Abstract
The aim was to study in vitro regulation of the IL-5 receptor α (IL-5Rα) on purified peripheral blood eosinophils from healthy subjects. The IL-5Rα was down-regulated, in a dose-dependent manner, by recombinant IL-5 and GM-CSF, with IL-5 being most potent. This down-regulation was not induced by autocrine release of GM-CSF or IL-5, respectively. Incubation of eosinophils with cell-free peritoneal dialysis fluid (PF) collected from a patient with peritoneal fluid eosinophilia (PFE), induced up-regulation of the proportion of CD69 positive eosinophils, in parallel with down-regulation of the proportion of IL-5Rα positive eosinophils. Experiments with neutralizing antibodies against IL-5 and GM-CSF, revealed that IL-5 was the principal cytokine responsible for the down-regulation of the IL-5Rα. When eosinophils were incubated with PF collected from the same patient in remission or with PF collected from a newly started patient or a patient with bacterial peritonitis, less down-regulation of the IL-5Rα was observed.
In conclusion our data indicate that IL-5, as opposed to its proposed action on eosinophil progenitors, down-regulates the IL-5Rα chain on mature eosinophils. We therefore suggest that an IL-5 driven inflammation generates an eosinophil tissue phenotype that is characterized by a low IL-5Rα expression. These aspects of IL-5 action on IL-5Rα expression could gain new insights into the mechanisms of specific immuno-modulatory therapies, such as anti-IL-5.
Keywords: CD69, eosinophils, GM-CSF, IL-5, IL-5 receptor alpha
INTRODUCTION
The eosinophil is regarded as an important effector cell in allergic inflammatory responses such as asthma [1], and an increased number of activated eosinophils has been observed in peripheral blood [2–5] and airways [1,6–9] during allergic responses. Moreover, the eosinophil has been implicated to be involved in other eosinophilic disorders like parasitic infection [10], hypereosinophilic syndrome [11], peritoneal fluid eosinophilia [12] and nasal polyposis [13]. In addition, data indicate that eosinophils might exert cytotoxic action on cancer cells [14].
Eosinophils exert their effect through the release of cytotoxic basic granule proteins like eosinophil cationic protein (ECP), major basic protein (MBP) and other eosinophil-derived products [15]. These inflammatory products are believed to contribute to the pathophysiology of asthma [6,16].
Interleukin (IL)-5 and granulocyte/macrophage-colony stimulating factor (GM-CSF) are haematopoietic growth factors essential for eosinophil development, activation and survival. Interleukin-5 acts specifically on eosinophils, while GM-CSF acts on many different cell types [17]. Increased levels of both cytokines have been detected in sera, bronchoalveolar lavage (BAL) fluid and lung biopsies from asthmatic individuals [18–21]. In guinea pigs, an intravenous injection of recombinant IL-5 resulted in rapid blood eosinophilia [22] and the level of IL-5 have been shown to significantly correlate to the number of eosinophils detected in the airways of asthmatics [20]. Furthermore, experiments with gene deletion and blocking antibodies have demonstrated IL-5's role to attenuate eosinophilic responses in different models of allergic inflammation [23,24]. Together these results suggest that IL-5 is a major determinant of eosinophilic inflammation.
The biological signal from IL-5 is mediated through a receptor consisting of a specific IL-5-binding α-chain, and a signal-transducing common β-chain, which is shared with the receptors for IL-3 and GM-CSF [25,26]. When the β-chain associates with the specific IL-5-binding α-chain, a high affinity complex is formed [27]. The IL-5 receptor α-subunit (IL-5Rα) can appear in either a membrane-bound active form or a soluble antagonistic form. The various expressions of these forms are regulated at the transcriptional level by alternative splicing [28].
