Abstract
We show here that, in the absence of a direct geniculostriate input in human subjects, causing loss of sight in the visual half-field contralateral to the damage, the pupil responds selectively to chromatic modulation toward the long-wavelength (red) region of the spectrum locus even when the stimulus is isoluminant for both rods and cones and entirely restricted to the subjects’ “blind” hemifields. We also show that other colors are less or wholly ineffective. Nevertheless, red afterimages, generated by chromatic modulation toward the green region of the spectrum locus, also cause constrictions of the pupil even when green stimuli are themselves completely ineffective in the blind hemifield. Moreover, human subjects with damage to or loss of V1 are typically completely unaware of the stimulus that generates the aftereffect or of the aftereffect itself, both of which can be seen clearly in normal vision. The results show that pupillary responses can reveal the processing of color afterimages in the absence of primary visual cortex and in the absence of acknowledged awareness. This phenomenon is therefore a striking example of “blindsight” and makes possible the formulation of a model that predicts well the observed properties of color afterimages.
It is commonly assumed that the primary function of the pupil of the eye is to respond to changes in the amount of light that enters the eye, with its major neural complex and the origin of its final efferent pathway residing in the midbrain. But recent studies have demonstrated that the pupil constricts in a systematic manner to stimulus attributes such as spatial structure, color, and movement, even when there is no change in mean light flux level or, indeed, even when there is a net reduction in light flux in a visual stimulus (1, 2). Such stimulus properties are associated with electrophysiological activity in extrastriate cortical neurones (3), and thus there might be a down-stream modulation of midbrain centers by the cortex. Moreover, there is a clear cortical contribution to the pupillary response because loss of striate cortex (V1) in human or monkey diminishes the pupil light reflex response (4, 5). However, even in such cases, the pupillary response to spatial structure and color remains, although reduced significantly in size (6). These observations suggest that the midbrain is influenced by the processing of specific stimulus attributes involving extrastriate visual areas. Psychophysical studies of residual vision in patients with damaged central visual pathways have demonstrated residual capacity for processing chromatic stimuli (6, 7), and this has been confirmed in functional MRI studies by using stimulus techniques that isolate the use of chromatic signals (8). In this study, we investigate pupil responses to “red” and “green” chromatic stimuli that are both photopically and scotopically isoluminant (9) and are restricted entirely to the subjects’ blind hemifields.
METHODS
Subjects.
The studies reported here were carried out in three normal subjects and in two subjects with hemianopia (subjects G, a 43-year-old male, and W, a 59-year-old male). Subject G had a car accident when 8 years old, which resulted in loss of vision in the right hemifield. Numerous psychophysical and MRI studies in subject G show unilateral damage in the posterior left hemisphere (10–12) and the corresponding loss of vision in his right hemifield, except for a small region of macular sparing (<3.5°) (13, 14). Subject W suddenly developed a right homonymous hemianopia at the age of 44. He has unilateral ischemic lesions that affect mostly the primary visual cortex in the left hemisphere with diffuse bilateral damage restricted to the ventral, occipitotemporal cortical regions. Measurements of visual-field sensitivity revealed right homonymous hemianopia with some less severe loss in W’s left upper quadrant. MRI in W shows a large left occipital infarct and a smaller ventral infarct on the right.
Pupil Responses to Single Flashes.
