Abstract
The secretory leucocyte proteinase inhibitor (SLPI) is a low molecular weight, tissue-specific inhibitor of proteases, such as elastase and cathepsin G. It is the major local protease inhibitor in the upper airways. Proteinase 3, the main autoantigen in Wegener's granulomatosis (WG), can degrade SLPI proteolytically. In addition, SLPI is sensitive to oxidative inactivation by myeloperoxidase-generated free oxygen radicals. SLPI also has an antimicrobial capacity that can be of interest, as infection is considered to play a role in the pathogenesis of WG. This study focuses on SLPI expression in patients suffering from WG, something that to our knowledge has not been explored hitherto. Serum samples and nasal biopsies were obtained from 12 Swedish WG patients, while buffy coats were obtained from 33 American WG patients. SLPI levels in serum were measured by means of ELISA and the protein was detected by means of immunohistochemistry in nasal biopsies. mRNA expression was studied by means of in situ hybridization on nasal biopsies and RT-PCR on leucocytes. IL-6 or ESR were measured as markers of inflammatory activity. Cystatin C or creatinine was measured as a marker of renal filtration. White blood cell counts were registered. In serum, we found close to normal SLPI levels, without any correlation to IL-6. Two patients had greatly elevated values, both of them suffering from severe renal engagement. Strong SLPI mRNA expression was found in nasal biopsies. RT-PCR on leucocyte mRNA showed normal or greatly elevated expression of SLPI mRNA, correlating with disease activity. Leukocyte SLPI expression seems to be up-regulated in active WG. Serum levels were measured in a small number of patients and were found to be close to normal. Lack of correlation to the acute phase response indicates a specific regulation. This might be linked to an altered protease/antiprotease balance. These findings could indicate that SLPI locally participates in the anti-inflammatory and perhaps antimicrobial response in WG.
Keywords: protease inhibitor SLPI vasculitis Wegener's granulomatosis ANCA
INTRODUCTION
Wegener's granulomatosis (WG) is a small vessel vasculitis, initially reported by Klinger in 1931 and later described in more detail by Friedrich Wegener [1]. The definitive description was provided in 1954 by Godman and Churg, who identified the classic triad of systemic angiitis, granulomatous inflammation of the respiratory tract and glomerulonephritis [2]. Anti-neutrophil cytoplasmic antibodies (ANCA) are autoantibodies directed against constituents of polymorphonuclear neutrophils and monocytes. ANCA directed against proteinase 3 results in a cytoplasmic staining pattern (C-ANCA), whereas ANCA against myeloperoxidase mostly show a perinuclear staining pattern (P-ANCA) by indirect immunofluorescence. The majority of patients with WG have a C-ANCA and there are different theories of how ANCA might contribute to the pathogenesis of vasculitis [3]. The C-ANCA antigen, proteinase 3, has the capacity to attack and degrade components of the extracellular vessel wall matrix and the glomerular basement membrane. In blood, an inhibitor, mainly alpha-1-antitrypsin, immediately binds to it. Patients with WG have an over-representation of the PiZ gene of alpha-1-antitrypsin and thus a shortage of the inhibitor [4]. Secretory leucocyte proteinase inhibitor (SLPI) (in earlier work named MPI, HUSI-1, antileucoprotease, CUSI, bronchial inhibitor) is a 107 amino acid non-glycosylated single chain protein with an estimated molecular weight of 12 kDa. The C-terminal is responsible for the inhibitory activity against elastase, cathepsin G, chymotrypsin and trypsin, while the N-terminal has been reported to possess antimicrobial capacity [5,6]. SLPI is also known to inhibit HIV type 1 infection by blocking viral DNA synthesis [7]. The inhibitor forms complexes with the proteases and has a half-life of 10 min in the circulation. The complexes formed locally dissociate in the plasma and the proteases are taken over by binding to the plasma protease inhibitors (alpha-1-antitrypsin, alpha-2-macroglobulin and antichymotrypsin) [5]. Furthermore, SLPI has been shown to inhibit elastin-bound elastase which may be of importance for the preservation of elastic lung tissue [8]. The inhibitor is also capable of up-regulation of macrophage production of the anti-inflammatory cytokines IL-10 and TGF-beta [9]. The SLPI gene contains binding sites for transcription factors induced by, e.g. TNFα and an up regulation of SLPI is seen in several inflammatory disorders [10, 11, 12, 13, 14, 15]. The inhibitor is sensitive to oxidative inactivation, e.g. by myeloperoxidase-generated free oxygen radicals. It can also be degraded proteolytically by proteinase 3 [16]. SLPI have no direct inhibitory capacity against proteinase 3. However, the progressive binding of elastase to SLPI instead of alpha1-antitrypsin has been shown to be paralleled by an increasing binding of proteinase 3 to alpha-1-antitrypsin [17]. SLPI is also an inhibitor of the production of matrix metalloproteinases (MMP), the expression and activity of which are greatly elevated in inflammatory disorders [18]. SLPI has been localized to the upper and lower respiratory tracts [5,19], the gastrointestinal tract [20,21], the male and female genital tracts [5], the intervertebral discs and articular cartilage [22], leucocytes [23,24] and the kidney [25]. SLPI's antimicrobial capacity is also of interest as infection is considered to play a role in the pathogenesis of WG [26,27]. SLPI production can be induced in response to LPS and has been shown to inhibit macrophage activation [28]. In this study, we started out with the hypothesis that a lack of SLPI could contribute to the pathophysiology of WG. Measurements of SLPI in serum from 12 Swedish patients with active disease gave no indication that this was the case. There was still the possibility of a local SLPI deficiency, e.g. in the nose where WG often makes its debut. Investigation of nasal biopsies from eight patients showed, on the contrary, a profuse SLPI expression. In order to further explore this, SLPI expression was measured in leucocytes from 33 American WG patients. This study focuses on SLPI expression in patients suffering from WG, something that to our knowledge has not hitherto been examined.
