Abstract
Differential aspects of the host immune response generated by Trypanosoma cruzi infection were examined in two different mouse strains, BALB/c (haplotype H2-Kd) which does not overcome the acute phase of the infection and C57BL/6 (haplotype H2-Kb) which survives to the acute phase. After infection an increase in CD3+ T cells was observed in both mouse strains in the peritoneal cavity. However, while the CD3+ T cells from the BALB/c mice showed an increase in the IL-4 cytokine expression level, the same type of cells from the C57BL/6 mice showed an increase in IFN-γ expression. In addition, only the macrophages from the C57BL/6 mice were activated secreting IL-12 and TNF-α and producing, moreover, high levels of nitrites. It was observed that also after parasite infection the expression of macrophage and dendritic cells CD40 and CD86 co-stimulation molecules from the spleen were diminished in BALB/c but not in C57BL/6 mice. In correlation with this observation the macrophages from the spleen of infected BALB/c mice secreted lower concentrations of nitrites than the C57BL/6 mouse cells. Also, the spleen dendritic cells from infected BALB/c mice had a small potential to present alloantigens in contrast to that observed in the infected C57BL/6 mouse cells.
Keywords: APCs, co-stimulatory molecules, cytokine, infection, Trypanosoma cruzi
INTRODUCTION
Infection of humans with the protozoan parasite Trypanosoma cruzi leads to Chagas’ disease, an acute and chronic illness that affects nearly 18 million people in Latin America, and for which no effective drugs or vaccines are available [1]. In mammalian hosts, the parasite life cycle includes both non-dividing trypomastigote forms, which circulate in the blood stream and infect different host cells (macrophages, fibroblasts, nervous and muscular system cells) and replicating intracellular amastigotes which reside in the cytoplasm of the infected cells [2]. Experimental murine models of Chagas’ disease have been developed in order to understand the course of infection and the pathology of the sickness. During the acute stage of the human natural infection and experimental murine infection, an intense suppression of the lymphoproliferative response to antigens and mitogens is produced. This alteration in the immune system has been related to many different mechanisms, including a lower IL-2 production and a reduction of IL-2R expression level by spleen cells [3]. The precise mechanisms involved in the antiparasitic response remain unclear. However, it is well known that the generation of functional CD4+ and CD8+ T cells is crucial for controlling parasitaemia and survival to the acute infection [4]. Although it has been reported that susceptibility or resistance to T. cruzi infection is not associated with an exclusive Th cytokine pattern [5], various studies have shown that IFN-γ, TNF-α and IL-12 are important cytokines for the control of the infection [6,7].
Macrophages and dendritic cells (DCs) are involved in antigen presentation to the immune system, providing two specific signals to achieve optimum activation of resting T cells [8]. The first signal is delivered through binding of the T-cell receptor (TCR) to peptide/MHC complexes on the antigen-presenting cell (APC) surface. The second and co-stimulatory signal is delivered by several proteins integrated on the APC surface binding to distinct receptors on the T cell surface. CD28 and CD40L receptors on T cells are known to interact, respectively, with CD80/CD86 and CD40 on APCs [9,10]. The APC ability to deliver the second co-stimulatory signal is mainly inducible by infectious agents. For example, expression of co-stimulatory molecules has been observed after exposure of macrophages to Listeria monocytogenes[11]. In contrast, it has been reported that the protozoan parasites could also modulate the accessory function of the APC in a negative way. In particular, the in vitro infection of human DC cells by Trypanosoma cruzi induces a defective maturation process reducing both the secretion of cytokines and the up-regulation of co-stimulatory molecules [12].
In the present paper we have studied the host immune response to T. cruzi experimental infection in susceptible BALB/c and resistant C57BL/6 mice. The results show that the spleen macrophages and DCs from infected BALB/c mice have defective co-stimulatory capacity when compared with the capacity of these cells from the infected C57BL/6 mice and that the peritoneal macrophages from the BALB/c mice were not activated. Moreover, the spleen and peritoneal macrophages from infected BALB/c mice secrete lower amounts of nitrites relative to the cells from the infected C57BL/6 mice. The allostimulatory capacity of the spleen dendritic cells from infected BALB/c mice is also lower than that of the spleen cells from infected C57BL/6 mice. The peritoneal CD3+ T cells from infected BALB/c mice secrete IL-4 while there is an increase of INF-γ expression level in C57BL/6 mice.