Previous studies have demonstrated that IL-5 specifically up-regulates the expression of the transmembrane IL-5Rα on CD34+ cord blood cells [29]. Moreover, there is an increase in the number of CD34+/IL-5Rα+ cells in the airways of asthmatics as well as in the bone marrow after allergen challenge of asthmatic subjects [30,31]. These results indicate that IL-5 could act early in eosinophil differentiation by up-regulating its own receptor on progenitors. However, the regulation of the transmembrane IL-5Rα on mature peripheral blood eosinophils is poorly studied. We have previously demonstrated that the transmembrane IL-5Rα is down-regulated on eosinophils in peritoneal dialysis fluid, as compared to blood eosinophils, from patients with peritoneal fluid eosinophilia [32], which indicates that the receptor on mature eosinophils can be dynamically regulated at the extravascular site.
Taken this observation into account, we sought to study the regulation of IL-5Rα on peripheral blood eosinophils from healthy individuals upon in vitro stimulation with relevant cytokines. We analysed the impact of the haematopoietic growth factors IL-5 and GM-CSF as well as peritoneal fluid from the site of eosinophilic inflammation, representing an eosinophilic promoting milieu.
MATERIALS AND METHODS
Preparation of peripheral blood eosinophils
Peripheral blood from healthy blood donors (age 18–64 years) was collected in tubes containing citrate (Vacutainer, 5 ml, with 0·5 ml 0·129 m 9NC, Becton Dickinson, San Jose, CA, USA). Eosinophils were purified by the magnetic cell separation system MidiMacs (Miltenyi Biotec, Bergisch Gladbach, Germany) [33]. Briefly, citrate blood was layered onto Percoll solution (Pharmacia-Upjohn, Uppsala, Sweden) and centrifuged (30 min, 1000 × g, 20°C). The mononuclear cells layer was removed and the remaining cell suspension was haemolysed in isotonic lysing solution (154 mm NH4Cl, 10 mm KHCO3, 0·1 mm EDTA, pH 7·2). Neutrophils and eosinophils were washed in PBS and anti-CD16 magnetic beads were added for 20 min at +4°C. The eosinophils were obtained by negative selection by using a separation column in a magnetic field where magnetically labelled cells (CD16+ neutrophils) were trapped and unlabelled cells (eosinophils) were collected.
This study was approved by the local ethics committee.
Preparation of peritoneal dialysis fluids (PF)
Newly drained effluents from night bags (Standard double bag system) were collected from three patients (Patient A-C, Table 1) treated with continuous ambulatory peritoneal dialysis (CAPD). Patient A had peritoneal fluid eosinophilia (PFE) and was referred with cloudy effluent and a negative bacterial culture but without abdominal pain. Effluents were collected both during disease and after remission of the disease. Patient B was a newly started CAPD-patient with clear PF. Patient C had bacterial peritonitis and appeared with cloudy dialysate and a positive bacterial culture but without abdominal pain. The fluids were centrifugated and supernatants were stored at − 70°C. The leukocyte counts were analysed by flow cytometry. The IL-5, IL-8, GM-CSF and eotaxin levels were measured with Quantikine human immunoassays (R & D Systems Inc, Minneapolis, MN, USA). Levels of ECP were measured with Pharmacia ECP CAP FEIA System (Pharmacia & Upjohn, Uppsala, Sweden). Leukocyte counts and soluble factors in PF are presented in Table 2 and have partly been reported previously [32].
Table 1.
Patient characteristics
| Patient | Age | Sex | Time on CAPD | Disease | Bag/manufacturer* |
|---|---|---|---|---|---|
| A | 52 | Female | 10 days | Diabetes mellitus | Dianeal,Physioneal/Baxter |
| B | 70 | Male | <3 days | Nephrosclerosis | GambrosolBio/Gambro |
| C | 56 | Male | 10 months | Renal obstruction | Lockolys/Fresenius |
Baxter, Deerfield, IL, USA; Gambro, Lund, Sweden; Fresenius, Bad Homburg, Germany.
Table 2.
