatively immature (CD83−). Following loading of antigen onto DCs, a further ‘danger signal’ is required to achieve maximal antigen presentation [9]. Examples of factors that have been shown to enhance maturation of DCs include tumour necrosis factor-alpha (TNF-α) [10], lipopolysaccharide (LPS) [11], polyriboinosinic polyribocytidylic acid (Poly(I:C)) [12] and interferon-α (IFN-α) [13]. In addition to this, there are recent data showing that interleukin-15 (IL-15) can enhance antigen specific proliferation in vitro[14], and correct the T cell dysfunction found in B cell malignancies such as multiple myeloma [15]. The current view is that effective antitumour responses necessitate the generation of both CD4+ HLA class II-restricted helper T cells and CD8+ HLA class I-restricted cytotoxic T cells (CTLs) [16]. However, the responses found in this B-CLL system were due predominantly to CD4+ HLA class-II restricted CTLs [1]. Effective priming of CD8+ HLA-class I-restricted CTLs requires the introduction of antigen into the cytoplasm [17,18]. A strategy that can achieve this is to fuse antigen-presenting cells with tumour cells. Whole cell vaccines have been produced in this manner for several different tumours [19–21]
In view of the above, (i) DCs were incubated with TNF-α, LPS, Poly(I:C) and IFN-α (ii) T cells were incubated with IL-15 and (iii) DCs were fused to autologous B-CLL B cells, in an attempt to increase the level of cytotoxicity of our system and generate CD8+ HLA class I-restricted CTLs.
MATERIALS AND METHODS
Patients and normal volunteers
Local research ethics committee permission and individual informed consent were obtained for these studies. A group of eight patients with B-CLL, six males and two females were selected at random from the out-patient clinic. Of the three patients used for IL-15 experiments, two were stage A/0 and one was stage C/III and had received previous treatment at least 6 months previously. White blood cell counts ranged between 12 and 45 × 109/l. The single patient used for electrofusion experiments was stage A/0 with a white blood cell count of 27 × 109/l and had not received treatment previously.
Dendritic cell isolation and culture
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation from peripheral blood. PBMC from patients with B-CLL and healthy volunteers were depleted of CD19+ cells using Pan B Dynabeads (Dynal, Merseyside, UK). The CD19-depleted PBMC were cultured in a 24-well tissue culture plate (Gibco, Life Technologies, Paisley, UK) at 37°C in 5% CO2 for 2 h. Culture medium consisted of RPMI-1640 (Gibco), 10% human AB serum, 2 mm glutamine (Sigma, Dorset, UK), 500 U/ml penicillin (Sigma) and 500 µg/ml streptomycin (Sigma). Non-adherent cells were removed by vigorous washing in culture medium. Adherent cells were incubated in culture medium with 800 U/ml granulocyte macrophage colony stimulating factor (GM-CSF) (Cambridge Bioscience, Cambridge, UK) and 1000 U/ml interleukin-4 (IL-4) (Cambridge Bioscience) at 37°C in 5% CO2 for 6 days. The cultures were fed every 2 days with fresh culture medium containing IL-4 and GM-CSF. On day 6, the culture medium was removed and the cells cultured in fresh culture medium with 800 U/ml GM-CSF and 100 µg/ml interleukin-12 (IL-12) (Cambridge Bioscience) for 16 h, washed and resuspended in culture medium. The incubation with IL-12 was performed on all DCs prior to pulsing with lysate or fusion.
Preparation of soluble cell lysate
The CD19+ B cells from the PBMC fraction were removed from Dynabeads using Pan B Detachabeads (Dynal). In the B-CLL patient these cells were 97% CD5+ and 92% CD20+. B cells were resuspended in 2 ml of lysis buffer (10 mm bicarbonate buffer pH 7·1 and 0·5 mm phenyl methyl sulphonyl fluoride) (Sigma) on ice. The cells were homogenized on ice using a Dounce Homogeniser (Jencons, Leighton Buzzard, UK) and then ultrasonicated using two 10-s bursts with a 15-s rest from a 50-W-Vibracell (Sonics and Materials Inc., Jencons). Soluble protein was collected after ultracentrifugation at 55 000 r.p.m. (100 000 g) for 1 h at 4°C. The protein concentration was quantified by the Bradford protein assay method using a protein determination kit (Biorad, Hertfordshire, UK). Soluble protein lysates were sterile filtered using 0·4 µfilters (Nalgene, Marathon Laboratory Supplies, London, UK) and stored at − 70°C. All cell lysates were exposed to CD19+ Dynabeads.
Pulsing dendritic cells with soluble B cell lysate
DCs were pulsed by the addition of soluble lysate to the culture medium at 10 µg/ml per 106 cells and incubated for 24 h at 37°C in 5% CO2. Control unpulsed DCs were incubated at this time with lysis buffer.