We measured pupil responses triggered by “red” and “green” stimuli generated in a white background field of luminance 12 cd/m2 and CIE (u′, v′)-chromaticity coordinates 0.179, 0.467 (see Inset to Fig. 1). The experiments were carried out on the P_SCAN system, which provides facilities for the generation of visual stimuli and for the measurement of pupil size (15). A number of stimulus durations were investigated. The colored stimulus was a disc of 14° diameter and was presented 15° in the periphery, and 4.6° above the horizontal meridian either to the left (“sighted” hemifield) or to the right (“blind” hemifield) of the fixation stimulus. To minimize the contribution of rod contrast signals to a pupil light-reflex response, the colors generated had zero scotopic contrast, in addition to being photopically isoluminant. This double-isoluminant constraint (9) restricts the number of possible colors that can be generated on the visual display to only two complementary hues. For the background chromaticity employed in this study, the two possible directions of chromatic displacement are one toward the “green,” and the other toward the “red” region of the spectrum locus (see Inset to Fig. 1). The distance away from background chromaticity along either one of these directions, as measured in the CIE (u′, v′)-uniform chromaticity diagram, was used to specify the chromatic saturation of the stimulus. Because the pupil response threshold is significantly higher than the corresponding psychophysical detection threshold, any small, residual luminance contrast signals that may not have been eliminated by the d-isoluminant condition are therefore unlikely to trigger a measurable pupil response. Light flux modulation yields significantly shorter pupil response latencies, even when the response amplitudes are comparable to those elicited by double-isoluminant stimuli.
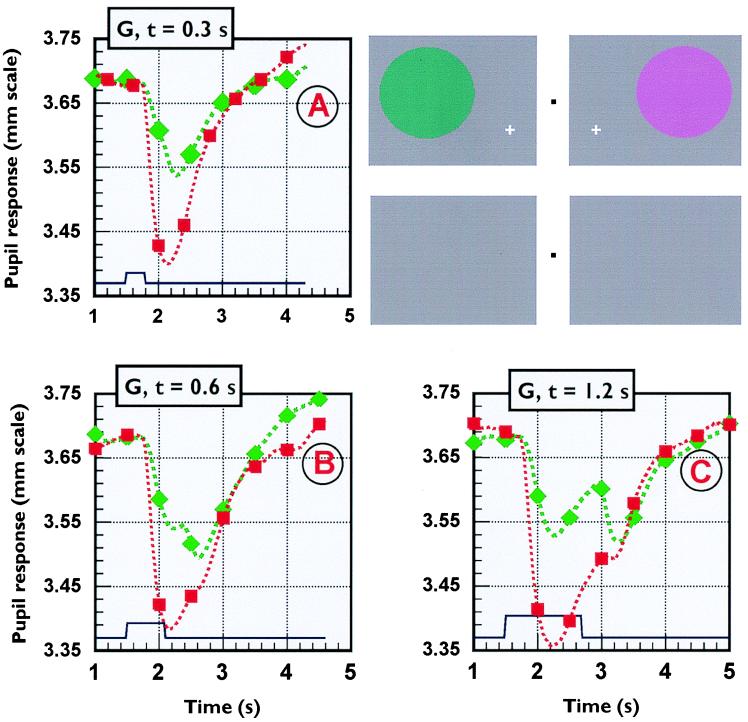
Figure 1.
Pupil responses to “red” and “green” stimuli generated in a white background field of luminance 12 cd/m2 and CIE (u′, v′)-chromaticity coordinates 0.179, 0.467 (see Inset). Each pupil response represents the average of 48 traces. In addition to the expected response to stimulus onset, the longer stimulus durations also show a response to stimulus offset that is particularly large for the “green” stimulus (C). The Inset on the top right hand side has been added to help the reader appreciate the subject’s perception of the stimulus and its chromatic afterimage during the test. To see these afterimages, place this figure at normal viewing distance in good illumination and look steadily for a few seconds at the upper black dot placed midway between the red and the green discs. Shift your gaze direction to the lower dot in between the uniform, gray fields and you should now again perceive the two colored discs, but with the red on the left and the green on the right. The red symbols show responses measured for a stimulus chromatic displacement 0.12 unit in the “red” direction, whereas the maximum chromatic saturation possible along the “green” direction was only 0.08 unit. The duration of the colored stimulus varied from 0.3 s to 1.2 s, as shown in each section. The results show that, for the longer stimulus durations, the d-isoluminant stimuli employed generate two constrictions of the pupil, with the second constriction amplitude being particularly large for the green stimulus. Comparable results were obtained in three normal subjects and in the sighted hemifield of the second hemianope (subject W).
Pupil Responses to Sinusoidal Modulation.