Cell counts and soluble factors in dialysis fluids from CAPD-patients
| Counts | Soluble factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Leukocytes (count ×103/ml) | Eosinophils % (count ×103/ml) | Lymphocytes % (count ×103/ml) | Monocytes % (count ×103/ml) | Neutrophils % (count ×103/ml) | ECP (µg/l) | IL-5 (pg/ml) | Eotaxin (pg/ml) | IL-8 (pg/ml) | GM-CSF (pg/ml) |
| A | 4500 | 44·8 (2016) | 13·0 (585) | 12·9 (581) | 28·4 (1278) | 314 | 454 | 996 | 52 | 49 |
| A* | 30 | 17·7 (5) | 28·8 (9) | 57·9 (17) | 0·0 (0) | 2·2 | 27 | 260 | <10 | <2·8 |
| B | 31 | 2·9 (1) | 11·8 (4) | 82·5 (26) | 2·8 (1) | <2·0 | <3·0 | 37 | <10 | 4·3 |
| C | 1080 | 2·2 (24) | 19·0 (205) | 14·2 (153) | 63·9 (690) | 2·1 | <3·0 | 501 | 47·1 | 13·9 |
ECP, Eosinophil Cationic Protein
Patient A after remission of disease.
In vitro incubation of purified eosinophils with recombinant IL-5, GM-CSF and peritoneal dialysis fluids
Purified eosinophils (0·4 × 106/ml) were incubated in recombinant human (rh) IL-5 (0·1–1000 ng/ml) (Immunokontact, Frankfurt, Germany) and/or rhGM-CSF (0·2–2000 ng/ml) diluted in HEPES (10 mm)-buffered RPMI 1640 medium (Gibco, Paisley, UK) supplemented with 10% heat-inactivated fetal calf serum (RPMI). In another assay eosinophils were resuspended in 500 µl undiluted PF from CAPD-patients (see above). As control, eosinophils were incubated in RPMI alone. The suspensions were incubated in 24-well plates for 120 min at +37°C in 5% CO2, supernatants were removed and the cells were washed twice in PBS (300 × g for 5 min at +4°C). In a time-related study eosinophils were incubated in rhIL-5 (10 ng/ml) or RPMI alone for 0–120 min at +37°C in 5% CO2, and then washed twice in PBS.
Inhibition of cytokines with neutralizing antibodies
Peritoneal dialysis fluid, rhIL-5 (10 ng/ml), rhGM-CSF (2 ng/ml) or RPMI (500 µl) were preincubated with the neutralizing antibodies anti-IL-5 (5 or 10 µg/ml) (R & D Systems), anti-GM-CSF (5 or 10 µg/ml) (R & D Systems) or the IgG1 isotypic control (5 or 10 µg/ml) (DAKO A/S, Glostrup, Denmark), for 60 min at +37°C, whereafter the eosinophils were added (0·4 × 106/ml). When both antibodies were used in PF (5 µg/ml each), the isotypic control antibody was used at 10 µg/ml. Eosinophils were subsequently cultured for 120 min at +37°C, resuspended and washed in PBS.
Immunofluorescence staining of eosinophils and flow cytometry
Surface antigens were incubated with nonconjugated monoclonal antibodies (mAb) to IL-5Rα (10 µg/ml, Clone: α16, non-neutralizing) [29] (Kind gift from Prof Jan Tavernier, University of Ghent, Belgium), fluoroscein isothiocyanate (FITC)-conjugated mAb to CD69 (5 µg/ml, Clone: L78) (Becton Dickinson, Meylan-Cedex, France) for 30 min at +4°C, and then washed in PBS. Secondary immunostaining was performed with FITC-conjugated rabbit-anti-mouse immunoglobulin, F(ab′)2 (50 µg/ml, Code: F313) (DAKO A/S), for 30 min at +4°C and washed with PBS. The nonspecific binding was determined with isotype-matched control antibodies in corresponding concentrations. Cells were finally diluted in 0·5 ml PBS and a minimum of 2000 eosinophils was analysed in an EPICS XL-MCL (Beckman Coulter Inc., Fullerton, CA, USA) flow cytometer. The flow cytometer was calibrated daily with Flow Check and Flow Set (Beckman Coulter).
Statistics
Non-parametric methods were used; data were analysed by one-way anova, followed by Wilcoxon paired within groups. P < 0·05 were considered significant. Results are presented as medians (interquartile ranges) and mean ± SD.