Patient material
Serum samples from 12 patients with active WG were obtained from the Department of Nephrology at Lund University Hospital. Nasal biopsy preparations were obtained from eight of these patients, then sectioned and mounted by the Department of Cytology and Pathology at Lund University Hospital. Blood samples were acquired from 33 consecutive patients (from October 1999 to March 2001) admitted to the Department of Nephrology, Chapel Hill, NC, USA with WG, according to the Chapel Hill Consensus Conference criteria. The latter patients were blindly classified as being either active (n = 24) or in remission (n = 9). In short, active disease was defined as occurrence of one of the following: (1) rapid rise in serum creatinine accompanied by active sediment, (2) necrosis or crescent formation detected by renal biopsy or necrotizing vasculitis identified in other tissue, (3) pulmonary haemorrhage or expanding nodules, (4) active vasculitis in the gut seen by endoscopy, (5) iritis or uveitis, or (6) new mononeuritis multiplex [29].
Material and chemicals
Normal rabbit serum was purchased from Dakopats AB (Copenhagen, Denmark). Swedish landrace goats were immunized with recombinant human SLPI, to produce anti-SLPI antiserum. Lamb anti-human granulocyte elastase serum was produced in our laboratory. Biotinylated rabbit anti-goat IgG and avidin biotinylated horseradish peroxidase complexes (ABC) were from Vector Laboratories (Burlingame, CA, USA). The AEC (3-amino-9-ethylcarbazole) substrate-chromogen system, DAB (3·3′-diamino-benzidine-tetrahydrochloride) and Faramount aqueous mounting medium were from Dako Corporation (Carpinteria, CA, USA). An oligonucleotide probe cocktail for SLPI and immunoassays for SLPI and IL-6 were from R & D systems (Abingdon, UK). Blocking reagent and anti-DIG-AP-conjugate (Fab fragments) were from Boehringer Mannheim (Mannheim, Germany). Visualizing substrates (bromochloroindolylphosphate, BCIP and nitroblue tetrazolium, NBT) were from Bio-Rad Laboratories (CA, USA). For leukocyte RNA purification, RNA STAT-60 from Teltest B (Friendswood, TX, USA) was used. The TaqMan EZ RT-PCR core reagents kit, as well as the primers and TaqMan probes for cyclophilin, were purchased from Applied Biosystems (Foster City, CA, USA). Primers and TaqMan probes for SLPI were designed by Gene Logic Inc. (Maryland, USA).
Elisa
SLPI
The Qantikine quantitative sandwich enzyme im-munoassay from R & D Systems, where a monoclonal antibody specific for SLPI had been precoated onto a microplate, was employed.
One hundred microlitres assay diluent RD1Q and 100 µl standard or sample were added to each well, followed by incubation for 2 h at room temperature. After three washes, 200 µl SLPI conjugate (a polyclonal anti-SLPI antibody conjugated to horseradish peroxidase) was added to each well and left to incubate for 2 h at room temperature. After another three washes, 200 µl substrate solution was added to each well, with an incubation time of 20 min at room temperature. Finally, 50 µl stop solution was added to each well. The absorbance was read at 450 nm by an automatic Titertek Multiskan photometer, and Titersoft EIA software was used for the calculations of SLPI levels.
Il-6
A quantitative sandwich enzyme immunoassay from R & D systems, where a monoclonal antibody specific for IL-6 had been precoated onto a microplate, was used. One hundred microlitres assay diluent RD1A and 100 µl standard or sample were added to each well and left to incubate for 2 h at room temperature. The plates were washed four times to eliminate any unbound substances and then 200 µl conjugate (a polyclonal anti IL-6 antibody conjugated to horseradish peroxidase) was added to each well for detection of the bound IL-6. After 2-h incubation at room temperature and washing four times, 200 µl substrate solution was added to each well. Twenty minutes incubation at room temperature allowed colour development in proportion to the amount of IL-6 bound in the initial step. Finally, 50 µl stop solution was added to each well and the intensity of the colouring was measured. The absorbance was read at 450 nm by an automatic Titertek Multiskan photometer, and Titersoft EIA software was used for calculations of IL-6 levels.
