Abstract
The present study investigates the modulating effects of nicotinamide on the cytokine response to endotoxin. In an in vitro model of endotoxaemia, human whole blood was stimulated for two hours with endotoxin at 1 ng/ml, achieving high levels of the proinflammatory cytokines IL-1β, IL-6, IL-8 and TNFα. When coincubating whole blood, endotoxin and the vitamin B3 derivative nicotinamide, all four cytokines measured were inhibited in a dose dependent manner. Inhibition was observed already at a nicotinamide concentration of 2 mmol/l. At a concentration of 40 mmol/l, the IL-1β, IL-6 and TNFα responses were reduced by more than 95% and the IL-8 levels reduced by 85%. Endotoxin stimulation activates poly(ADP-ribose)polymerase (PARP), a nuclear DNA repair enzyme. It has been hypothesized that the anti-inflammatory properties of nicotinamide are due to PARP inhibition. In the present study, the endotoxin induced PARP activation was dose dependently decreased with 4–40 mmol/l nicotinamide or 4–100 µmol/l 6(5H) phenanthridinone, a specific PARP inhibitor. 6(5H)phenanthridinone however, failed to inhibit the proinflammatory cytokines. Thus, the mechanism behind the cytokine inhibition in our model seems not to be due to PARP inhibition. In conclusion, the present study could not only confirm previous reports of a down-regulatory effect on TNFα, but demonstrates that nicotinamide is a potent modulator of several proinflammatory cytokines. These findings demonstrate that nicotinamide has a potent immunomodulatory effect in vitro, and may have great potential for treatment of human inflammatory disease.
Keywords: sepsis, inflammation, nicotinamide, endotoxin, poly(ADPribose)polymerase
INTRODUCTION
Endotoxin or lipopolysaccharide (LPS) from the outer membrane of gram negative bacteria stimulates the production and release of proinflammatory cytokines via binding of LPS binding protein, LBP, to monocyte membrane bound CD14 [1–3]. The monocyte expression of cytokine genes following stimulation with endotoxin is regulated by the nuclear transcription factor NFκβ/Rel complexes [4]. The proinflammatory cytokines play a central role in the patophysiology of gram negative sepsis [5], and have been demonstrated to appear early after endotoxin injection in healthy volunteers [6]. There are a number of reports on proinflammatory cytokines contributing to disease severity, organ failure and poor outcome in sepsis and septic shock [7–10]. The individual cytokine response following exposure to endotoxin, e.g. in sepsis, however, shows considerable variation [10].
Nicotinamide, the amide derivative of vitamin B3, has been shown to exert a number of anti-inflammatory properties, e.g. inhibition of inducible NO synthase (iNOS) [11], free radical scavenging [12], suppression of MHC class II expression [13] and intracellular adhesion molecule ICAM-1 expression on endothelial cells [14], all possibly due to the ability of nicotinamide to inhibit poly (ADPribose) polymerase (PARP) [15]. PARP is a nuclear DNA binding enzyme involved in DNA repair in response to genotoxic stress [16,17]. Activation of PARP, which has been shown to occur upon endotoxin administration [17] depletes intracellular NAD+, slowing down the rate of glycolysis, electron transport and ATP formation, which can result in cell dysfunction and cell death.
Over the years, nicotinamide has been used in daily doses of 1–12 gram to treat various diseases such as pellagra, psoriasis, schizophrenia and diabetes type I [18–21]. In the present study we assess the potential beneficial effect of nicotinamide in an in vitro model of endotoxaemia, by incubating whole blood with endotoxin, nicotinamide and the specific PARP inhibitor 6(5H)phenanthridinone [22], analysing the effects on the proinflammatory cytokines IL-1β, IL-6, IL-8 and TNFα.
MATERIALS AND METHODS
Healthy volunteers
Six male volunteers, healthy according to medical history and routine haemostatic and biochemical screening, were included in the study. Two additional healthy volunteers were sampled for initial titration experiments. For the PARP experiments, two healthy volunteers were included. Informed consent was obtained from all volunteers.
The local ethics committee approved the study.