Addition of maturation agents to dendritic cells
One hundred ng/ml LPS (Sigma) was added to DCs from patient 3 after 7 days culture for 24 h at 37°C in 5% CO2. Fifty µg/ml Poly(I:C) (Sigma) was added to DCs from patient 2 after 7 days' culture for 3 days at 37°C in 5% CO2. TNF-α (Cambridge Bioscience) was added to DCs from patient 4 at 10 ng/ml after 7 days' culture for 2 days at 37°C in 5% CO2. IFN-α (Cambridge Bioscience) was added to DCs from patient 1 at 2 mg/ml after 7 days' culture for 24 h at 37°C in 5% CO2. DCs were removed from 24 well tissue culture plates by 0·2% EDTA in PBS (Sigma) and washed in culture medium prior to addition to T cell co-cultures.
Fusion of dendritic cells and B-CLL B cells
DCs were isolated as described previously. The electrofusion method was based upon findings of Scott-Taylor et al. [22]. B-CLL B cells and dendritic cells were resuspended in 0·3 m sodium sucrose solution at a cell density of 5 × 105/ml; 0·4 ml of B-CLL B cells and dendritic cells were added to a 0·8-ml electro-plated cuvette (Bio-Rad). An exponential pulse of 250 V at 25 µFd with a time constant averaging between 3·4 and 4 milliseconds was delivered using the Gene Pulser Transfection Apparatus (Bio-Rad). Additional B-CLL B cells and dendritic cells were pulsed with an exponential pulse of 500 V at 25 µFd with a time constant of 8·7 milliseconds. B-CLL B cells and dendritic cells were mixed in electroplated cuvette but not pulsed as a control for non-specific uptake of membrane fragments. Cells were washed once in Hepes buffered saline solution. Fused cells were separated on 10% wv dextran solution 500 × g for 5 min while some remained non-separated as a control. The layer containing hybrid cells was washed in HBSS. Cells in the process of ‘round-up’ were observed in the separated cultures using × 20 objective of a Hund Wetzlar phase contrast inverted microscope (Wilovert, Jencons). Cells were resuspended and cultured in culture medium + 10% AB serum + IL-12 and GM-CSF overnight at 37°C in 5% CO2. The total viable cell yield was established from the count of cells in an aliquot mixed 1 : 10 with 0·4% Trypan blue using a haemocytometer after 16 h in culture. This was a more accurate estimate of long-term survival of the cells, as electroporation can make live cells permeable to exclusion dyes such as Trypan blue [23]. Comparison of the total cell yield in separated and non-separated cultures gave a crude estimation of fusion efficiency. Cells were assessed for the presence of CD86 and CD20 upon the cell surface by flow cytometry after 16 h in culture. Due to the low yield, fused separated cells exposed to the two different voltages were combined and added to T cell co-cultures. A summary of cell yields and fusion efficiencies is shown in Table 1.
Table 1.
Summary of electrofusion efficiencies and yields
| Fusion method | No. of viable cells | % fused cells | Total fused cell yield |
|---|---|---|---|
| DC alone | 3·5 × 105 | 3 | – |
| Mixed DC and B-CLL non-fused (negative control) | 4·3 × 105 | 6·6 | |
| (passive uptake) | 2·8 × 104 | ||
| 250 V non-separated | 3 × 105 | 10·4 | 3·1 × 104 |
| 250 V separated on 10% dextran | 1 × 104 | 50·5 | 5 × 103 |
| 500 V non-separated | 1 × 105 | 45·0 | 4·5 × 104 |
| 500 V separated on 10% dextran | 5 × 104 | 83·1 | 4·1 × 104 |
B-CLL B cells from patient 8 were electrofused using Gene Pulser Transfection Apparatus (Biorad). Viability counts were estimated by counting cells excluding Trypan blue dye using haemocytometer 16 h after voltage application. Percentage of cells fused was calculated from flow cytometry duel-labelling with CD20-RPE and CD86-FITC antibodies under stated conditions 16 h after voltage application.
T cell isolation
T cells were isolated indirectly from the PBMC fraction by depleting adherent and CD19+ cells. The purity of the T cells was, on average, 60% when assessed by flow cytometry using anti-CD3-FITC conjugated antibodies.
Pretreatment of T cells with IL-15
T cells were plated at 8 × 105/ml in 24-well plates (Life Technologies, Invitrogen) in culture medium + 10% AB serum + 10 ng/ml IL-15 (Cambridge Bioscience). Cells were incubated for 16 h at 37°C in 5% CO2. T cells were washed in culture medium twice to remove any residual IL-15 before coculture with dendritic cells.