Cystatin C
The Clinical Chemistry Laboratory at Lund University Hospital performed analysis by means of immunoturbimetric methods on the instrument Hitachi 917 Pluto using antibodies from Dakopat AB (Copenhagen, Denmark).
Immunohistochemistry for SLPI and elastase
After rehydration, the paraffin embedded biopsy sections were exposed to pepsin (4 mg/ml in 0·01 m Tris-HCl buffer) for 20 min at 37°C, in order to unmask the target sequences. The sections were then washed between every step in Tris-buffered (TBS) saline 3 × 5 min. Endogenous peroxidase activity was prevented by incubation of the specimens in 0·3% H2O2 in methanol for 30 min at room temperature. Normal rabbit serum (3% in TBS) was applied to block non-specific staining. The next step, after draining, was to incubate the specimens with primary antibody (goat anti-SLPI, 1/1000, 30 min). For detection we used biotinylated rabbit anti-goat IgG (5 µg/ml), followed by the application of avidin biotinylated horseradish peroxidase complexes for 30 min. Visualization was accomplished by the addition of AEC (0·75 mg/ml 3-amino-9-ethylcarbazole in 2·5% N,N-dimethylformamide and 50 mm acetate buffer), which was left to incubate for 15 min. The slides were washed and counterstained before mounting. All the incubations were carried out at room temperature. Adsorbed anti-SLPI-antiserum, obtained by affinity chromatography on a SLPI conjugated Sepharose 4B-column, was used as a negative control and applied instead of the primary antibody. Similar procedures were used for detection of elastase. However, in order to unmask the target sequences, the slides were pre-treated with pepsin (4 mg/ml in 0·01 m Tris-HCl buffer). Anti-serum adsorbed with granulocyte elastase was used for controls. The results were compared with those from an earlier study on healthy nasal mucosa [19].
In situ hybridization, SLPI
A cocktail of three 30-bases-long oligonucleotide probes (5′-TCTTAGGAGGACAGACTCCAGCTTTGAAGG-3′; 5′-ATTT CCCACACATGCCCATGCAACACTTCA-3′; 5′-AAC ATCTC TTCTTCCCTGGACACTGCCAGT-3′), based on the anti-sense sequence of SLPI and labelled with digoxigenin (DIG) at the 5′ end was used for the in situ hybridization. DEPC (diethylpyrocarbonate) was added to the millipore water and all solutions to block RNAse activity. All solutions were autoclaved or sterilized by filtration. After rehydration, the nasal biopsy specimens were washed in PBS for 2 × 5 min. Incubation in 0·2 m HCl for 20 min was done to remove protein. To unmask the target sequences, the slides were rinsed in PBS for 2 × 5 min, followed by incubation in 0·02% pepsin in 0·2 m HCl in a 37°C water-bath for 20 min. To preserve morphology, the sections were post fixed in 4% paraformaldehyd in PBS for 5 min at 4°C. Pepsin activity was blocked by incubation in PBS with 0·2% glycine for 2 × 5 min. After being washed in PBS for 2 × 5 min, the slides were exposed to 0·1 m triethanolamine/0·9% NaCl/0·25% acetanhydrid for 10 min. Triethanolamine blocks the endogenous activity of alkaline phosphatase and acetanhydrid reduces probe stickiness. The samples were then washed in 2 × standard saline citrate (SSC) for 5 min. Pre-hybridization was performed in a moist chamber. Thirty microlitres hybridization buffer was applied to each section and the sections were then placed in an incubator at 42°C for 1 h. To prevent evaporation, the specimens were covered with parafilm. The probe cocktail (10 ng in 30 µl hybridization buffer) was added and left to hybridize overnight at 37–42°C (Tm 65°C). After washing in 4 × SSC (2 × 5 min) at room temperature, 1 × SSC (3 × 15 min) at 50°C and 0·1 SSC (10 min) at room temperature, the slides were immersed in buffer I (100 mm Tris-HCl, 150 mm NaCl, pH 7·5) for 2 min equilibration. This was followed by treatment with buffer II (buffer I plus 0·5% blocking reagent) to block non-specific anti-DIG binding. After rinsing in buffer I for 2 min, the sections were placed in a moist chamber and the anti-DIG-AP-conjugate (1/500 in buffer I) was applied. The buffer I washing was repeated for 2 × 15 min. The slides were then placed in buffer III (i.e. 100 mm Tris-HCl, 100 mm NaCl, 50 mm MgCl2, pH 9·5) for 10 min. Visualization was achieved by incubation in 50 ml buffer III, 228 µl NBT, 170 µl BCIP and 50 µl Levamisole (240 mg/ml) for 24 h. The substrate reaction was interrupted by the addition of buffer IV (10 mm Tris-HCl, 1 mm EDTA, pH 8·0) for 10 min and the slides were then mounted directly. Negative controls were incubated with hybridization buffer without probes as well as with oligonucleotide probes based on the sense sequence of SLPI. The results were compared with those from an earlier study on healthy nasal mucosa [19].