MATERIALS AND METHODS
Mice, parasites and experimental infection
BALB/c (H2-Kd) and C57BL/6 (H2-Kb) mice were obtained directly from IFFA-CREDO (Crifa, Lyon, France). Females were held under clean conventional conditions and used between 6 and 8 weeks of age. T. cruzi infection was produced by intraperitoneal injection with blood trypomastigotes of the T. cruzi Y strain. Groups of six BALB/c mice were inoculated with 5 × 102 or 2·5 × 103 parasites and groups of six C57BL/6 mice were infected with 104 or 2·5 × 104 parasites.
All protocols using animals were approved by the CSIC Ethical Committee.
Parasitaemia and mortality rates
Number of circulating parasites was counted in fresh blood obtained from each mouse every 2 or 4 days during the first 20 days after the T. cruzi infection. Mean values were determined by averaging the parasitaemias for at least three mice per group. Mortality was recorded regularly.
Antibodies
The following antibodies were purchased from PharMingen (San Diego, CA, USA): fluorescein isothiocyanate (FITC) and R-phycoerythrin (PE)-anti-CD3∈ (145–2C11), FITC-anti-CD4 (GK1·5), FITC-anti-CD8 (53–6·7), PE-anti-IL-2 (S4B6), PE-anti-IL-4 (BVD4–1D11), PE-anti-IL-10 (JES5–16E3), PE-anti-IL-12 (C15·6), PE-anti-IFN-γ (XMG1·2), PE-anti-TNF-α (MP6-XT22), PE-anti-CD11c (HL3), FITC-anti-Mac3 (M3/84), PE-anti-Mac3 (M3/84), FITC and PE-anti-CD40 (HM40-3), FITC and PE-anti-CD86 (GL1). The 2·4G2 hybridoma, producing anti-FcγR MoAbs was kindly provided by Dr C. Terhorst (Beth Israel Deaccones Medical Center, Boston, MA, USA).
Spleen and peritoneum cell cultures
Spleens from infected and non-infected BALB/c and C57BL/6 mice were removed aseptically 10 and 15 days after infection by T. cruzi (2·5 × 103 and 104 parasites in BALB/c and C57BL/6, respectively). Single-cell suspensions were prepared in complete medium, Dulbecco's modified Eagle medium (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) (Life Technologies), 2 mm l-glutamine (Gibco BRL), 50 µm 2-mercaptoethanol (Sigma, Germany), 100 IU/ml penicillin (Sigma, Germany) and 100 µg/ml streptomycin (Sigma, Germany). For obtaining spleen macrophages, splenocytes were cultured in Petri dishes (5 × 106 cells/ml) and incubated at 37°C in a 5% CO2-in-air for 16 h. Then, the plates were washed and the adherent population was carefully collected. Subsequently the cells were cultured in complete medium in 24-well plates (1 × 106 cells/ml) for nitrite determination. Peritoneal cells from infected and non-infected animals were harvested 15 days postinfection using cold PBS. After depletion of erythrocytes with lysis buffer (0·15 m NH4Cl, 1 mm KHCO3, 0·01 mm Na2EDTA) adherent and non-adherent cells were separated after 2–4 h of incubation at 37°C in tissue culture plates by exhaustive washing with complete medium. Both cell types were used for the analysis of surface molecules and intracellular cytokine expression.
Determination of secreted nitrite
Spleen and the peritoneal macrophages from infected and non-infected C57BL/6 and BALB/c mice were purified as described above and cultured (1 × 106 cells/ml) in 24-well plates. The nitrite concentration was determined in the supernatants after 24 h (peritoneal macrophages) or 48 h (spleen macrophages) using the Griess reagent. One hundred µl of each supernatant was mixed with 100 µl of the Griess reagent. The O.D. was determined at a wavelength of 540 nm using a Multiskan Plus plate reader (Labsystem, Helsinki, Finland).