T cell cultures
DCs were aliquoted at a concentration of 103 cells per well to 96-well round-bottom tissue culture plates. T cells were added to give a T cell : DC ratio of at least 20 : 1. Cultures destined for cytotoxicity assays were fed with 5 U/ml of interleukin-2 (IL-2) (Cambridge Bioscience) on days 3, 7, 10, 14 and 17. DCs in cultures destined for cytotoxicity assays were re-stimulated by the addition of 1 µg/ml soluble B cell lysate or lysis buffer on days 7 and 14. Cultures were continued for a total of 28 days at 37°C in 5% CO2 in culture medium + 5% AB serum. Cultures used to assess cytokine secretion were not fed IL-2 or restimulated with soluble lysate.
Immunophenotyping
The following monoclonal antibodies were used for immunophenotyping cells: CD4-FITC (Serotec, Oxford, UK), CD8-PE (Serotec), CD3-FITC (Serotec), CD16-FITC (Serotec), CD56-FITC (Serotec), HLA-DR-PE (Serotec), CD83-FITC (Immunotech, Coulter, Luton, UK), CD40-PE (Serotec), CD86-FITC (Serotec), CD14-PE/CD45-FITC (Becton Dickinson, Oxford, UK), CD11c-PE (Serotec), CD20-PE (Serotec), CD5-PE (Serotec), CD19-FITC (Serotec), CD1a-FITC (Serotec), anti-IgG1-PE and anti-IgG1-FITC (Serotec). Cells were washed twice in PBS and then twice in PBS + 0·05% BSA (Sigma). Directly conjugated antibodies were added at 10 µl per 106 cells and incubated for 15 min at room temperature. Cells were washed twice in PBS and twice in PBS + 0·05% BSA. Positive antibody binding was assessed in terms of gates set at 2% of relative isotype controls using an Epics Elite flow cytometer (Coulter).
Activation assay
T cell activation was measured quantifying cells co-expressing CD3 and CD25 (IL-2 receptor) by double-labelled flow cytometry. The methodology outlined by Loken and Wells [24] was employed using anti-CD3-FITC (Serotec) and anti-CD25-PE (Serotec) conjugated monoclonal antibodies. Positive antibody binding was assessed in terms of gates set at 2% of anti-IgG1-PE and anti-IgG1-FITC-labelled cells. Anti-CD3-FITC (Serotec) and anti-CD25-PE (Serotec) conjugated monoclonal antibodies were added individually to controls to set compensation.
Quantification of cytokine secretion
Cell-free tissue culture supernatants were harvested on days 1–5 and stored at −70°C until required. When convenient, the supernatants were thawed and the concentrations of IFN-γ measured in duplicate by ELISA (Pelkline, Eurogenetics, Hampton, UK). Sensitivity limits for the assays were 2–6 pg/ml for IFN-γ.
Cytotoxicity assay
Cytotoxicity was measured by a flow cytometric method using LIVE/DEAD cell-mediated cytotoxicity kit (Molecular Probes, Cambridge Bioscience). Target cells were labelled with 4 µl per 5 × 105 cells of diOC18 for 2 h at 37°C in 5% CO2 and washed twice in culture medium. Effector cells were harvested from the tissue culture and placed in flow cytometry tubes (Falcon, Marathon Laboratory Supplies) at the appropriate effector : target ratios. A minimum of 104 labelled targets was added. Propidium iodide was added to each tube. Targets and effectors were mixed gently and centrifuged at 500 g for 1 min. Targets and effectors were incubated together for 4 h at 37°C in 5% CO2. Flow cytometry standard gates were set on unlabelled targets stained with propidium iodide and diOC18-labelled targets without propidium iodide. Non-specific cell death (spontaneous apoptosis) was measured by the cytotoxicity of diOC18-labelled targets stained with propidium iodide without effectors. Cytotoxicity was expressed as the number of dead targets (cells staining positive for propidium iodide and diOC18) divided by the total number of targets (cells staining positive for diOC18). Percentage specific cytotoxicity was measured as follows:
The B-CLL B cell targets were 97% CD5+ and 92% CD20+. The K562 cell line were purchased from European Collection of Cell Cultures (ECACC) (Sigma) and underwent four passages in RPMI-1640, 10% fetal calf serum (Sigma), 2 mm glutamine, 500 U/ml penicillin and 500 mg/ml streptomycin before use as a target sensitive to cytotoxicity mediated by natural killer cells.
Antibody blocking studies
Antibody blocking experiments involved the addition anti-CD4 (Serotec) and anti-CD8 (Serotec) monoclonal antibodies at 100 µg/ml at the commencement of the 4-h incubation of effectors and targets.
Statistical analysis
Direct comparisons between treatment groups were analysed using Student's t-test. Overall effects of treatment were analysed by one-way analysis of variance (anova). Statistics were generated using Statsgraphics Plus software.