Leukocyte purification
Leukocytes were obtained from blood collected in EDTA tubes. Erythrocytes in the blood were lysed with lysis buffer (0·83% NH4Cl, 10 mm KHCO3, and 0·1 mm EDTA) and the remaining leucocytes washed with Hanks' Balanced Salt Solution (HBSS). Total RNA was directly isolated from patient leucocytes with RNA STAT-60 using supplied protocol [30]. High purity and good integrity were determined by optical density (OD) 260/280 nm spectrophotometric ratios and staining of ribosomal 18 and 28 s RNA. In the latter procedure, RNA was denatured by heating in the gel loading buffer (250 µl formamide, 72·5 µl formaldehyde, 5 µl sodium phosphate dibasic, 0·5 µl 0·5 m EDTA), 67°C for 10 min. Five to ten micrograms per millilitre ethidium bromide was added for visualization. The samples were then loaded onto an agarose gel, 0·5 g agarose in 50 ml running buffer (DEPC H2O with 10% 10 × TBE). After 35 min electrophoresis at 100 V, ribosomal RNAs (rRNAs) showed up as intense, sharp bands. The larger ribosomal bands were more intense than the smaller ones. Degradation of the RNA is indicated if this is not true or if the rRNA bands are smeared. DNA contamination of the RNA preparation will be evident as higher molecular weight ethidium bromide-staining material migrating above the larger rRNA band.
Quantitative PCR assay
For determination of changes in gene transcription, leucocyte RNA was analysed by quantitative PCR (TaqMan®) for the relative expression of the gene, SLPI, using cyclophilin expression levels for normalization. Quantitative PCR assays were performed on an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA) with a TaqMan EZ RT-PCR core reagents kit using the standard conditions determined by the company. A unique combination of forward and reverse primers and fluorescent probe oligos was used. Twenty-five nanograms RNA was used per well and the reactions were performed in duplicate. The level of expression was calculated based upon the PCR cycle number (Ct) at which a linear increase in fluorescence from the probe was found. Relative expression was determined by the difference in the Ct values for the SLPI gene after normalization to RNA input levels using cyclophilin Ct values. Relative quantification was determined by standard 2(–ΔΔCt) calculations [30]. Leukocyte RNA samples from 23 normal donors and from 33 WG patients were compared.
Statistics
All statistics were performed in StatView 5·01. For correlation analysis, the non-parametric Spearman rank correlation test was used in order to reduce the impact of outliers. A one-way analysis of variance was done using the non-parametric Kruskal–Wallis test as well as (on logarithmic data) anova with Fisher's PLSD.
RESULTS
Levels of SLPI in serum
Serum samples were collected from 12 Swedish WG patients at the time of diagnosis. Their SLPI levels (median 44 µg/l; range 31–243) were found to be close to normal (36 µg/l; 27–47, n = 40) (Table 1a). No obvious correlation was seen between their SLPI levels and their degree of inflammation, measuring IL-6 as an inflammatory marker (rho = 0·22; P = 0·13) (Table 1b). Two of the patients showed very high SLPI levels. These two had, according to their files, at the time of diagnosis, developed a more serious form of the disease than the others. Their renal function was lower and their organ involvement was more severe overall. Additional serum samples from these patients were tested during a 4-year follow up, showing consistently high SLPI levels.
Table 1a.
SLPI in serum, 12 Swedish WG patients
| Patient | SLPI (µg/l) | Cyst C (mg/l) | IL-6 (ng/l) |
|---|---|---|---|
| 1 | 56 | 0·74 | 1·0 |
| 2 | 33 | 0·76 | 10·1 |
| 3 | 35 | 0·76 | 2·4 |
| 4 | 46 | 0·87 | 4·2 |
| 5 | 42 | 1·11 | 14·9 |
| 6 | 41 | 1·13 | 0·4 |
| 7 | 43 | 1·17 | 2·4 |
| 8 | 31 | 1·27 | 3·5 |
| 9 | 52 | 1·38 | 36·8 |
| 10 | 46 | 1·44 | 1·5 |
| 11 | 243 | 4·31 | 19·5 |
| 12 | 237 | 4·34 | 3·9 |
| Median (range) | 44 (31–243) | 1·15 (0·74–4·34) | 3·7 (0·4–36·8) |
Table 1b.
Spearman rank correlation, SLPI versus Cystatin C and IL-6
| Rho | P-value | |
|---|---|---|
| SLPI, Cystatin C | 0·46 | 0·13 (NS) |
| SLPI, IL-6 | 0·22 | 0·46 (NS) |
NS = non significant.