Experimental procedure
Nicotinamide (prepared by the Karolinska Hospital pharmacy) was dissolved at a concentration of 0·5 g/ml in TBS, pH 7·4. The TBS was endotoxin free, i.e. contained < 0·125 IU/ml endotoxin assayed with CoaTest Endotoxin chromogenic limulus amoebocyte lysate assay (Chromogenix, Mölndal, Sweden), where 1 IU is the activity of 0·1 ng US Standard Endotoxin EC-5. LPS endotoxin E. Coli O26B6, was purchased from Difco (Detroit, MI, USA) and diluted in TBS to reach a final concentration of 1 ng/ml in the whole blood samples. 6(5H)phenanthridinone (Sigma Aldrich, St.Louis, MI, USA) was dissolved to a stock solution of 40 mmol/l in dimetylsulphoxide, and diluted in TBS to 4 mmol/l before each experiment. Blood was drawn from the antecubital vein with 19 gauge needles, into 4 ml sterile heparinized tubes (Becton Dickinson, NJ, USA), and endotoxin, TBS and different concentrations of either nicotinamide (n = 6) or 6(5H)phenanthridinone (n = 2) were added. The total volume added (reagents diluted in TBS) was 220 µl per sample containing 4 ml blood. Mixing was achieved by carefully inverting the tubes. Whole blood samples were thereafter incubated in 5% CO2, 37°C for 2 h. Leucocytes, prepared by lymphoprep (Nycomed Pharma, Oslo, Norway) or lysed with NH4Cl, showed>99% viability after the two hour incubation.
From each sample, 2 ml blood was centrifuged at 2000 × g for 15 min, plasma was aliqoted and stored at −70°C until cytokine analysis.
Poly (ADP-ribose)polymerase (PARP) assay
Leukocytes from two healthy volunteers were prepared by lymphoprep, and thereafter treated with MgCl2, sonicated, and analysed with the PARP activity kit (Trevigen, Gaithersburg, MD, USA) according to manufacturer, adding an extra wash with 5% trichloroacetic acid. 32PNAD was purchased from BioNuclear, Stockholm, Sweden, and used within one week. Protein concentration in the samples (0·35–0·65 mg/ml) was measured according to Lowry [23].
Cytokine measurements
TNFα, IL-1β, IL-6 and IL-8 were measured in plasma by a chemiluminescent immunometric assay using an IMMULITE instrument and reagents from DPC Diagnostic Products Corporation, Los Angeles, CA, USA [24]. Reference levels in normal subjects, according to manufacturer, were <8·1, <5, <9·7 and <62 pg/ml, respectively, with detection limits of 1·7, 1·5, 5 and 2 pg/ml, respectively.
Statistics
Wilcoxon matched pair test was used for statistical analyses. Significance was set to the level of P < 0·05.
RESULTS
In unstimulated whole blood from six healthy volunteers, as well as in whole blood incubated for two hours with nicotinamide, no cytokine response was seen (results not shown). Incubation of whole blood with added endotoxin, however, showed a marked increase of all four cytokines at two hours. Already at endotoxin levels of 0·1 ng/ml, there was a massive cytokine response, which was further enhanced by increasing endotoxin concentrations. In the presence of 1 ng/ml endotoxin and increasing concentrations of nicotinamide, there was a dose dependent decrease in the cytokine response (Fig. 1a–d).
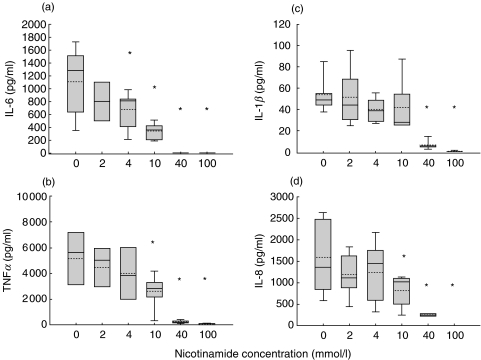
Fig. 1.
(a) IL-6, (b) TNFα,(c) IL-1β and (d) IL-8 responses to a two hour incubation of whole blood with 1 ng/ml endotoxin at 37°C with 5% CO2. A dose dependent down-regulation by 2–100 mmol/l nicotinamide is observed. Box plots are shown with 5th & 95th percentiles. *statistically significant P < 0·05 compared to initial levels.
IL-6 increased from undetectable to mean 1100 pg/ml (range 351–1728) levels during the 2 h incubation with endotoxin. Coincubation with nicotinamide resulted in a dose dependent decrease of IL-6, lowering the cytokine response to mean 670 pg/ml (range 185–996) already at a nicotinamide concentration of 4 mmol/l. At a nicotinamide concentration of 40 mmol/l, there was no detectable IL-6 response (Fig. 1a).