In these experiments, the chromatic saturation of each stimulus was modulated sinusoidally at a frequency of 0.8 Hz. Each stimulus consisted of eight cycles of modulation with maximum chromatic saturation amplitude of 0.071 unit. To minimize the effects of sharp transients in the pupil response at stimulus onset, a Hanning window (16) was applied to the stimulus trace. The stimuli were interleaved, and 16 traces were averaged for each stimulus condition. The signal power and phase shift at the modulation frequency, together with a measure of signal/noise ratio and response nonlinearity (N/L) were then computed from the discrete Fourier transform of each averaged trace. The N/L parameter was defined as the ratio of the summed signal power in all harmonics and the signal power at the stimulus modulation frequency.
RESULTS
The data in Fig. 1 were obtained with single stimuli presented to G’s sighted (left) hemifield. Each pupil response represents the average of 48 traces. Comparison of the red and green traces in Fig. 1 shows that pupil responses to a “green” chromatic stimulus are only about half that observed for the “red” stimulus. The results also show that, for the longer stimulus durations, there are two constrictions of the pupil, one following the stimulus onset and the other following its offset, the amplitude of the second constriction being relatively large for the green stimulus compared with the red. Both the control and the hemianopic subjects (with stimuli presented to their normal visual fields) reported the perception of strong colored afterimages at each stimulus offset. The colored stimuli, included as an Inset to Fig. 1, are intended to demonstrate these afterimages (see legend to Fig. 1).
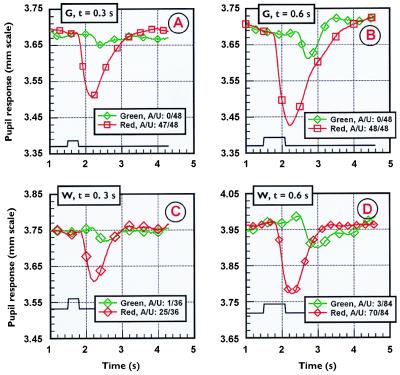
Fig. 2 shows pupil responses to the same red and green stimuli presented to the “blind” hemifields of subjects G and W. There is a robust response to the red stimulus, but little or none to green. The pupil response to red in the blind hemifield is somewhat reduced by comparison with the sighted hemifield (≈20%). As there is also a relative reduction to green in the sighted hemifield (Fig. 1), a smaller pupil response amplitude to the onset of the green stimulus in the blind hemifield would also be predicted, but the complete absence of a response is unexpected. Even more surprising is the response in the blind field at the offset of the green stimulus, just when there would normally be the appearance of a red afterimage; this is especially evident as duration of the stimulus increases. G was unaware of any of the green stimuli or their aftereffects, whereas W was aware only in a small number of trials. The red stimulus, in contrast, produced a majority of reports of awareness for both subjects. It should be noted that, even when these subjects reported an awareness of something presented in the blind hemifields, awareness was devoid of color as such. It was akin to a “feeling” or a “knowing” that an event had occurred.
Figure 2.
Pupil responses to the “red” and “green” stimuli described in caption to Fig. 1. Two subjects with homonymous hemianopia took part in this study (see text). For these experiments, the stimuli were located at the same eccentricity, but in the subjects’ blind hemifields. Neither of the two subjects was able to see either of the two stimuli. However, both subjects were aware of something presented into the blind hemifield for the red stimulus, but not for the green stimulus. The aware/unaware (A/U) scores for the red and the green stimuli employed are given in the Insets.