Cell population, cytokine and co-stimulation molecule analysis
The distribution of T cells, dendritic cells (DCs) and macrophages in the spleen and peritoneum of infected and healthy BALB/c and of C57BL/6 mice and of the cytokine and co-stimulation molecules expression was carried out in a FACScan flow cytometer (Becton Dickinson, Mountain View, CA, USA). A single-cell suspension from splenocytes and adherent and non-adherent cells from peritoneum was prepared in complete medium. For the cytokine expression analysis 10 µg/ml brefeldin A (Sigma, Germany) were added to the medium in order to block Golgi protein transport. Aliquots of 106 cells/well were washed with phosphate-buffered saline (PBS) and resuspended in FcR block for 10 min on ice. Afterwards, surface staining was performed in each case by cell incubation with specific labelled monoclonal antibodies, anti-CD3∈, anti-CD4, anti-CD8, anti-CD11c, anti-Mac3, anti-CD40 and/or anti-CD86. After 20 min the cells were washed and analysed. The cell samples used for the intracellular cytokine analysis were fixed with 4% paraformaldehide (PFA) and permeabilized (0·2% saponine in PBS). Then, the cells were stained for 20–30 min with the PE-anticytokine antibodies, anti-IFN-γ, anti-IL-2, anti-IL-4, anti-IL10, anti-IL-12 or anti-TNF-α. The cells were suspended in PBS. Cytometry data were analysed with a WinMDI 2·8 software.
Purification of spleen dendritic cells and mixed leucocyte reaction (MRL)
To analyse the functional activity of spleen DC, allogeneic MLR assays were carried out using mouse T splenocytes as responder cells. On days 10 and 15 postinfection the dendritic cells from the spleen of four C57BL/6 and BALB/c mice were purified using the MidiMACS separation system (Miltenyi Biotec, Germany) following the manufacture's recommendation. Allogeneic T cells were obtained from C57BL/6 and BALB/c using a nylon wool column. The purity of the T cell and the dendritic cell population was checked by flow cytometry. Dendritic cells were incubated with allogeneic T cells in 96-well plates at different responder : stimulator ratios in a final volume of 200 µl/well. Control wells contained DCs or T cells alone. Plates were held in a CO2 incubator at 37°C for 5 days. After the addition of methyl-[3H]-thymidine (0·5 µCi/well) the cells were incubated for 8 h at 37°C. DNA was immobilized in glass fibre filtermats using an Inothech harvester (Dottikon, Switzerland). The [3H] incorporation was measured in a Wallac 1450 microbeta counter device (Turku, Finland).
RESULTS
Parasitaemia and survival rates of infected C57BL/6 and BALB/c mice
The course of T. cruzi infection was studied in C57BL/6 and BALB/c mice. Groups of six animals were infected intraperitoneally with different amounts of T. cruzi blood trypomastigotes (see Materials and methods). Following infection, circulating parasites were measured in blood from each mouse at different time-points (Fig. 1a). Parasitaemia peaks appeared in both mouse strains on days 8–10 after challenge. In BALB/c mice the parasitaemia peaks reached values fivefold higher than those observed in C57BL/6 mice. After day 8 in the blood stream of C57BL/6 mice there was a dramatic decrease in the level of parasites. Three weeks postinfection circulating parasites were controlled to undetectable levels as observed by microscopic examination. BALB/c mice were unable to control the parasitaemia and all of them died during the third week postinfection (Fig. 1b).
Fig 1.
Parasitaemia levels and survivor numbers in susceptible and resistant mice after T. cruzi infection. C57BL/6 mice were intraperitoneally infected with 104 (□) or 2·5 × 104 (×) and BALB/c mice with 5 × 102 (Δ) or 2·5 × 103 (○) peripheral blood T. cruzi trypomastigotes (Y strain). (a) Parasitaemia level in bloodstream of infected mice was determined by parasite counting in a Neubauer chamber. Values represent mean ± s.d. of six mice. (b) Survival rates of infected mice.