Upon endotoxin stimulation, TNFα levels increased to mean 5200 pg/ml (1150–8270). Nicotinamide, at a concentration of 40 mmol/l, caused a mean decrease of>95%, and at a concentration of 100 mmol/l, no TNFα response was observed (Fig. 1b).
The IL-1β levels in response to 2 h incubation with endotoxin were mean 54 pg/ml (range 37–88). At 40 mmol/l nicotinamide, five of six experiments showed IL-1β levels below reference range, and one slightly above reference. Also IL-1β was totally abolished at a nicotinamide concentration of 100 mmol/l (Fig. 1c).
IL-8 reached a mean level of 1800 pg/ml (586–2630) after 2 h of endotoxin stimulation (Fig. 1d). The IL-8 level decreased by 50% in the presence of 10 mmol/l nicotinamide, 85% at 40 mmol/l, and was totally abolished at 100 mmol/l.
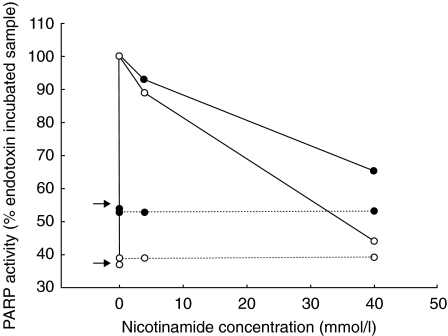
PARP activity was measured in leucocyte cell extracts from two healthy volunteers. Samples incubated without endotoxin for 2 h showed no PARP activity, while endotoxin at 1 ng/ml induced PARP activity in the samples (Fig. 2). This PARP activity was inhibited by 40 mmol/l nicotinamide (Fig. 2) as well as by 40–100 µmol/l of the specific PARP inhibitor 6(5H) phenanthridinone (results not shown). 6(5H)phenan has previously been shown to dose dependently inhibit PARP, with a 80% inhibition at 50 µmol/l and 90% inhibition at 100 µmol/l [22].
Fig. 2.
PARP activity was inhibited by nicotinamide. PARP activity induced by 1 ng/ml endotoxin was set to 100%. Arrows indicate samples incubated for two hours without endotoxin or nicotinamide. …… negative control for each experiment. • and ○ symbolize the two healthy volunteers.
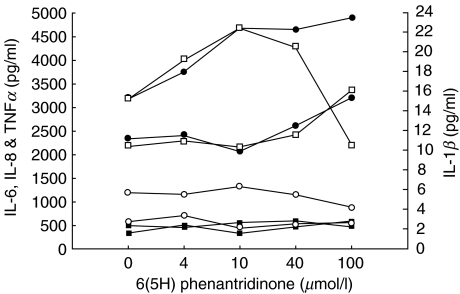
However, when incubating whole blood from two healthy volunteers with endotoxin and 4–100 µmol/l of the PARP inhibitor, no cytokine inhibition could be detected, with the exception that in one individual a TNFα decrease from initial 3100 pg/ml to 2300 pg/ml was seen (Fig. 3).
Fig. 3.
IL-1β, IL-6, IL-8 and TNFα responses to 1 ng/ml endotoxin in whole blood, into which different amounts of the specific PARP inhibitor 6(5H)phenanthridinone had been added to five different tubes. n= 2, each line represents one healthy volunteer. Left y-axis: □ TNFα, ○ IL-8, ▪ IL-6; right side y-axis • IL-1β.
DISCUSSION
In the present study, a two-hour incubation of whole blood with 1 ng/ml endotoxin resulted in a massive increase of the inflammatory cytokines IL-1β, TNFα, IL-6 and IL-8. Already at an in vitro concentration of 4 mmol/l nicotinamide, there was a significant reduction of the IL-6 response, and at 40 mmol/l nicotinamide, the IL-1β, IL-6, and TNFα responses were reduced by more than 95%, and the IL-8 levels reduced by 85%. The endotoxin concentration used for stimulation and the cytokine concentrations obtained were of similar magnitude to what is observed in human endotoxaemia [7,9,10]. These results demonstrate that nicotinamide has the capacity to dose dependently down-regulate the cytokine response in a model with several similarities to human inflammatory disease.