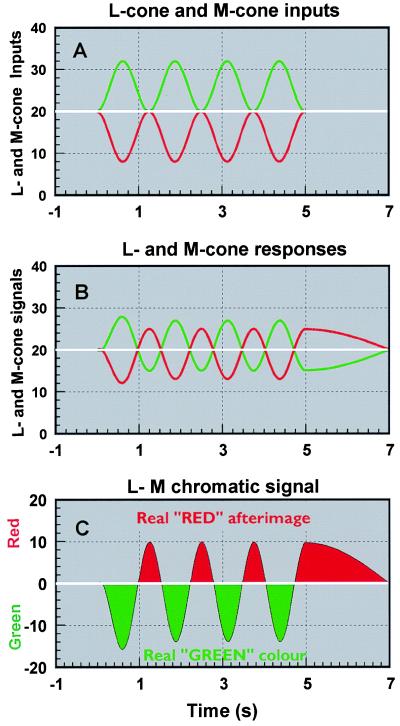
Useful theoretical properties emerge from an analysis and modeling of the cone receptor responses to the isoluminant, colored stimuli employed in this study. Periodic sinusoidal modulation of the “green” stimulus would generate period modulation of M (medium wavelength) cones above that generated by the steady-state adapting field. Such a periodically modulated signal is shown in green in Fig. 3A. To achieve isoluminance, the complementary L (long wavelength) signals are modulated correspondingly below the background signal level, so that the sum of L-cone and M-cone signals remains unchanged (17). The increased stimulation of M-cones and the corresponding decreased stimulation of L-cones will cause an opposite shift in the cellular adaptation of these receptors (18, 19). This adaptation predicts a decrease in the mean signal level of M-receptors and a corresponding increase in the L-receptor responses, without disturbing isoluminance (Fig. 3B). It is assumed, further, that the formation of the red/green chromatic channel requires the difference between L and M cone signals (L − M), as shown in Fig. 3C. In addition to the “green” episodic signal, this simple model predicts the periodic “red” afterimage, lagging behind the “green” signal by a half-cycle.
Figure 3.
The diagrams show a simple model for prediction of colored afterimages based on cellular receptor adaptation (18, 19). A shows in green and red the stimulus input to the M-cone (middle wavelength-sensitive), and L-cone (long wavelength-sensitive) receptors, respectively. The signal generated in M-cones is increased and that in L-cones is decreased, as imposed by the d-isoluminant constraints. The model is based on the predicted changes in mean response level of M- and L-cone receptors during modulation: an increase in mean signal response for L-cones and a corresponding decrease in M-cone responses, as shown in B. The output of a red-green chromatic channel based on the difference between L- and M-cone signals is shown in C. The assumptions made are sufficient to predict the observed afterimages and the half-cycle increase in afterimage response latency (as shown in C). When the modulation terminates and the stimulus returns to background level, the normal response properties are restored with a time constant characteristic of build-up and decay of afterimages. The model also predicts that the signal level in the luminance channel that is based on summation of L- and M-signals remains unaffected during the modulation. This explains the absence of perceived achromatic afterimages when adapting to isoluminant stimuli (27).
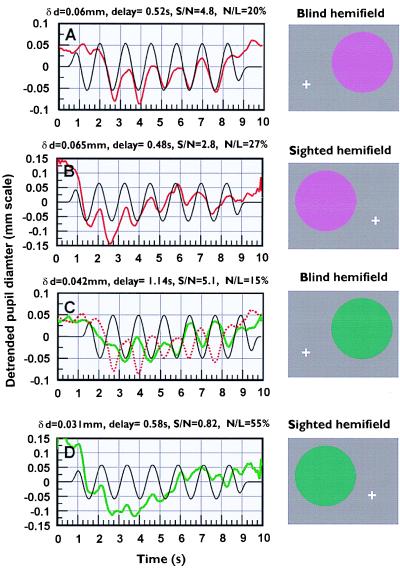
To confirm that the pupil response triggered by the offset of the “green” stimulus is indeed related to its aftereffect, and to examine the prediction of a half-cycle lag, an experiment was carried out by using sinusoidally modulated series of green or red isoluminant stimuli that were also isoluminant for rods (see Insets to Fig. 4). The latency of response with respect to the stimulus was computed from the phase lag of the fundamental frequency component of the pupil response. The pupillary responses to “green” modulation in the blind hemifield of subject G show a clear response at the modulation frequency, but with a time delay of 1.14 s, in contrast to the delay for the “red” stimulus of 0.52 s. The time delay for “green” is precisely half a cycle (i.e., 0.625 s) longer (Fig. 4C) than the latency measured for the modulated “red” series (i.e., 0.625 + 0.52 = 1.14 s). The absence of a pupil response to “green” modulation in the blind hemifields is of great advantage because it helps to reveal a clear pupil response to the red afterimage. This is evident in Fig. 4D, which shows the much reduced pupillary modulation and the strong harmonic at twice the modulation frequency caused by pupil responses to both the green stimulus and its red afterimage, when the stimulus is presented in the sighted hemifield. The strong harmonic at twice the modulation frequency observed in the sighted hemifield is consistent with combined pupil response to both the green component and the corresponding red afterimage. The results suggest that, when the chromatic saturation of the stimulus is modulated sinusoidally toward the green region of the spectrum locus in the blind hemifield, the pupil responds only to the red afterimage.