RESULTS
Addition of dendritic cell maturation agents
Cell surface expression of CD83 was increased (maximum 12%) by incubating DCs with Poly(I:C), LPS and TNF-α but not with IFN-α. No increase in IFN-γ secretion was demonstrated when DCs were incubated with IFN-α, Poly(I:C) or LPS. Increased specific cytotoxicity to B-CLL targets was demonstrated when DCs were incubated with Poly(I:C), but not with IFN-α or LPS (data not shown).
Treatment of T cells with IL-15 prior to stimulation with lysate-pulsed dendritic cells
T cells from two B-CLL patients cultured with lysate-pulsed autologous DCs demonstrated a significant increase in numbers of activated cells at 168 h, compared to T cells cultured with buffer-pulsed DCs (P = 0·030) (Fig. 1). Pretreatment of T cells with IL-15, prior to culturing with lysate-pulsed autologous DCs, gave rise to a further increase in numbers of activated T cells at 168 h, which was significantly higher than T cells not treated with IL-15 (P = 0·038) and IL-15-treated T cells cultured with buffer-pulsed DCs (P = 0·029) (Fig. 1).
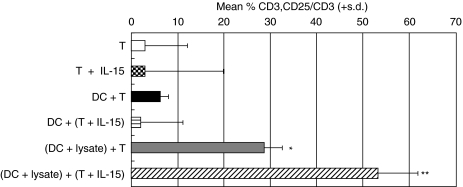
Fig 1.
Effect of IL-15 pretreatment on activation markers. Numbers of CD3/CD25 positive T cells were measured in two patients at 168 h. T cells were cultured alone (T) or with autologous DCs pulsed with lysate [(DC + lysate) + T] or lysis buffer [DC + T]. T cells were preincubated with IL-15 (10 ng/ml) for 16 h and then cultured alone (T + IL-15) or with autologous DCs pulsed with lysate [(DC + lysate) + (T + IL-15)] or lysis buffer [DC + (T + IL-15)]. T cells cultured with lysate-pulsed DCs demonstrated a significant increase in T cell activation, compared to T cells cultured with lysis-buffer pulsed DCs (P = 0·030). Pretreatment of T cells with IL-15, prior to culturing with lysate-pulsed DCs, gave rise to a further increase in numbers of activated T cells, which was significantly higher than T cells not treated with IL-15 (P = 0·038). One measurement per patient was made. Mean values were tested using unpaired Students' t-test. *P = 0·030; **P = 0·038.
T cells from two B-CLL patients cultured with lysate-pulsed autologous DCs demonstrated an increase in IFN-γ secretion which peaked at 96 h and which was significantly greater than T cells cultured with buffer-pulsed DCs (P = 0·038) (Fig. 2). Pretreatment of T cells with IL-15, prior to culturing with lysate-pulsed autologous DCs, gave rise to a significant increase in IFN-γ secretion, which peaked at 24 h, compared to T cells not treated with IL-15 (P = 0·030) and IL-15-treated T cells cultured with buffer-pulsed DCs (P = 0·018) (Fig. 2).
Fig 2.
Effect of IL-15 pretreatment on IFN-γ secretion. Culture supernatants from two patients were assayed for secreted IFN-γ by ELISA at (a) 24, (b) 48, (c) 72 and (d) 96 h. T cells were cultured alone (T) or with autologous DCs pulsed with lysate [(DC + lysate) + T] or lysis buffer (DC + T). T cells were preincubated with IL-15 (10 ng/ml) for 16 h and then cultured alone [T + IL-15] or with autologous DCs pulsed with lysate [(DC + lysate) + (T + IL-15)] or lysis buffer [DC + (T + IL-15)]. T cells cultured with lysate-pulsed DCs demonstrated an increase in IFN-γ secretion which peaked at 96 h and which was significantly greater than T cells cultured with lysis buffer-pulsed DCs (P = 0·038). Pretreatment of T cells with IL-15, prior to culturing with lysate-pulsed DCs, gave rise to a significant increase in IFN-γ secretion, which peaked at 24 h, compared to T cells not treated with IL-15 (P = 0·030). Each treatment group per patient was measured in duplicate. Mean values were tested using unpaired Students' t-test; *P = 0·038, **P = 0·030.
T cells from three B-CLL patients cultured with lysate-pulsed DCs showed significantly higher levels of specific cytotoxicity to autologous B-CLL B cell targets at the 40 : 1 effector : target ratio compared to DCs pulsed with lysis buffer (P = 0·019) (Fig. 3). Pretreatment of T cells with IL-15, prior to culture with lysate-pulsed autologous DCs, showed a further rise in specific cytotoxicity to B-CLL B cell targets, which was significantly higher than T cells not treated with IL-15 (P = 0·006) and with IL-15-treated T cells cultured with buffer-pulsed DCs (P = 0·002) (Fig. 3). K562 cells were used as a target in our cytotoxicity assay to ensure that the effect seen with IL-15 pretreatment of T cells was not a result of NK cell activity. No significant killing to K562 cells was shown by T cells cultured with autologous lysate-pulsed DCs whether pretreated with IL-15 or not (data not shown).