Local production of SLPI
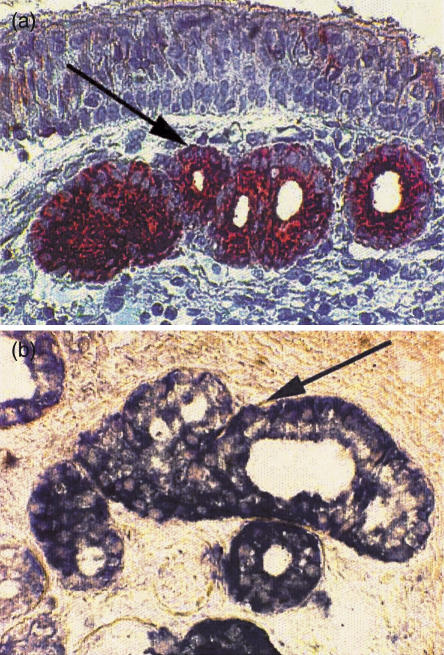
Immunohistochemical staining for SLPI was done on nasal biopsies from eight Swedish WG patients with normal SLPI levels in serum. Leukocyte elastase was used as a marker of the degree of inflammation and stained by similar procedures. Positive staining for SLPI was seen in the serous cells of the nasal mucosa. In comparison with earlier studies [19], the staining tended to be more profuse in the WG. In negative control sections incubated with adsorbed anti-SLPI-antiserum the positive staining was completely absent. A positive hybridization signal was obtained in the serous cells of the mucosa after hybridization with an oligonucleotide probe cocktail. No staining was observed in the control biopsies, where either no probes or sense probes had been added (Fig. 1a,b).
Fig 1.
(a) Immunohistochemical demonstration of SLPI in nasal biopsies from patients suffering from Wegener's granulomatosis. Utilizing a goat anti-SLPI diluted 1/1000 and DAB as a chromogen, positive staining is seen in the serous cells. Magnification 1 : 1250. (b) Demonstration of SLPI-mRNA by in situ hybridization of nasal biopsies from patients with Wegener's granulomatosis. The specimens were hybridized using an equimolar cocktail of three 30-base-long anti-sense, digoxigenin-labelled oligonucleotides as probes. Positive staining is seen in the serous cells of the mucosa. Magnification 1 : 5000.
Leukocyte SLPI expression
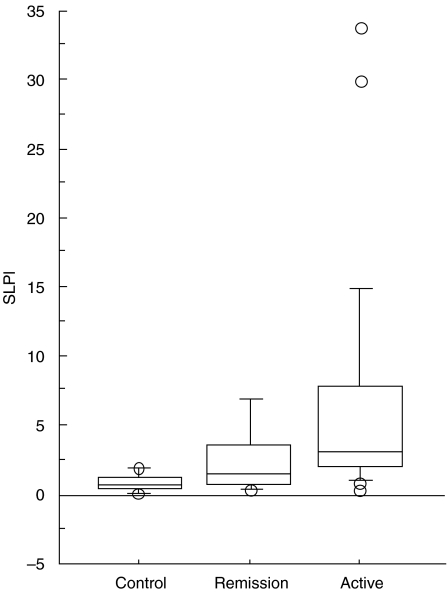
Leukocytes were purified from 47 blood samples from 33 American patients with WG. All data were compared with and expressed in relation to one healthy individual, set as standard, = 1. The patients demonstrated elevated SLPI expression in comparison with 23 healthy individuals. Patients with active disease had significantly higher SLPI expression than patients in remission (median 3·0; range 0·3–33·7, n = 13 versus median 0·8; range 0·3–8·1, n = 34). Both patient groups also had significantly higher SLPI expression than healthy controls (median 0·7; range 0·1–1·9, n = 23). There was a positive correlation between SLPI expression and the patients blood leucocyte count (rho = 0·85; P < 0·001, n = 18). No significant correlation was seen with ESR (rho = 0·13; P = 0·66, n = 14) or creatinine (rho = 0·7; P = 0·36, n = 30) (Fig. 2, Tables 2a,b,c.).
Fig 2.
SLPI gene expression, as measured by a Taqman RT-PCR in 33 patients with Wegener's granulomatosis and 23 healthy controls. The level of expression was calculated from the PCR cycle number (Ct) at which a linear increase in fluorescence from the probe was found and normalized to RNA input levels using cyclophilin Ct values. The relative level was determined by the equation 2(−ΔΔCt). All data were expressed in relation to a healthy control, selected as standard (= 1·0).
Table 2a.
SLPI mRNA in leucocytes, 33 Chapel Hill WG patients
| Patients | SLPI (folds of control) | ESR (mm) | WBC (× 109) | S-Creatinine (mmol/l) |
|---|---|---|---|---|
| WG active | Median 3·0 | Median 49 | Median 10·0 | Median 1·6 |
| (n = 24) | Range 0·3–33·7 | Range 17–58 | Range 4·7–20·7 | Range 0·6–9·2 |
| WG remission | 0·8 | 20 | 6·0 | 1·8 |
| (n = 9) | 0·3–8·1 | 2–75 | 3·7–9·4 | 0·9–2·4 |
| Healthy controls | 0·7 | – | – | – |
| (n = 23) | 0·1–1·9 |
Table 2b.