Figure 4.
Pupil responses to sinusoidal modulation of the same “red” and “green” stimuli presented to either the blind or the sighted hemifields in subject G as shown by the Panels on the right. The luminance of the background field was 24 cd/m2 and (u′, v′)-chromaticity coordinates 0.179, 0.467. The luminance of the background field remained unchanged throughout, and the colored stimuli were again d-isoluminant. The chromatic saturation of each stimulus was modulated sinusoidally at a frequency of 0.8 Hz. Each stimulus consisted of eight cycles of modulation with maximum chromatic saturation amplitude of 0.071 unit. The stimuli were interleaved, and 16 traces were averaged for each stimulus condition. The pupil modulation amplitude, δd, and phase shift at the modulation frequency, together with a measure of signal/noise ratio (S/N) and response nonlinearity (N/L) were then computed from the discrete Fourier transform of each averaged trace. These parameters, the measured, detrended pupil trace, and the corresponding reference waveform (phase-shifted to match the phase of the 0.8 Hz frequency component) are shown in each section. The results show that the pupil responds well to chromatic modulation toward the long-wavelength region of the spectrum locus for stimulus locations both in the sighted and the blind hemifields. Pupil responses to the green stimulus imaged in the sighted hemifield are heavily contaminated by a harmonic component at twice the modulation frequency that produces 55% nonlinearity (D). The dotted red trace in C shows the blind hemifield response to “red” modulation taken from A. Comparison of the two traces confirms the 180° phase shift between red and green responses as predicted by the afterimage model shown in Fig. 3C.
DISCUSSION
The results demonstrate that selective chromatic signals and their aftereffects can be detected in the pupil response. They can be measured in the “blind” hemifield of subjects with V1 damage, even though they report no awareness of a green stimulus or its red aftereffect, a striking example of residual function in “blindsight.” They demonstrate that pupillary control mechanisms can be modulated by neural activity that survives the destruction of V1. As pathways exist that allow visual information to reach all of the remaining visual association areas (such as V2 to V5 and IT) (20–22), it is possible that these cortical areas modulate midbrain pupillary structures in a “downstream” manner. Normal color aftereffects generated monocularly do not transfer to the other eye, and this is in agreement with the receptor adaptation model for afterimage generation proposed here. One cannot, however, exclude the possibility that the midbrain pupillary neural control nuclei may be subject to modulation not only by a direct retinal input, but also by a downstream effect from cortical areas, especially as the pupillary response to luminance increments and to isoluminant gratings is definitely altered by V1 lesions in human subjects (5, 23). The observed effects are likely to have passed unnoticed in experiments restricted to normal subjects who show sensitivity for pupil responses for both colors. Interestingly, however, both normal subjects and the hemianopes show greater pupil responses to red than to green stimuli, even when these are matched for chromatic saturation, for reasons that are not clearly understood at present (24–26).
The question of whether a cortical effect is essential to generate the observed stimulus specific pupil responses could be settled by studying such chromatic aftereffects in hemispherectomized subjects, but this remains to be done.
Acknowledgments
This research was supported by grants from the Medical Research Council of Great Britain and the Wellcome Trust.