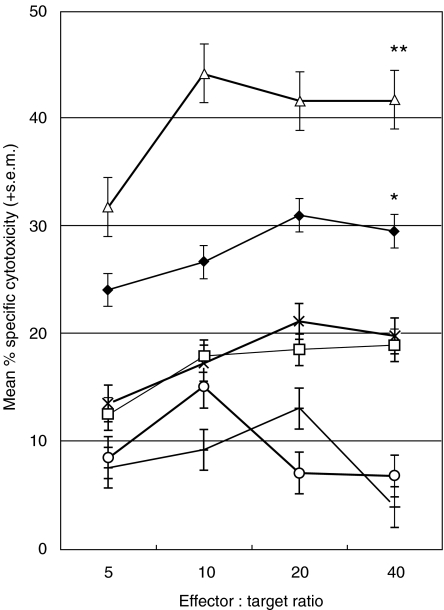
Fig 3.
Effect of IL-15 pretreatment on cytotoxicity. Effectors from three patients were cultured for 28 days and tested for cytotoxicity against autologous B-CLL B cell targets. T cells were cultured alone (+) or with autologous DCs pulsed with lysate (♦) or lysis buffer (□). T cells were preincubated with IL-15 (10 ng/ml) for 16 h and then cultured alone (○) or with autologous DCs pulsed with a soluble B-CLL lysate (▵) or lysis buffer (×). T cells cultured with lysate-pulsed DCs showed significantly higher levels of specific cytotoxicity to B-CLL B cell targets compared to lysis buffer-pulsed DCs (P = 0·019). Pretreatment of T cells with IL-15, prior to culture with lysate-pulsed DCs, showed a further rise in specific cytotoxicity to B-CLL B cell targets, which was significantly higher than T cells not treated with IL-15 (P = 0·006). Data was analysed by one-way anova; *P = 0·019, **P = 0·006.
Fusion of B-CLL B cells with monocyte-derived dendritic cells
Cell yields and fusion efficiencies are summarized in Table 1. T cells from one B-CLL patient cultured with lysate-pulsed DCs showed significantly higher levels of specific cytotoxicity to autologous B-CLL B cell targets compared to DCs pulsed with lysis buffer at the 40 : 1 effector : target ratio (P = 0·037) (Fig. 4). Culturing T cells with autologous fused DC–B-CLL hybrids resulted in a further rise in specific cytotoxicity to B-CLL targets which was significantly higher than T cells cultured with lysate-pulsed DCs at the 40 : 1 effector : target ratio (P = 0·013) (Fig. 4). In addition, culturing T cells with autologous fused DC–B-CLL hybrids gave rise to significantly greater specific cytotoxicity to B-CLL targets than T cells cultured with a non-fused mix of DCs and B-CLL cells at the 40 : 1 effector : target ratio (P = 0·016) (Fig. 4).
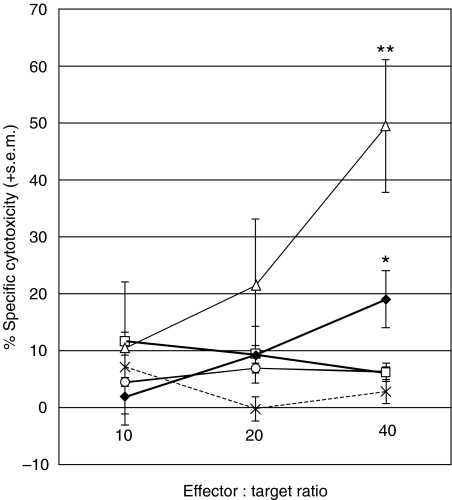
Fig. 4.
Comparison of lysate-pulsed DCs and electrofused DCs. Effectors from one patient were cultured for 28 days and tested for cytotoxicity against autologous B-CLL B cell targets. T cells were cultured alone (○), with autologous DCs pulsed with lysate (♦) or lysis buffer (□), with DCs mixed with B-CLL B cells (×) or with DCs electrofused with B-CLL B cells (▵). T cells cultured with lysate-pulsed DCs showed significantly higher levels of specific cytotoxicity to B-CLL B cell targets compared to lysis buffer-pulsed DCs (P = 0·037) at the 40 : 1 ratio. T cells cultured with fused DC–B-CLL hybrids resulted in a further rise in specific cytotoxicity to B-CLL targets, which was significantly higher than T cells cultured with lysate-pulsed DCs (P = 0·013) at the 40 : 1 ratio. Each treatment group was measured in duplicate. Data were analysed by unpaired Students' t-test; *P = 0·037, **P = 0·013.