An inhibitory effect of nicotinamide on endotoxin induced TNFα has previously been described by Pero et al.[15] using a mouse model, and by Fukuzawa et al.[25], in mice and in human peripheral blood mononuclear cells (PBMC). In PBMC, Fukuzawa et al. [25] found a significant inhibition of TNFα with nicotinamide concentrations of 1 mmol/l or more, but no significant inhibition of IL-1β or IL-6. There are, however, several differences in the experimental conditions. In our study, an endotoxin concentration of 1 ng/ml was used, a concentration similar to that observed in septic patients [7,9,10], while Fukuzawa et al.[25] used 20 µg/ml endotoxin. Additionally, our study used whole blood while Fukuzawa et al. [25] used PBMC in the absence of plasma, i.e. without lipopolysaccharide binding protein, LBP. LBP, is important for the monocyte response to endotoxin [1], and is essential for lethal endotoxaemia [3].
Cytokines are regulators of host responses to infection, immune responses, inflammation, and trauma, and are thus needed for optimal function of important host defense mechanisms. In some severe inflammatory diseases, modulation of the cytokine response is considered an essential part of treatment. TNFα, IL-6 and also IL-1β have been shown to correlate to disease severity and outcome in septic patients [7,8,10,15]. Administration of antibodies to IL-6 attenuates the hypercoagulation seen in endotoxaemia [26], and antibodies to TNFα prevent endotoxin lethality in mice and baboon models [27,28]. Antibodies against endotoxin can be used, but only prophylactically, to counteract the endotoxin effect [29]. There are, however, problems with the use of antibodies and similar biological response modifiers, having short plasma half-life and requiring high doses. Since inflammatory disease is the net result of the interaction of many endogenous mediators, a broader pharmacological intervention, such as nicotinamide, is of interest.
Activation of PARP is a central mechanism of endotoxin induced acute pulmonary inflammation [30], and PARP activation was observed after endotoxin stimulation in our endotoxaemia model. The hypothesis of PARP inhibition being the mechanism behind the anti-inflammatory properties of nicotinamide originated from several earlier studies describing that PARP inhibition has anti-inflammatory effects. PARP -/- mice survive endotoxin-mediated shock [31], and inhibition of PARP with the specific PARP inhibitors 3-aminobenzamide or 5-iodo-6-amino-1,2-benzopyrone improves survival rate of mice subjected to endotoxin [32,33]. In the present study, nicotinamide (4–40 mmol/l) and 6(5H) phenanthridinone (4–100 µmol/l) dose dependently inhibited PARP activity. However, 6(5H)phenanthridinone at doses inhibiting PARP activity, was unable to mimic the cytokine inhibition exerted by nicotinamide. Interestingly, another specific PARP inhibitor, PJ34, based on a modified 6(5H)phenanthridinone structure, increased endotoxic shock survival rate in rats [34] and had various cytoprotective and anti-inflammatory effects in animal models of endotoxaemia [34,35]. The sequence of events leading to endotoxin induced shock include the endotoxin ability to activate the nuclear transcription factor NFκβ[4]. It has been shown that activation of PARP is required for activation of NFκβ, and that the two form a stable immunoprecipitable nuclear complex [36], reacting functionally upstream the synthesis of proinflammatory mediators [36,37]. One possible mechanism could be that some PARP inhibitors can inhibit this complex whereas others do not, and that this influences their anti-inflammatory properties. The strong and broad cytokine inhibitory effect of nicotinamide in the present study suggests that inhibition of NFκβ is part of the mechanism.
A PARP inhibition dependent anti-inflammatory effect on, e.g. cytokine protein synthesis could not be ruled out in this study, since only cytokine release was measured. There may be PARP inhibition effects exerted that occur later or that we have not measured.
Nicotinamide, originally discovered as a pellagra preventive factor [38], has over the years been used in a broad spectrum of disease [18–21]. A drug safety study was conducted for long-time nicotinamide treatment of up to 3 g daily [39], showing no side-effects. Earlier studies using considerably higher doses (1–12 g daily for months) reported side-effects of nausea and gastrointestinal effects to be rare [40].
As the proinflammatory cytokine response of IL-1β, IL-6, IL-8 and TNFα following endotoxin stimulation of human whole blood is profoundly inhibited by nicotinamide, nicotinamide may have a therapeutic potential as a modulator of cytokine effects in inflammatory disease.