RESULTS
Dose-response curves of IL-5Rα expression
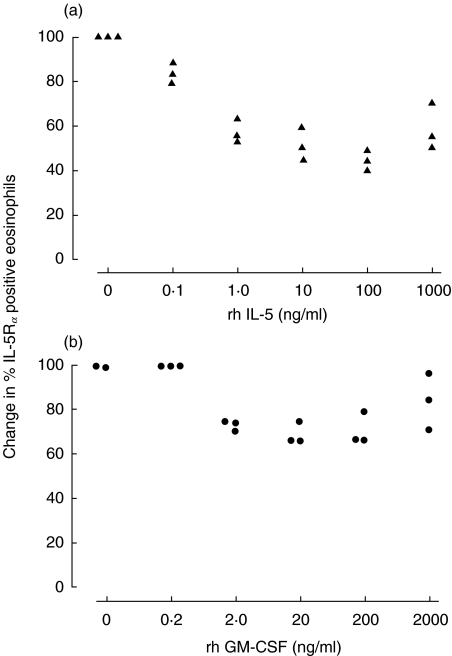
The proportion of IL-5Rα positive eosinophils was down-regulated in a dose-dependent manner by rhIL-5 (Fig. 1a) and GM-CSF (Fig. 1b) when incubated for 120 min at +37°C in 5% CO2 (n = 3). For the following experiments 10 ng/ml of rhIL-5 and 2 ng/ml of rhGM-CSF were selected.
Fig. 1.
(a) Dose–response curve (n = 3). Purified peripheral blood eosinophils (0·4 × 106/ml) from healthy subjects were incubated with rhIL-5 (0–1000 ng/ml) for 120 min at +37°C. Results are presented as change in the proportion of IL-5Rα positive eosinophils as compared to the RPMI control. (b) Dose–response curve (n = 3). Purified peripheral blood eosinophils (0·4 × 106/ml) from healthy subjects were incubated with rhGM-CSF (0–2000 ng/ml) for 120 min at +37°C. Results are presented as change in the proportion of IL-5Rα positive eosinophils as compared to the RPMI control.
Time-related expression of IL-5Rα after incubation with rhIL-5
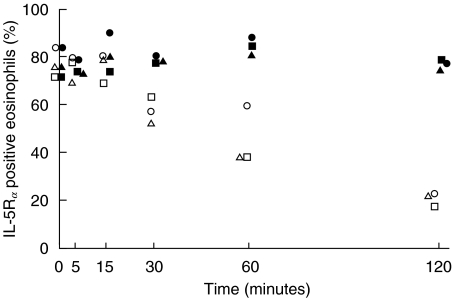
The kinetics of IL-5-induced down-regulation of IL-5Rα positive eosinophils was analysed after incubation of eosinophils with rhIL-5 or RPMI alone for 0, 5, 15, 30, 60 and 120 min (n = 3). RhIL-5 induced a down-regulation of the IL-5Rα at 30 min, which was further pronounced at 60 and 120 min (Fig. 2). Incubation of eosinophils with RPMI for different time points did not effect the IL-5Rα expression.
Fig. 2.
Purified peripheral blood eosinophils (0·4 × 106/ml) from healthy subjects (n = 3) were incubated with rhIL-5 (10 ng/ml) (○,□,▵) or RPMI (•,▪,▴) for 0–120 min at +37°C. Results are presented as the proportion of IL-5Rα positive eosinophils.