References
- 1.Barbur J L, Forsyth P M. Clin Vision Sci. 1986;1:107–111. [Google Scholar]
- 2.Barbur J L, Harlow A J, Sahraie A. Ophthalmic Physiol Opt. 1992;12:137–141. doi: 10.1111/j.1475-1313.1992.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeki S. A Vision of the Brain. Oxford: Blackwell Scientific; 1993. [Google Scholar]
- 4.Barbur J L. J Psychophysiol. 1991;5:259–263. [Google Scholar]
- 5.Weiskrantz L, Cowey A, LeMare C. Brain. 1998;121:1065–1072. doi: 10.1093/brain/121.6.1065. [DOI] [PubMed] [Google Scholar]
- 6.Weiskrantz L, Cowey A, Barbur J L. Brain. 1999;122:1533–1538. doi: 10.1093/brain/122.8.1533. [DOI] [PubMed] [Google Scholar]
- 7.Brent P J, Kennard C, Ruddock K H. Proc R Soc Lond Ser B. 1994;256:219–225. doi: 10.1098/rspb.1994.0073. [DOI] [PubMed] [Google Scholar]
- 8.Barbur J L, Sahraie A, Simmons A, Weiskrantz L, Williams S C R. Vision Res. 1998;38:3447–3453. [PubMed] [Google Scholar]
- 9.Young R, Teller D. J Opt Soc Am. 1991;8:2048–2052. doi: 10.1364/josaa.8.002048. [DOI] [PubMed] [Google Scholar]
- 10.Barbur J L, Forsyth P M, Findlay J M. Brain. 1988;111:63–82. doi: 10.1093/brain/111.1.63. [DOI] [PubMed] [Google Scholar]
- 11.Barbur J L, Watson J D G, Frackowiak R S J, Zeki S. Brain. 1993;116:1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- 12.Sahraie A, Weiskrantz L, Barbur J L, Simmons A, Williams S C R, Brammer M J. Proc Natl Acad Sci USA. 1997;94:9406–9411. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbur J L, Ruddock K H, Waterfield V A. Brain. 1980;103:905–928. doi: 10.1093/brain/103.4.905. [DOI] [PubMed] [Google Scholar]
- 14.Blythe I M, Kennard C, Ruddock K H. Brain. 1987;110:887–905. doi: 10.1093/brain/110.4.887. [DOI] [PubMed] [Google Scholar]
- 15.Alexandridis E, Leendertz J A, Barbur J L. J Psychophysiol. 1991;5:223–239. [Google Scholar]
- 16.Lynn P. An Introduction to the Analysis and Processing of Signals. London: Macmillan; 1982. [Google Scholar]
- 17.Lennie P, Pokorny J, Smith V C. J Opt Soc Am A. 1993;10:1283–1293. doi: 10.1364/josaa.10.001283. [DOI] [PubMed] [Google Scholar]
- 18.Normann R A, Perlman I. J Physiol (London) 1979;286:491–507. doi: 10.1113/jphysiol.1979.sp012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valeton M J, van Norren D. Vision Res. 1983;23:1539–1547. doi: 10.1016/0042-6989(83)90167-0. [DOI] [PubMed] [Google Scholar]
- 20.Shipp S, Zeki S. Nature (London) 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- 21.Van Essen D C, Newsome W T, Maunsell J H R, Bixby J L. J Comp Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura H, Gattass R, Desimone R, Ungerleider L G. J Neurosci. 1993;13:3681–3691. doi: 10.1523/JNEUROSCI.13-09-03681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbur J L. In: Basic and Clinical Perspectives in Vision Research. Robbins J G, Djamgoz M B A, Taylor A, editors. New York: Plenum; 1995. pp. 3–18. [Google Scholar]
- 24.Kadoya S, Wolin L R, Massopust L C. Brain Res. 1971;32:251–254. doi: 10.1016/0006-8993(71)90175-2. [DOI] [PubMed] [Google Scholar]
- 25.Felsten G, Benevento L A, Burman D. Brain Res. 1983;288:363–367. doi: 10.1016/0006-8993(83)90119-1. [DOI] [PubMed] [Google Scholar]
- 26.Roorda A, Williams D R. Nature (London) 1999;397:520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- 27.Kelly D H, Martinez-Uriegas E. J Opt Soc Am A. 1993;10:29–37. doi: 10.1364/josaa.10.000029. [DOI] [PubMed] [Google Scholar]