Acknowledgments
The authors thank Mrs Rumjana Djilali-Merzoug for excellent technical assistance. Baxter, Karolinska Institutet funds, and the Swedish Heart and Lung foundation supported this study.
References
- 1.Schumann RR, Latz E. Lipopolysaccharide-binding protein. Chem Immunol. 2000;74:42–60. doi: 10.1159/000058760. [DOI] [PubMed] [Google Scholar]
- 2.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 3.Verbon A, Dekkers PE, ten Hove T, et al. IC14, an anti-CD14 antibody, inhibits endotoxin-mediated symptoms and inflammatory responses in humans. J Immunol. 2001;166:3599–605. doi: 10.4049/jimmunol.166.5.3599. [DOI] [PubMed] [Google Scholar]
- 4.Muller JM, Ziegler-Heitbrock HWL, Bauerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–56. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Lowry SF. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990;55:157–70. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- 6.Martich GD, Boujoukos AJ, Suffredini AF. Response of man to endotoxin. Immunobiology. 1993;187:403–16. doi: 10.1016/S0171-2985(11)80353-0. [DOI] [PubMed] [Google Scholar]
- 7.Gardlund B, Sjolin J, Nilsson A, Roll M, Wickerts CJ, Wretlind B. Plasma levels of cytokines in primary septic shock in humans: correlation with disease severity. J Infect Dis. 1995;172:296–301. doi: 10.1093/infdis/172.1.296. [DOI] [PubMed] [Google Scholar]
- 8.Waage A, Halstensen A, Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;1:355–7. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 9.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–76. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–75. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 11.Andersen HU, Jorgensen KH, Egeberg J, Mandrup-Poulsen T, Nerup J. Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes. 1994;43:770–7. doi: 10.2337/diab.43.6.770. [DOI] [PubMed] [Google Scholar]
- 12.Burkart V, Koike T, Brenner HH, Kolb H. Oxygen radicals generated by the enzyme xanthine oxidase lyse rat pancreatic islet cells in vitro. Diabetologia. 1992;35:1028–34. doi: 10.1007/BF02221677. [DOI] [PubMed] [Google Scholar]
- 13.Otsuka A, Hanafusa T, Miyagawa J, Kono N, Tarui S. Nicotinamide and 3-aminobenzamide reduce interferon-gamma-induced class II MHC (HLA-DR and -DP) molecule expression on cultured human endothelial cells and fibroblasts. Immunopharmacol Immunotoxicol. 1991;13:263–80. doi: 10.3109/08923979109019705. [DOI] [PubMed] [Google Scholar]
- 14.Hiromatsu Y, Sato M, Tanaka K, Ishisaka N, Kamachi J, Nonaka K. Inhibitory effects of nicotinamide on intercellular adhesion molecule-1 expression on cultured human thyroid cells. Immunology. 1993;80:330–2. [PMC free article] [PubMed] [Google Scholar]
- 15.Pero RW, Axelsson B, Siemann D, Chaplin D, Dougherty G. Newly discovered anti-inflammatory properties of the benzamides and nicotinamides. Mol Cell Biochem. 1999;193:119–25. [PubMed] [Google Scholar]
- 16.Szabo C. Boca Raton: CRC Press; 2000. Cell death: the role of PARP. [Google Scholar]
- 17.DeMurcia G, Shall S. Oxford: Oxford University Press; 2000. From DNA Damage and Stress Signaling to Cell Death; Poly ADP-Ribosylation Reactions. [Google Scholar]
- 18.Green RG. Subclinical pellagra. its diagnosis and treatment. Schizophrenia. 1970;2:70–9. [Google Scholar]
- 19.Hawkins DR. Treatment of schizophrenia based on the medical model. J Schizophr. 1968;2:3–10. [Google Scholar]
- 20.Vague P, Picq R, Bernal M, Lassmann-Vague V, Vialettes B. Effect of nicotinamide treatment on the residual insulin secretion in type 1 (insulin-dependent) diabetic patients. Diabetologia. 1989;32:316–21. doi: 10.1007/BF00265549. [DOI] [PubMed] [Google Scholar]
- 21.Zackheim HS. Topical 6-aminonicotinamide plus oral niacinamide therapy for psoriasis. Arch Dermatol. 1978;114:1632–8. [PubMed] [Google Scholar]