IL-5Rα and CD69 expression after incubation with rhIL-5 and GM-CSF
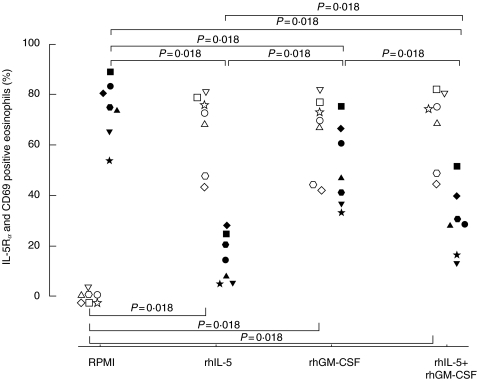
When purified eosinophils from seven healthy subjects were incubated with rhIL-5, rhGM-CSF or the combination of both for 120 min, the proportion of IL-5Rα positive eosinophils was significantly (P = 0·018 for all) down-regulated (14·2%, range 5·2–24·8; 46·7%, 36·3–66·6; 28·8%, 15·8–39·8; respectively) as compared to the RPMI control (74·8%, range 65·3–82·9) (Fig. 3). The proportion of CD69 positive eosinophils was significantly (P = 0·018 for all) up-regulated (72·4%, range 47·7–78·5; 70·4%, 44·0–76·9; 74·2%, 48·3–80·7; respectively) as compared to the RPMI control (0%, range 0–0·2) (Fig. 3). The proportion of IL-5Rα and CD69 positive eosinophils obtained at +4°C, (79·2%, 69·6–87·8; 0·4%, 0–0·9; respectively) did not significantly differ from the values obtained after incubation with RPMI (see above).
Fig. 3.
Purified peripheral blood eosinophils (0·4 × 106/ml) from healthy subjects (n = 7) were incubated with RPMI, rhIL-5 (10 ng/ml), rhGM-CSF (2 ng/ml) or the combination of both cytokines for 120 min at +37°C. Results are presented as the proportion of IL-5Rα (filled symbols) and CD69 (open symbols) positive eosinophils.
Effect of addition of neutralizing antibodies to rhIL-5 and rhGM-CSF suspensions
To analyse whether the results from the incubation with rhIL-5 could be an effect of an autocrine production of GM-CSF and vice versa, neutralizing antibodies were added to the cell suspensions. However, anti-GM-CSF antibodies did not effect the IL-5-induced down-regulation of the IL-5Rα, 22·2% ± 1·1 versus 22·1% ± 1·2, and anti-IL-5 did not restore the GM-CSF-induced down-regulation of the IL-5Rα, 75·9% ± 4·3 versus 69·8% ± 6·7, as compared to RPMI, 87·3% ± 3·8 (n = 3).
IL-5Rα and CD69 expression after incubation with peritoneal dialysis fluids
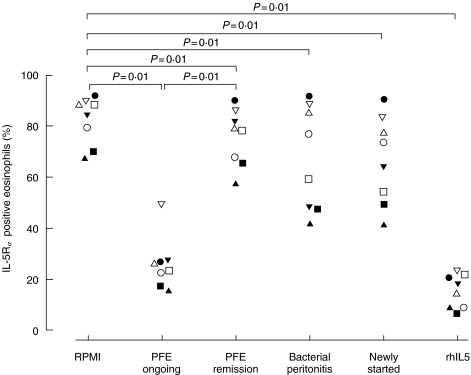
Eosinophils from eight healthy subjects were incubated with PF from CAPD-patients (Tables 1 and 2) for 120 min. The proportion of IL-5Rα positive eosinophils was significantly (P = 0·01) down-regulated by PF from the patient with PFE (patient A) both during disease, 24·5% (range 19·7–26·9), and after remission of the disease (patient A), 78·7% (66·8–84·1), when compared to RPMI, 86·6% (74·5–89·7) (Fig. 4). The PF collected during disease did however, induce a significantly (P = 0·01) lower IL-5Rα expression as compared to PF collected after remission. A small but significant (P = 0·01) down-regulation of the IL-5Rα was found when eosinophils were incubated with PF from the patient with bacterial peritonitis (patient C), 67·8% (47·9–87·3) as well as from the patient newly started on CAPD (patient B), 69·1% (52·0–80·4), as compared to RPMI (see above) (Fig. 4). Recombinant IL-5 was used as a positive control and down-regulated the receptor significantly (P = 0·01) to 16·1% (8·4–21·2).
Fig. 4.
Purified peripheral blood eosinophils (0·4 × 106/ml) from healthy subjects (n = 8) were incubated with PF from the CAPD-patient with PFE, both during and after remission of the disease, or PF from a bacterial peritonitis patient or a newly started patient, for 120 min at +37°C. RhIL-5 (10 ng/ml) was used as a control. Results are presented as the proportion of IL-5Rα positive eosinophils. Statistics are based on the comparison with the RPMI control and the comparison between during and after remission of PFE.