Spearman rank correlation SLPI mRNA, ESR, WBC, Creatinine
| Rho | P-value | |
|---|---|---|
| SLPI, ESR | 0·13 | 0·66 |
| SLPI, WBC | 0·85 | <0·001 |
| SLPI, Creatinine | 0·17 | 0·36 |
Table 2c.
One-way analysis of variance, SLPI mRNA
| Parametric analysis, logarithmic data | anova; power 1·0, F-value 18, P < 0.0001 Fishers PLSD: |
|---|---|
| WG active, WG remission | P = 0·32 (NS) |
| WG active, controls | P = 0·002 |
| WG remission, controls | P = < 0·0001 |
| Non-parametric analysis | Kruskal–Wallis; P < 0·0001 |
| Mean ranks: | |
| WG active | 43 |
| WG remission | 24 |
| Controls | 19 |
NS = non significant.
DISCUSSION
The elucidation of pathophysiologic mechanisms might help identify future means of therapy for the small vessel vasculitis WG. Earlier research has largely focused upon the anti-neutrophil cytoplasmic antibodies and their antigens [31–33] and, to some extent, the systemic protease inhibitors [4]. Patients with WG often have upper and lower airway disease manifestations such as nasal crusting, mucosal ulceration and necrotizing pulmonary inflammation. SLPI is a major local protease inhibitor in the ENT ear, nose, throat (ENT) region, the upper and lower airways [5, 11, 19, 34]. Furthermore, SLPI is present in neutrophil cytosol as an internal protective shield [13]. This shield can, however, be broken by the main autoantigen in WG, proteinase 3, degrading SLPI proteolytically. Our initial hypothesis was that a shortage of SLPI could contribute to the pathophysiology of WG. In the Swedish patient group (n = 12), however, we found close to normal SLPI levels in serum, without any correlation to inflammation. Two exceptions had greatly elevated levels; both suffered from severe renal engagement. All serum samples were collected at the time of diagnosis and thus represent patients with active disease. There was still the possibility of local SLPI deficiency, e.g. in the nose where WG often makes its debut. In situ hybridization demonstrated, however, strong SLPI mRNA expression in nasal biopsies from patients with normal SLPI levels in serum (n = 8). In order to further investigate this, SLPI expression was measured in leucocytes from 33 American WG patients. These patients showed moderately to greatly elevated SLPI mRNA expression in their leucocytes, correlating well with disease activity. No significant positive correlation was, however, seen with ESR, indicating that the high production was not a side-effect of an ongoing acute phase reaction. There was a positive correlation between the patients' blood leucocyte count and SLPI expression in the leucocytes, suggesting some kind of joint regulation and making it interesting to isolate the leucocyte subpopulations.
Taken together, we thus found signs of raised SLPI mRNA expression without a corresponding increase in the protein level in serum. Manifest inflammatory changes in the lungs are known to cause elevated levels of SLPI and inflammation per se may give an up-regulation of SLPI production, however, the protein does not act like an acute phase reactant [10,35]. Something that has to be considered is the possibility of a glucocorticoid-induced increase of SLPI mRNA levels, as glucocorticoids are part of these patients' treatment [36]. SLPI's suggested role as an internal leucocyte shield could theoretically be in higher demand in patients with WG and therefore would call for increased transcription. Moreover, inflammation leads to a tilted protease-antiprotease balance and the transcriptional increase of SLPI could be induced by proteinase activated receptors (PAR) [37–39]. PAR represent a novel class of seven transmembrane domain G-protein coupled receptors, activated by proteolytic cleavage. The lack of raised SLPI levels in serum could, theoretically, be due to proteinase 3 induced degradation. Although SLPI has no direct inhibitory capacity against proteinase 3, it has been shown that the progressive binding of elastase to SLPI instead of alpha1-antitrypsin is paralleled by an increased binding of proteinase 3 to alpha-1-anti-trypsin [17]. With this in mind, high SLPI levels may indirectly aid in the binding and inhibition of proteinase 3. High SLPI production may thus represent an increased demand for protease inhibition, locally as well as systemically. Our results also make it interesting to study circulating levels and production of proteinase 3. Except for protease inhibition, SLPI is capable of up-regulation of anti-inflammatory cytokines and has antimicrobial capacity, all qualities useful in the battle against chronic inflammation [5,6,9,18].
In summary, we have found raised leucocyte mRNA expression of the secretory leucocyte proteinase inhibitor, SLPI, in patients with WG. A small number of serum sample analyses could not confirm raised levels in the protein level. A possible explanation would be local SLPI consumption. Further studies will focus on why SLPI is up-regulated in these patients.
Acknowledgments
This study was supported by grants from the Swedish Medical Research Council (projects 3910, 71X-09487), the foundations of the Royal Physiographic society and Thelma Zoega, the Foundation for Strategic Research and the Medical Faculty, Lund University. Thanks to Carina Linder and Irena Ljungkrantz for their skilful laboratory work.