- 22.Weltin D, Picard V, Aupeix K, Varin M, Oth D, Marchal J, Dufour P, Bischoff P. Immunosuppressive activities of 6(5H)-phenanthridinone, a new poly (ADP-ribose) polymerase inhibitor. Int J Immunopharmacol. 1995;17:265–71. doi: 10.1016/0192-0561(95)00007-o. [DOI] [PubMed] [Google Scholar]
- 23.Hartree EF. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–7. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 24.Babson AL, Olson DR, Palmieri T, Ross AF, Becker DM, Mulqueen PJ. The IMMULITE assay tube: a new approach to heterogeneous ligand assay. Clin Chem. 1991;37:1521–2. [PubMed] [Google Scholar]
- 25.Fukuzawa M, Satoh J, Muto G, Muto Y, Nishimura S, Miyaguchi S, Qiang XL, Toyota T. Inhibitory effect of nicotinamide on in vitro and in vivo production of tumor necrosis factor-alpha. Immunol Lett. 1997;59:7–11. doi: 10.1016/s0165-2478(97)00088-6. [DOI] [PubMed] [Google Scholar]
- 26.van der Poll T, Levi M, Hack CE, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–9. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashkenazi A, Marsters SA, Capon DJ, et al. Protection against endotoxic shock by a tumor necrosis factor receptor immunoadhesin. Proc Natl Acad Sci USA. 1991;88:10535–9. doi: 10.1073/pnas.88.23.10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emerson TE, Jr, Lindsey DC, Jesmok GJ, Duerr ML, Fournel MA. Efficacy of monoclonal antibody against tumor necrosis factor alpha in an endotoxemic baboon model. Circ Shock. 1992;38:75–84. [PubMed] [Google Scholar]
- 29.Calandra T, Baumgartner JD, Glauser MP. Anti-lipopolysaccharide and anti-tumor necrosis factor/cachectin antibodies for the treatment of gram-negative bacteremia and septic shock. Prog Clin Biol Res. 1991;367:141–59. [PubMed] [Google Scholar]
- 30.Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C. Activation of poly (ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165:372–7. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- 31.Kuhnle S, Nicotera P, Wendel A, Leist M. Prevention of endotoxin-induced lethality, but not of liver apoptosis in poly (ADP-ribose) polymerase-deficient mice. Biochem Biophys Res Commun. 1999;263:433–8. doi: 10.1006/bbrc.1999.1393. [DOI] [PubMed] [Google Scholar]
- 32.Szabo C, Wong HR, Bauer PI, et al. Regulation of components of the inflammatory response by 5-iodo-6-amino-1,2-benzopyrone, an inhibitor of poly (ADP-ribose) synthetase and pleiotropic modifier of cellular signal pathways. Int J Oncol. 1997;10:1093–101. doi: 10.3892/ijo.10.6.1093. [DOI] [PubMed] [Google Scholar]
- 33.Szabo C, Zingarelli B, Salzman AL. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ Res. 1996;78:1051–63. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- 34.Jagtap P, Soriano FG, Virag L, et al. Novel phenanthridinone inhibitors of poly (adenosine 5′-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit Care Med. 2002;30:1071–82. doi: 10.1097/00003246-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 35.Mabley JG, Jagtap P, Perretti M, et al. Anti-inflammatory effects of a novel, potent inhibitor of poly (ADP-ribose) polymerase. Inflamm Res. 2001;50:561–9. doi: 10.1007/PL00000234. [DOI] [PubMed] [Google Scholar]
- 36.Hassa PO, Hottiger MO. A role of poly (ADP-ribose) polymerase in NF-kappaB transcriptional activation. Biol Chem. 1999;380:953–9. doi: 10.1515/BC.1999.118. [DOI] [PubMed] [Google Scholar]
- 37.Oliver FJ, Menissier-de Murcia J, Nacci C, et al. Resistance to endotoxic shock as a consequence of defective NF-kappaB activation in poly (ADP-ribose) polymerase-1 deficient mice. Embo J. 1999;18:4446–54. doi: 10.1093/emboj/18.16.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horwitt MK. Niacin-tryptophan relationships in the development of pellagra. Am J Nutrition. 1955;3:244. doi: 10.1093/ajcn/3.3.244. [DOI] [PubMed] [Google Scholar]
- 39.Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, Gale EA. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–45. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 40.Zackheim HS. Reactions to nicotinamide. J Am Assoc Dermol. 1981;4:736–7. [Google Scholar]