In three experiments, the proportion of CD69 positive eosinophils was up-regulated by PF from the patient with PFE (patient A) during disease, 70·5% ± 12·6, and after incubation with recombinant IL-5, 70·3% ± 10·7, when compared to RPMI where no CD69 positive eosinophils could be detected. In addition, no CD69 positive eosinophils was observed after incubation with PF from the patient with PFE after remission of the disease, from the newly started CAPD-patient, or the patient with bacterial peritonitis (all values < 4·5%).
Effect of addition of neutralizing antibodies to PF from the patient with PFE
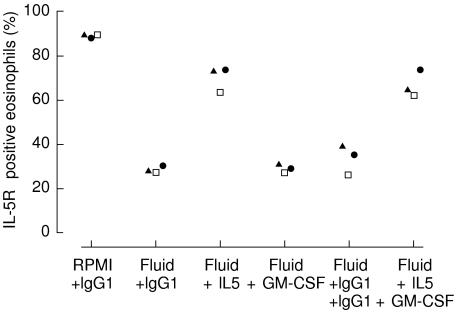
The PF-induced decrease in the proportion of IL-5Rα positive eosinophils, 28·3% ± 1·2, was almost abolished by the addition of anti-IL-5 antibodies, 70·1% ± 5·9, compared to RPMI, 89·1% ± 0·6, whereas no change was observed by the addition of anti-GM-CSF antibodies, 28·9% ± 1·6 (n = 3) (Fig. 5). When both anti-IL-5 and anti-GM-CSF antibodies were added, 67·2% ± 6·0, the inhibition was as marked as when only anti-IL-5 was added (n = 3) (Fig. 5).
Fig. 5.
RPMI or PF (500 µl) from the patient with PFE was preincubated with anti-IL-5 (5 µg/ml), anti-GM-CSF (5 µg/ml) or the IgG1 isotypic control (5 µg/ml), for 60 min at +37°C. Thereafter purified peripheral blood eosinophils (0·4 × 106/ml) from healthy subjects (n = 3) were added. When both antibodies were used in PF (5 µg/ml each), the isotypic control antibody was used at 10 µg/ml. Eosinophils were subsequently cultured for 120 min. Results are presented as the proportion of IL-5Rα positive eosinophils.
DISCUSSION
The main finding in the present study is that IL-5 and GM-CSF down-regulate the membrane-bound IL-5Rα on peripheral blood eosinophils from healthy individuals. In addition, extravascular fluid from a pro-eosinophilic milieu has the potential to down-regulate the IL-5Rα, and the effect is mediated through the action of IL-5. The kinetics for IL-5-induced down-regulation of IL-5Rα is rapid, beginning within 30 min and reaches its lowest expression at 120 min.
Interleukin-5 is the main actor in the activation and survival of mature eosinophils and its action is mediated through binding to the IL-5Rα[25,26]. Previous data suggest that there is an increase in the IL-5Rα expression on bone marrow-derived CD34+ cells from asthmatic subjects [31] and that IL-5 is able to up-regulate its own receptor on progenitors [29]. However, the mechanisms involved in the regulation of the IL-5Rα on mature eosinophils and the potential role of IL-5 in this respect, are poorly known.