References
- 1.Wegener F. Über eine eigenartige rhinogene granulomatose mit besonderer Beteilgung des Arteriensystems und den Nieren. Beitr Pathol Anat Allg Pathol. 1939;102:36–68. [Google Scholar]
- 2.Godman GC, Churg J. Wegener's granulomatosis: pathology and review of the literature. Arch Pathol. 1954;58:533–53. [PubMed] [Google Scholar]
- 3.Segelmark M. Lund: Lund University; 1995. Rapidly progressive glomerulonephritis. PhD thesis. [Google Scholar]
- 4.Segelmark M, Elzouki AN, Wieslander J, et al. The PiZ gene of alpha 1-antitrypsin as a determinant of outcome in PR3-ANCA-positive vasculitis. Kidney Int. 1995;48:844–50. doi: 10.1038/ki.1995.360. [DOI] [PubMed] [Google Scholar]
- 5.Carmichael DF, Ohlsson K. Chemistry and biology of secretory leukoprotease inhibitor. In: Crystal RG, editor. Alpha-1-antitrypsin Deficiency: Biology, Pathogenesis, Clinical Manifestations. New York, Basel, Hong Kong: Mercel Dekker Inc.; 1996. pp. 193–208. [Google Scholar]
- 6.Hiemstra PS, Maassen RJ, Stolk J, et al. Antimicrobial activity of antileucoprotease. Infect Immun. 1996;64:4520–4. doi: 10.1128/iai.64.11.4520-4524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNeely TB, Shugars DC, Rosendahl M, et al. Inhibition of human immunodeficiency virus type 1 infectivity by secretory leukocyte protease inhibitor occurs prior to viral reverse transcription. Blood. 1997;90:1141–9. [PubMed] [Google Scholar]
- 8.Bruch M, Bieth JG. Influence of elastin on the inhibition of leucocyte elastase by alpha-1-proteinase inhibitor and bronchial inhibitor. Potent inhibition of elastin-bound elastase by bronchial inhibitor. Biochem J. 1986;238:269–73. doi: 10.1042/bj2380269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano C, Shimizu K, Sato H, et al. Effects of secretory leukocyte protease inhibitor on the production of the anti-inflammatory cytokines IL-10 and transforming growth factor-beta (TGF-β) Clin Exp Immunol. 2000;121:77–85. doi: 10.1046/j.1365-2249.2000.01269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maruyama M, Hay JG, Yoshimura, et al. Modulation of secretory leukoprotease inhibitor gene expression in human bronchial epithelial cells by phorbol ester. J Clin Invest. 1994;94:368–75. doi: 10.1172/JCI117331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bingle L, Tetley TD. Secretory leukoprotease inhibitor: partnering alpha 1-proteinase inhibitor to combat pulmonary inflammation. Thorax. 1996;51:1273–4. doi: 10.1136/thx.51.12.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grobmyer SR, Barie PS, Nathan CF, et al. Secretory leukocyte protease inhibitor, an inhibitor of neutrophil activation, is elevated in serum in human sepsis and experimental endotoxemia. Crit Care Med. 2000;28:1276–82. doi: 10.1097/00003246-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Sallenave JM, Donnelly SC, Grant IS, et al. Secretory leukocyte proteinase inhibitor is preferentially increased in patients with acute respiratory distress syndrome. Eur Respir J. 1999;13:1029–36. doi: 10.1034/j.1399-3003.1999.13e16.x. [DOI] [PubMed] [Google Scholar]
- 14.Shimoya K, Moriyama A, Ogata I, et al. Increased concentrations of secretory leukocyte protease inhibitor in peritoneal fluid of women with endometriosis. Mol Hum Reprod. 2000;6:829–34. doi: 10.1093/molehr/6.9.829. [DOI] [PubMed] [Google Scholar]
- 15.Trefz G, Schliesser J, Heck B. Alpha 1-proteinase inhibitor and mucus proteinase inhibitor in human lung emphysema. Clin Invest. 1992;70:269–76. doi: 10.1007/BF00184661. [DOI] [PubMed] [Google Scholar]
- 16.Rao NV, Marshall BC, Gray BH. Interaction of secretory leukocyte protease inhibitor with proteinase-3. Am J Respir Cell Mol Biol. 1993;8:612–6. doi: 10.1165/ajrcmb/8.6.612. [DOI] [PubMed] [Google Scholar]
- 17.Bergenfeldt M, Axelsson L, Ohlsson K. Release of neutrophil proteinase 4(3) and leukocyte elastase during phagocytosis and their interaction with proteinase inhibitors. Scand J Clin Laboratory Invest. 1992;52:823–9. doi: 10.3109/00365519209088387. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, De Witt DL, McNeely TB. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase-2, prostaglandin E2, and matrix metalloproteinases. J Clin Invest. 1997;99:894–900. doi: 10.1172/JCI119254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westin U, Fryksmark U, Polling Å, et al. Localisation of secretory leucocyte proteinase inhibitor mRNA in nasal mucosa. Acta Otolaryngol. 1994;114:199–202. doi: 10.3109/00016489409126042. [DOI] [PubMed] [Google Scholar]