Cytokine production in the peritoneal cavity of infected BALB/c and C57BL/6 mice
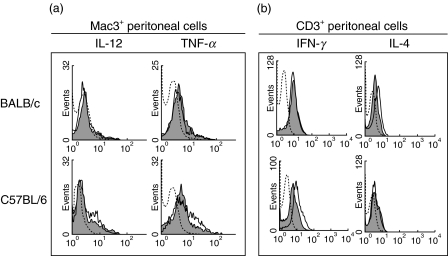
We were interested in comparing the immune events taking place in the mouse peritoneal cavity in infected BALB/c and C57BL/6 mice. We observed that, following T. cruzi infection, there was an increase in the CD3+ T cell population in both mouse strains but only in the C57BL/6 mice there was an increase in the percentage of macrophages (Mac3+ cells) (Fig. 2a, b). In order to analyse whether the expanded peritoneal macrophages were activated we checked the cytokine profile of the Mac3+ cells. The results, shown in Fig. 3a, indicate a IL-12 and TNF-α up-regulation of the macrophages from C57BL/6 mice but not of the BALB/c mice. Moreover, the non-adherent peritoneal CD3+ cells also showed a differential expression of cytokines (Fig. 3b). In BALB/c mice there was, after T. cruzi infection, an increase in the percentage of cells expressing IL-4, while C57BL/6 mice presented an increase in the peritoneal CD3+ cells producing IFN-γ.
Fig 2.
Macrophages and CD3+ T cells in the peritoneal cavity of BALB/c and C57BL/6 mice. For this and all subsequent experiments, BALB/c and C57BL/6 mice were intraperitoneally infected with 2·5 × 103 or 104 T. cruzi trypomastigotes, respectively. For each mouse strain, two T. cruzi infected mice (2 weeks postinfection) and two non-infected mice were sacrificed and peritoneal cells isolated. Non-adherent and adherent cells were stained for CD3 and Mac3, respectively, and analysed by flow cytometry. The shaded histograms show Mac3+ (a) and CD3+ (b) expression on the harvested cells and dotted line histograms show the staining with labelled isotype control MoAbs. The percentage of positive cells showing specific MoAb binding is indicated in the histograms. This set of results is representative of three separate experiments.
Fig 3.
Cytokine expression level in peritoneal cavity. 15 days postinfection, two infected and two non-infected mice from BALB/c and C57BL/6 strains were sacrificed, peritoneal cells isolated and adherent and non-adherent cells were separated. (a) Double staining in the adherent population for Mac3 and IL-12 or TNF-α cytokines. (b) Double staining in the non-adherent fraction for CD3 and IFN-γ or IL-4 cytokines. The figure shows the cytokine expression on the gated cells (Mac3+ and CD3+, respectively) from infected (solid line histograms) and non-infected (shaded histograms) mice, relative to staining with labelled isotype control MoAbs (dotted line histograms). For each staining the same number of cells was gated in infected and non-infected mice. This set of results is representative of three separate experiments.
Analysis of the co-stimulation molecules and cytokine expression in APCs of spleen
In an attempt to analyse whether APCs co-stimulatory signals from T. cruzi infected BALB/c and C57BL/6 mice were altered we checked the surface expression pattern of CD40 and CD86 molecules in macrophages and dendritic cells. Infection by T. cruzi led, in both mouse strains, to a large increase in the percentage of both Mac3+ and CD11+ spleen cells (Fig. 4a). Remarkably, as shown in Fig. 4b and 4c, 15 days postinfection the expression of CD86 and CD40 molecules was notably reduced in macrophages (b) and dendritic cells (c) from infected BALB/c relative to control mice. The CD86 and CD40 expression was also inhibited 10 days postinfection (figure not shown). However, after infection of C57BL/6 mice, there was no inhibition of the expression of CD40 and CD86 either in macrophages or in dendritic cells. Furthermore, concerning to the spleen CD3+ cells the analysis of the cytokine expression showed that the infected C57BL/6 mice presented a high number of cells expressing IL-2 and TNF-α while CD3+ from infected BALB/c were expressing IL-4 and IL-5 (Fig. 5).
Fig 4.