Antibody blocking studies
Specific cytotoxicity to B-CLL targets, by T cell effectors from a single B-CLL patient generated by culture with lysate-pulsed autologous DCs, was inhibited significantly by anti-CD4 (P = 0·039) but not by anti-CD8 monoclonal antibodies (Fig. 5). However, specific cytotoxicity by T cell effectors from the same B-CLL patient generated by culture with autologous DC–B-CLL hybrids was inhibited by anti-CD4 (P = 0·062) and inhibited significantly by anti-CD8 (P = 0·018) monoclonal antibodies (Fig. 5).
Fig 5.
Antibody blocking of cytotoxicity assay. Effectors from one patient were cultured alone ( ), with autologous DCs pulsed with lysate (□) or lysate buffer (
), with autologous DCs pulsed with lysate (□) or lysate buffer ( ), with autologous DCs electrofused with B-CLL B cells (▪) and with autologous DCs mixed with B-CLL B cells (
), with autologous DCs electrofused with B-CLL B cells (▪) and with autologous DCs mixed with B-CLL B cells ( ). Anti-CD4 and anti-CD8 monoclonal antibodies were added to effectors incubated with autologous B-CLL B cell targets at an effector : target ratio of 40 : 1. Specific cytotoxicity to B-CLL targets, by T cell effectors generated from culture with lysate-pulsed autologous DCs, was significantly inhibited by anti-CD4 (P = 0·039) but not by anti-CD8 monoclonal antibodies. However, specific cytotoxicity by T cell effectors generated from culture with autologous DC–B-CLL hybrids was inhibited by anti-CD4 (P = 0·062) and inhibited significantly by anti-CD8 (P = 0·018) monoclonal antibodies. Each treatment group was measured in duplicate. Data were analysed by unpaired Students' t-test; *P = 0·039, **P = 0·062, ***P = 0·018.
). Anti-CD4 and anti-CD8 monoclonal antibodies were added to effectors incubated with autologous B-CLL B cell targets at an effector : target ratio of 40 : 1. Specific cytotoxicity to B-CLL targets, by T cell effectors generated from culture with lysate-pulsed autologous DCs, was significantly inhibited by anti-CD4 (P = 0·039) but not by anti-CD8 monoclonal antibodies. However, specific cytotoxicity by T cell effectors generated from culture with autologous DC–B-CLL hybrids was inhibited by anti-CD4 (P = 0·062) and inhibited significantly by anti-CD8 (P = 0·018) monoclonal antibodies. Each treatment group was measured in duplicate. Data were analysed by unpaired Students' t-test; *P = 0·039, **P = 0·062, ***P = 0·018.
DISCUSSION
HLA class II-restricted proliferative and cytotoxic T cell responses to B cell chronic lymphocytic leukaemia (B-CLL) can be generated by pulsing autologous dendritic cells (DCs) with tumour cell lysate [1]. Other studies have also demonstrated that functional monocyte-derived DCs can be generated in B-CLL [25]. This study was undertaken to optimize further these in vitro cell responses. The addition of TNF-α, Poly(I:C) and LPS to autologous dendritic cells resulted in the emergence of only a small percentage of CD83+ dendritic cells, IFN-α having no demonstrable effect. Only the addition of Poly(I:C) to DCs resulted in modestly increased specific cytotoxicity to B-CLL targets, IFN-α and LPS having no effect.
Pretreatment of T cells with IL-15, prior to culturing with lysate-pulsed autologous DCs, however, gave rise to significant increases in T cell activation, IFN-γ secretion and specific cytotoxicity to B-CLL targets. The lack of cytotoxicity against K562 targets excludes activation of NK cells. T cell function in patients with B-CLL is dysregulated [7]. This effect could be due to factors such as IL-10 [26], transforming growth factor-β (TGF-β) [27] and vascular endothelial growth factor (VEGF) [28], which can suppress DCs and/or T cell function. B cells from B-CLL patients have been shown to secrete TGF-β[29] and VEGF [30]. TGF-β secreted from tumour cells has been shown to suppress T cell responsiveness to IL-2 by blocking signal transducers and activators of transcription (STAT)3 and (STAT)5 phosphorylation [15]. IL-15 may restore signalling of T cells, affected by in vivo exposure to inhibitory factors such as TGF-β, through (STAT)3 and (STAT)5 phosphorylation. This has been demonstrated in multiple myeloma [15]. Another mechanism for the action of IL-15 could be through reversal of T cell anergy induced by the B-CLL. IL-15 is capable of selectively activating primed T cells and naive CD8+ cells but not naive CD4+ cells [31].