- 20.Bergenfeldt M, et al. Localization of immunoreactive secretory leukocyte protease inhibitor (SLPI) in intestinal mucosa. J Gastroenterol. 1996;31:18–23. doi: 10.1007/BF01211182. [DOI] [PubMed] [Google Scholar]
- 21.Nyström M, Bergenfeldt M, Ljungcrantz I, et al. Production of secretory leucocyte protease inhibitor (SLPI) in human pancreatic beta-cells. Med Inflamm. 1999;8:147–51. doi: 10.1080/09629359990478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohlsson S, Tufresson B, Polling Å, et al. Distribution of the secretory leucocyte proteinase inhibitor in human articular cartilage. Biol Chem. 1997;378:1055–8. doi: 10.1515/bchm.1997.378.9.1055. [DOI] [PubMed] [Google Scholar]
- 23.Westin U, Polling Å, Ljungcrantz I, et al. Identification of SLPI (secretory leukocyte protease inhibitor) in human mast cells using immunohistochemistry and in situ hybridisation. Biol Chem. 1999;380:489–93. doi: 10.1515/BC.1999.063. [DOI] [PubMed] [Google Scholar]
- 24.Sallenave JM, Si-Tahar M, Cox G, et al. Secretory leukocyte proteinase inhibitor is a major leukocyte elastase inhibitor in human neutrophils. J Leukoc Biol. 1997;61:695–702. doi: 10.1002/jlb.61.6.695. [DOI] [PubMed] [Google Scholar]
- 25.Ohlsson S, Ljungkrantz I, Ohlsson K, Segelmark M, Wieslander J. Novel distribution of the secretory leucocyte proteinase inhibitor in kidney. Med Inflamm. 2001;10:347–50. doi: 10.1080/09629350120102389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayet WJ, Marker-Hermann E, Schlaak J, et al. Irregular cytokine pattern of CD4+ T lymphocytes in response to Staphylococcus aureus in patients with Wegener's granulomatosis. Scand J Immunol. 1999;49:585–94. doi: 10.1046/j.1365-3083.1999.00544.x. [DOI] [PubMed] [Google Scholar]
- 27.George J, Levy Y, Kallenberg CG, et al. Infections and Wegener's granulomatosis – a cause and effect relationship? QJM. 1997;90:367–73. doi: 10.1093/qjmed/90.5.367. [DOI] [PubMed] [Google Scholar]
- 28.Jin FY, Nathan C, Radzioch D, et al. Secretory leukocyte protease inhibitor: a macrophage product induced by and antagonistic to bacterial lipopolysaccharide. Cell. 1997;88:417–26. doi: 10.1016/s0092-8674(00)81880-2. [DOI] [PubMed] [Google Scholar]
- 29.Nachman PH, Hogan SL, Jennette JC, Falk RJ. Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol. 1996;7:33–9. doi: 10.1681/ASN.V7133. [DOI] [PubMed] [Google Scholar]
- 30.Pendergraft WF, Alcorta DA, Segelmark M, et al. ANCA antigens, proteinase 3 and myeloperoxidase, are not expressed in endothelial cells. Kidney International. 2000;57:1981–90. doi: 10.1046/j.1523-1755.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- 31.Gross WL, Csernok E, Helmchen U. Antineutrophil cytoplasmic autoantibodies, autoantigens, and systemic vasculitis. Apmis. 1995;103:81–97. doi: 10.1111/j.1699-0463.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 32.Harper L, Savage CO. Pathogenesis of ANCA-associated systemic vasculitis. J Pathol. 2000;190:349–59. doi: 10.1002/(SICI)1096-9896(200002)190:3<349::AID-PATH524>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 33.Homer RJ. Antineutrophil cytoplasmic antibodies as markers for systemic autoimmune disease. Clin Chest Med. 1998;19:627–39. doi: 10.1016/s0272-5231(05)70107-6. [DOI] [PubMed] [Google Scholar]
- 34.Fryksmark U, Jannert M, Ohlsson K, et al. Secretory leukocyte protease inhibitor in normal, allergic and virus induced nasal secretions. Rhinology. 1989;27:97–103. [PubMed] [Google Scholar]
- 35.Fryksmark U, Prellner T, Tegner H, Ohlsson K. Studies on the role of antileukoprotease in respiratory tract diseases. Eur J Respir Dis. 1984;65:201–9. [PubMed] [Google Scholar]
- 36.Abbinante-Nissen JM, Simpson LG, Leikauf GD. Corticosteroids increase secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am J Physiol. 1995;268:L601–6. doi: 10.1152/ajplung.1995.268.4.L601. [DOI] [PubMed] [Google Scholar]
- 37.Dery O, Corvera CU, Steinhoff M, et al. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–52. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- 38.Howells GL, Macey MG, Chinni C, et al. Proteinase-activated receptor-2: expression by human neutrophils. J Cell Sci. 1997;110:881–7. doi: 10.1242/jcs.110.7.881. [DOI] [PubMed] [Google Scholar]
- 39.Macfarlane SR, Seatter MJ, Kanke T, et al. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]