Expression of CD86 and CD40 co-stimulation molecules in spleen macrophages and dendritic cells. (a) Level of macrophages and dendritic cells detected in spleen from BALB/c and C57BL/6 mice infected with T. cruzi ( ) and non-infected (□). The bars indicate the positive cell percentages obtained in the dot plots. Results represent the mean ± s.d. value of four mice per group. (b,c) CD86 and CD40 expression in the Mac3+ (b) and CD11+ (c) spleen cells. Solid line histograms represent the cell population in infected mice and shaded histograms represent the cell population in non-infected mice, relative in both cases to staining with labelled isotype control MoAbs (dotted line histograms). For each staining the same number of cells was gated in infected and non-infected mice. The percentages of positive cells showing specific MoAb binding, from infected (I) or non-infected mice (NI) are indicated in the histograms. This set of results is representative of three separate experiments.
) and non-infected (□). The bars indicate the positive cell percentages obtained in the dot plots. Results represent the mean ± s.d. value of four mice per group. (b,c) CD86 and CD40 expression in the Mac3+ (b) and CD11+ (c) spleen cells. Solid line histograms represent the cell population in infected mice and shaded histograms represent the cell population in non-infected mice, relative in both cases to staining with labelled isotype control MoAbs (dotted line histograms). For each staining the same number of cells was gated in infected and non-infected mice. The percentages of positive cells showing specific MoAb binding, from infected (I) or non-infected mice (NI) are indicated in the histograms. This set of results is representative of three separate experiments.
Fig 5.
Cytokine expression by CD3+ splenocytes from infected BALB/c and C57BL/6 mice. Spleens from infected BALB/c ( ) and C57BL/6 (
) and C57BL/6 ( ) mice together with uninfected BALB/c (□) and C57BL/6 (▪) control mice were, respectively, removed 15 days postinfection. Splenocytes were surface-stained with a FITC-anti-CD3 antibody and intracellular stained with PE-anticytokine MoAbs. Graphic bars represent the percentage of positive cells for each cytokine among CD3+ cell population. Results represent the mean ± s.d. of four mice per group. Data correspond to one of three independent experiments.
) mice together with uninfected BALB/c (□) and C57BL/6 (▪) control mice were, respectively, removed 15 days postinfection. Splenocytes were surface-stained with a FITC-anti-CD3 antibody and intracellular stained with PE-anticytokine MoAbs. Graphic bars represent the percentage of positive cells for each cytokine among CD3+ cell population. Results represent the mean ± s.d. of four mice per group. Data correspond to one of three independent experiments.
Nitrite secretion by peritoneal and spleen macrophages
It is well established that activated macrophages synthesize nitric oxide due to the induction of the enzyme nitric oxide synthase II (NOS II) [13]. Nitric oxide has toxic capacity and is responsible for the parasite killing inside the macrophage. This effector mechanism is triggered when cells are activated by cytokines, IFN-γ and TNF-α, released by CD4+ T cells. A higher nitrite secretion was detected in the supernatant of peritoneal and spleen macrophage cultures from infected C57BL/6 than that observed in infected BALB/c (Fig. 6). As it was expected, macrophages from healthy BALB/c and C57BL/6 mice did not secrete nitrites to the medium as an indication of not being activated.
Fig 6.
Nitrite secretion in supernatants of macrophage cultures from infected BALB/c and C57BL/6 mice. 15 days postinfection the peritoneal and spleen macrophages from three infected and healthy BALB/c and C57BL/6 mice were isolated and processed as described in Materials and methods. Macrophages from the spleen and the peritoneum were cultured at 1 × 106 cells/ml in a CO2 incubator at 37°C. For the nitrite determination culture supernatants were collected after 24 h (for peritoneum macrophages) and 48 h (for spleen macrophages). The nitrite determination was carried out using the Griess reagent. NaNO2 was used to produce the standard curve. Data represent the mean concentration ± s.d. of triplicates; (a) peritoneum macrophages and (b) spleen macrophages. Results are representative of two independent experiments.