Of particular interest was the finding in this study that, when T cells from B-CLL patients were cultured with autologous fused DC–B-CLL hybrids, significantly higher levels of specific cytotoxicity were generated against B-CLL targets than T cells cultured with lysate-pulsed DCs or a mix of DCs and B-CLL cells. True fusions between human tumour cells and CD34+ cells have resulted in hybrid cells capable of efficient growth [22] and whole cell vaccines have been produced in this manner for several different tumours [19–21]. Use of human allogeneic DCs fused with tumour cells has shown more promising results [32,33]. Although analysis of fusion rates, by flow cytometric dyes and antibody staining, can overestimate hybrid efficiencies [34], for the purposes of this study it provided relevant information about ‘membrane mixing’ between two specific cell types in a heterogeneous mix of cells. In this system, fusion rates for B-CLL cells were low but this was due probably to the well-known spontaneous apoptosis of B-CLL cells in vitro. Further optimization of the voltage and current duration or the use of different fusion methods [35] could possibly increase yields of fused DC–B-CLL B cells. For these experimental purposes, introduction of antigens into the cytosol of the DCs by ‘membrane mixing’ was the main objective rather than the establishment of a proliferating hybrid cell.
Finally, despite suboptimal electrofusion conditions, it was possible to generate fused DC–B-CLL B cells which stimulated both CD8+ and CD4+ CTLs, compared to lysate-pulsed DCs which predominantly stimulated HLA class II-restricted CTLs. This is in keeping with current opinion that DCs process and present exogenous proteins by the HLA class II pathway and internalize antigen from apoptotic cells to stimulate MHC class I-restricted cytotoxic T lymphocytes [9]. It is also in keeping with the view that effective antitumour responses necessitate the generation of a combination of both CD4+ HLA class II-restricted helper T cells and CD8+ HLA class I-restricted CTLs [16].
In summary, in vitro B-CLL-specific T cell responses can be enhanced further by preincubating T cells with IL-15 or using autologous fused DC–B-CLL hybrids instead of autologous lysate-pulsed DCs. In addition, the latter results in the generation of MHC class I- and II-restricted effector T cells. These preliminary data require confirmation in studies of larger numbers of patients. Such an approach, however, may eventually provide effective immunotherapy for treatment of B-CLL.
Acknowledgments
This work was jointly funded by the Plymouth Postgraduate Medical School (PPMS) and the Plymouth and District Leukaemia Fund (PDLF).
References
- 1.Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. Generation in vitro of B-cell chronic lymphocytic leukaemia- proliferative and specific HLA class-II-restricted cytotoxic T-cell responses using autologous dendritic cells pulsed with tumour cell lysate. Clin Exp Immunol. 2001;126:16–28. doi: 10.1046/j.1365-2249.2001.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Elsas A, van der Burg SH, van der Minne CE, et al. Peptide-pulsed dendritic cells induce tumoricidal cytotoxic T lymphocytes from healthy donors against stable HLA-A*0201-binding peptides from the Melan-A/MART-1 self antigen. Eur J Immunol. 1996;26:1683–9. doi: 10.1002/eji.1830260803. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury A, Gajewski JL, Liang JC, et al. Use of leukemic dendritic cells for the generation of anti-leukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1997;89:1133–42. [PubMed] [Google Scholar]
- 4.Eibl B, Ebner S, Duba C, et al. Dendritic cells generated from blood precursors of chronic myelogenous leukaemia patients carry the translocation and can induce a CML-specific primary cytotoxic T-cell response. Genes Chromosomes Cancer. 1997;20:215–23. doi: 10.1002/(sici)1098-2264(199711)20:3<215::aid-gcc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Nieda M, Nicol A, Kikuchi A, et al. Dendritic cells stimulate the expansion of bcr-abl specific CD8+ T cells with cytotoxic activity against leukemic cells from patients with chronic myeloid leukemia. Blood. 1998;91:977–83. [PubMed] [Google Scholar]
- 6.Tsai V, Kawashima I, Keogh E, Daly K, Sette A, Cellis E. In vitro immunization and expansion of antigen-specific cytotoxic T lymphocytes for adoptive immunotherapy using peptide-pulsed dendritic cells. Crit Rev Immunol. 1998;18:65–75. doi: 10.1615/critrevimmunol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 7.Scrivener S, Kaminski ER, Demaine A, Prentice AG. Analysis of the expression of critical activation/interaction markers on peripheral blood T cells in B-cell chronic lymphocytic leukaemia: evidence of immune dysregulation. Br J Haematol. 2001;112:959–64. doi: 10.1046/j.1365-2141.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou LJ, Tedder T. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35. [PubMed] [Google Scholar]
- 9.Avigan D. Dendritic cells: development, function and potential use for cancer immunotherapy. Blood Rev. 1999;13:51–63. doi: 10.1016/s0268-960x(99)90023-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–93. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells. J Immunol. 1997;158:2919–25. [PubMed] [Google Scholar]
- 12.Verdijk RM, Mutis T, Esendam B, et al. Polyriboinosinic polyribocytidylic acid (Poly(I:C) induces stable maturation of functionally active human dendritic cells. J Immunol. 1999;163:57–61. [PubMed] [Google Scholar]
- 13.Luft T, Pang KC, Thomas E, et al. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 14.Dooms H, Desmedt M, Vancaeneghem S, et al. Quiescence-inducing and anti-apoptotic activities of IL-15 enhance secondary CD4+ T cell responsiveness to antigen. J Immunol. 1998;161:2141–50. [PubMed] [Google Scholar]
- 15.Campbell JD, Cook G, Robertson SE, et al. Suppression of IL-2-induced T cell proliferation and phosphorylation of STAT3 and STAT5 by tumor-derived TGF beta is reversed by IL-15. J Immunol. 2001;167:553–61. doi: 10.4049/jimmunol.167.1.553. [DOI] [PubMed] [Google Scholar]
- 16.Reid CDL. Dendritic cells and immunotherapy for malignant disease. Br J Haematol. 2001;112:874–88. doi: 10.1046/j.1365-2141.2001.02626.x. [DOI] [PubMed] [Google Scholar]
- 17.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–85. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 18.Yewdell JW, Bennink JR, Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988;239:637–40. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]
- 19.Gong J, Chen D, Kashiwaba M, Kufe D. Induction of anti-tumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–61. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Wu M, Chen H, et al. Effective tumour vaccine generated by fusion of hepatoma cells with activated B cells. Science. 1994;263:518–20. doi: 10.1126/science.7507262. [DOI] [PubMed] [Google Scholar]
- 21.Stuhler G, Trefzer U, Walden P. Hybrid cell vaccination in cancer immunotherapy. Recruitment and activation of T cell help for induction of anti-tumour cytotoxic T cells. Adv Exp Med Biol. 1998;451:277–82. [PubMed] [Google Scholar]
- 22.Scott-Taylor TH, Pettengell R, Clarke I, et al. Human tumour and dendritic cell hybrids generated by electrofusion: potential for cancer vaccines. Biochim Biophys Acta. 2000;1500:265–79. doi: 10.1016/s0925-4439(99)00108-8. [DOI] [PubMed] [Google Scholar]
- 23.Jaroszeski MJ, Gilbert R, Fallon PG, Heller R. Mechanically facilitated cell–cell electrofusion. Biophys J. 1994;67:1574–81. doi: 10.1016/S0006-3495(94)80630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loken MR, Wells DA. Immunofluorescence of surface markers. In: Ormerod MG, editor. Flow cytometryA practical approach. 2. Oxford: IRL Press; 1994. [Google Scholar]
- 25.Vuillier F, Maloum K, Thomas EK, Jouanne C, Dighiero G, Scott-Algara D. Functional monocyte-derived dendritic cells can be generated in B-CLL. Br J Haematol. 2001;115:831–44. doi: 10.1046/j.1365-2141.2001.03223.x. [DOI] [PubMed] [Google Scholar]
- 26.Hersey P. Impediments to successful immunotherapy. Pharmacol Ther. 1999;81:111–9. doi: 10.1016/s0163-7258(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 27.de Visser K, Kast WM. Effects of TGF-β on the immune system: implications for cancer immunotherapy. Leukemia. 1999;13:1188–99. doi: 10.1038/sj.leu.2401477. [DOI] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–2003. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 29.Kremer JP, Reisbach G, Neri C, Dormer P. B-cell chronic lymphocytic leukaemia cells express and release transforming growth factor-beta. Br J Haemotol. 1992;80:480–7. doi: 10.1111/j.1365-2141.1992.tb04561.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Treweeke AT, West DC, et al. In vitro and in vivo production of vascular endothelial growth factor by chronic lymphocytic leukemia cells. Blood. 2000;96:3181–7. [PubMed] [Google Scholar]
- 31.Dooms H, Van Belle T, Desmedt M, Rottiers P, Grooten J. Interleukin-15 redirects the outcome of a tolerizing T-cell stimulus from apoptosis to anergy. Blood. 2000;96:1006–12. [PubMed] [Google Scholar]
- 32.Kugler A, Stuhler G, Walden P, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumour cell-dendritic cell hybrids. Nat Med. 2000;6:332–6. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 33.Gong J, Nikrui N, Chen, et al. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumour immunity. J Immunol. 2000;165:1705–11. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 34.Li LH, Henson ML, Zhao YL, Hui SW. Electrofusion between heterogenous-sized mammalian cells in a pellet: potential applications in drug delivery and hybridoma formation. Biophys J. 1996;71:479–86. doi: 10.1016/S0006-3495(96)79249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindner M, Schirrmacher V. Tumour cell–dendritic cell fusion for cancer immunotherapy. comparison of therapeutic efficiency of polyethylene-glycol versus electro-fusion protocols. Eur J Clin Invest. 2002;32:207–17. doi: 10.1046/j.1365-2362.2002.00968.x. [DOI] [PubMed] [Google Scholar]