Allostimulatory capacity of spleen DCs
In order to analyse whether the diminished co-stimulation molecule expression level observed in spleen DCs from infected BALB/c mice was correlated with immunological functional alterations, purified dendritic cells from the spleen of infected BALB/c and C57BL/6 mice were added to allogeneic splenocytes for 5 days. The results, displayed in Fig. 7, demonstrate that 10 and 15 days after the infection the DCs from the C57BL/6 mice have a capacity for allostimulating T cells two times higher than the allostimulating capacity of the DCs from BALB/c mice.
Fig 7.
Stimulatory ability of dendritic cells from infected BALB/c and C57BL/6 mice. Ten and 15 days postinfection, BALB/c and C57BL/6 mice (n = 4) were sacrificed and the spleens removed. DCs resident in the spleens were purified by magnetic separation (Materials and methods) and incubated at different stimulator/responder ratios with 2·5 × 105 allogeneic T cells, purified by nylon column (the results show ratio 1 : 40). After 5 days in a CO2 incubator at 37°C, methyl-[3H] thymidine (0·5 µCi/well) was added to the cells and cultures incubated for 8 h at 37°C. The control cultures contained DCs alone or splenocytes alone. The figure represents the mean of the cpm obtained. Error bars give the s.d. of triplicates. Results are representative of two independent experiments.
DISCUSSION
Understanding of how the T. cruzi parasite eludes the immune system has important applications for the design of effective immunotherapies against Chagas’ disease. In this context, establishing infection models in mice is extremely helpful for studying the development and progression of infection and the immunopathology of the disease. Under our experimental conditions, intraperitoneal infection of BALB/c and C57BL/6 mice with the Y strain of T. cruzi has proved to be, respectively, a good model of the analysis of susceptibility and resistance to the parasite infection. Analysis of cytokine expression by peritoneal CD3+ T cells after T. cruzi infection showed that cells from the BALB/c mice express IL-4 while the cells from C57BL/6 mice express IFN-γ. It is well known that IFN-γ, a major Th1 cytokine, has a remarkable ability to activate macrophages and to inhibit intracellular parasite replication by inducing nitric oxide synthesis [14,15]. Thus, this cytokine plays a critical role in the development of resistance to T. cruzi. Remarkably, spleen and peritoneal macrophages from C57BL/6 mice secrete three times higher nitrite concentration than that observed in BALB/c mice. Furthermore, we have detected an overexpression of IL-12 and TNF-α cytokines in peritoneal macrophages of infected C57BL/6. This overexpression was not observed in the peritoneal macrophages from BALB/c mice. IL-12 and TNF-α contribute to parasite clearance and sickness control as it has been described previously [16,17].
The observation that splenocytes from both infected mouse strains produce a wide range of Th1, Th2-type and proinflammatory cytokines suggests that the differences in cytokine secretion associated with a resistance phenotype could be mainly quantitative or related to a specific secretion kinetic in some of them. Our results show that 15 days postinfection, splenocytes from C57BL/6 mice present a larger percentage of cells expressing IL-2 and TNF-α than that observed in cells from BALB/c mice which, in turn, express preferentially IL-4 and IL-5. In this context, Zhang and Tarleton [5] described that early IL-2 production and late IL-4 and IL-5 production by spleen cells seems to be associated with resistance to T. cruzi infection.
Interestingly, in this study the analysis carried out on co-stimulation molecules in the spleen APCs suggests that an altered expression of these molecules in the acute phase of the illness would be associated with susceptibility to T. cruzi infection. Thus, we have observed that the expression rate of CD40 and CD86 diminishes both in macrophages and dendritic cells from the infected BALB/c mice, a phenomenon not detected in C57BL/6 mice. It is well known that the absence of co-stimulatory signals during antigen presentation to resting T cells, may reduce T cell stimulation or lead to an anergic state [18]. In fact, our data show that the allostimulatory capacity of the DCs from infected BALB/c mice is reduced relative to that of the DCs from infected C57BL/6 mice. Studies carried out on Leishmania infantum revealed that in infected canine macrophages, the expression of CD80/86 molecules decreased and that this decrease is associated to a lower specific proliferative T response and a drop in IFN-γ secretion [19]. Recently, T. cruzi infection has been reported to interfere with antigen presentation capability and may well be the basis of defective APC function [20]. In conclusion, it seems evident that impaired function by macrophages and DC (co-stimulation molecules and cytokines production) could be a contributing factor to the nonrecovery of BALB/c mice following T. cruzi infection.
Acknowledgments
The authors give special thanks to A. López-Barajas, D. Beriso, F. Ferrer and V. Iniesta for their excellent technical assistance. The authors also thank to Dr C. Terhorst for providing us the 2·4G2 hybridoma. This work was supported by Plan Nacional I +D–FEDER (DGESIC) (grant 1FD1997-0630-C02-01) Spain and Servicio Andaluz de Salud (grant 145/2001) Spain.
References
- 1.World Health Organization. WHO Tropical Disease Research. Geneva, Switzerland: World Health Organization; 1995. Twelfth Program Report of the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases; pp. 125–6. [Google Scholar]
- 2.Burleigh BA, Andrews NW. The mechanisms of Trypanosoma cruzi invasion of mammalian cells. Annu Rev Microbiol. 1995;49:175–200. doi: 10.1146/annurev.mi.49.100195.001135. [DOI] [PubMed] [Google Scholar]
- 3.Rottenberg ME, Bakhiet M, Olsson T, et al. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–33. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DosReis GA. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol Today. 1997;13:335–42. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Tarleton RL. Characterization of cytokine production in murine Trypanosoma cruzi infection by in situ immunocytochemistry: lack of association between susceptibility and type 2 cytokine production. Eur J Immunol. 1996;26:102–9. doi: 10.1002/eji.1830260116. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–44. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 7.Minoprio P, el Cheikh MC, Murphy E, et al. Xid-associated resistance to experimental Chagas’ disease is IFN-gamma dependent. J Immunol. 1993;151:4200–8. [PubMed] [Google Scholar]
- 8.McAdam AJ, Schweitzer AN, Sharpe AH. The role of B7 co-stimulation in activation and differentiation of CD4+ and CD8+ T cells. Immunol Rev. 1998;165:231–47. doi: 10.1111/j.1600-065x.1998.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 co-stimulation: a review. Crit Rev Immunol. 1998;18:389–418. doi: 10.1615/critrevimmunol.v18.i5.10. [DOI] [PubMed] [Google Scholar]
- 10.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 11.Kaye PM, Rogers NJ, Curry AJ, et al. Deficient expression of co-stimulatory molecules on Leishmania-infected macrophages. Eur J Immunol. 1994;24:2850–4. doi: 10.1002/eji.1830241140. [DOI] [PubMed] [Google Scholar]
- 12.Van Overtvelt L, Vanderheyde N, Verhasselts V, et al. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and co-stimulatory molecules. Infect Immun. 1999;67:4033–40. doi: 10.1128/iai.67.8.4033-4040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065x.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- 14.Hölscher C, Kohler G, Muller U, et al. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–15. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torrico F, Heremans H, Rivera MT, et al. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–32. [PubMed] [Google Scholar]
- 16.Aliberti JC, Cardoso MA, Martins GA, et al. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–7. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima EC, García I, Vicentelli MH, et al. Evidence for a protective role of tumor necrosis factor in the acute phase of Trypanosoma cruzi infection in mice. Infect Immun. 1997;65:457–65. doi: 10.1128/iai.65.2.457-465.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boussiotis VA, Freeman GJ, Gribben JG, et al. The role of B7–1/B7–2: CD28/CLTA-4 pathways in the prevention of anergy, induction of productive immunity and down-regulation of the immune response. Immunol Rev. 1996;153:5–26. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 19.Pinelli E, Rutten VP, Bruysters M, et al. Compensation for decreased expression of B7 molecules on Leishmania infantum-infected canine macrophages results in restoration of parasite-specific T-cell proliferation and gamma interferon production. Infect Immun. 1999;67:237–43. doi: 10.1128/iai.67.1.237-243.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.La Flamme AC, Kahn SJ, Rudensky AY, et al. Trypanosoma cruzi-infected macrophages are defective in major histocompatibility complex class II antigen presentation. Eur J Immunol. 1997;27:3085–94. doi: 10.1002/eji.1830271202. [DOI] [PubMed] [Google Scholar]