In this study the membrane-bound IL-5Rα was expressed on the majority of isolated peripheral blood eosinophils from healthy subjects. In vitro exposure to recombinant IL-5 for 120 min induced an extensive dose-dependent down-regulation of IL-5Rα positive eosinophils. Recombinant GM-CSF down-regulated the IL-5Rα in a dose-dependent manner but to a less extent than recombinant IL-5. The IL-5-induced down-regulation of the IL-5Rα was initiated within 30 min and continued gradually for up to 120 min. These results are supported by Wang et al. [34] who demonstrated that the membrane-bound IL-5Rα mRNA is down-regulated by IL-5 and GM-CSF after incubation for four hours. The combination of IL-5 and GM-CSF induced a significant less down-regulation of the IL-5Rα than IL-5 alone and a plausible explanation is a competitive inhibition of the beta-chain which is shared between the IL-5, IL-3 and GM-CSF receptors [25–27]. In contrast to these results, the proportion of CD69 positive eosinophils, which was used as a marker for eosinophil activation [35], was significantly up-regulated to the same level by IL-5, GM-CSF as well as the combination of both cytokines. Our interpretation of data is that different concentrations of IL-5 are needed to achieve CD69 up-regulation and IL-5Rα down-regulation, respectively. When both IL-5 and GM-CSF are added the amount of IL-5 engaging β-chains is sufficient for maximal up-regulation of CD69, but not for maximal down-regulation of IL-5Rα. We therefor suggest that activation of eosinophils, judged by CD69 up-regulation, is not mandatory linked to IL-5Rα down-regulation.
Since both GM-CSF and IL-5 can be produced by eosinophils [36,37], we tested the hypothesis that IL-5 and GM-CSF-induced IL-5Rα down-regulation was mediated through autocrine action of released GM-CSF and IL-5, respectively. However, our results do not support this mode of action, and data point towards a direct effect of IL-5 and GM-CSF on IL-5Rα expression.
To further extend our in vitro observations, we addressed the question whether a pro-eosinophilic milieu has the propensity to generate an IL-5Rα-low phenotype. Indeed, an IL-5Rα-low/CD69-high phenotype was generated when eosinophils were incubated with cell-free peritoneal dialysis fluid from a CAPD-patient with on-going peritoneal fluid eosinophilia. Our data also indicate that IL-5 in the fluid was the principal cytokine responsible for this effect. These data support our results using recombinant cytokines and are also in concordance with our previous data showing down-regulated IL-5Rα expression on peritoneal eosinophils from patients with peritoneal fluid eosinophilia [32]. However, since dialysis fluids from CAPD-patients with no peritoneal fluid eosinophilia had low levels of IL-5 and GM-CSF but still induced a down-regulation of the IL-5Rα on eosinophils, we can not exclude the possibility that other unidentified factors in the fluid act on IL-5Rα-expression. The existence of tissue eosinophils with an IL-5Rα-low phenotype is supported by data from a murine model, which demonstrated that BAL eosinophils stained negative for IL-5Rα[38].
The potential physiological consequence of a down-regulated IL-5Rα on extravasated eosinophils is not fully delineated. We raise the hypothesis that a down-regulated IL-5Rα implies a reduced responsiveness to IL-5 in tissues. The rational for this hypothesis is based on our notion that eosinophils with a down-regulated IL-5Rα have a reduced in vitro responsiveness to recombinant IL-5 [39]. This, however, does not exclude other stimuli like eotaxin and GM-CSF to exert action on tissue-dwelling eosinophils.
In conclusion our data indicate that IL-5, as opposed to its proposed action on eosinophil progenitors, down-regulates the IL-5Rα chain on mature eosinophils. In addition, we suggest that tissue-dwelling eosinophils have a weaker expression of the IL-5Rα, as compared to blood eosinophils, rendering the eosinophils a reduced responsiveness towards IL-5. Collectively, these data suggest that the net outcome of IL-5 on IL-5Rα expression is critically dependent on maturation stage as well as localization of eosinophils and their progenitors. These aspects of IL-5 action on IL-5Rα expression have to be taken into account, when evaluating the effects of specific anti-eosinophilic therapies, such as anti-IL-5.
Acknowledgments
We wish to thank Professor Jan Tavernier for the kind gift of the IL-5Rα monoclonal antibody. This study was supported by the Swedish Asthma and Allergy Association, the Swedish Foundation for Health Care Sciences and Allergy Research, The Hesselman Foundation, the Swedish Society of Medicine, and the Karolinska Institute. The laboratory equipment was funded by the Tornspiran Foundation.
References
- 1.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 2.Griffin E, Håkansson L, Formgren H, Jörgensen K, Peterson C, Venge P. Blood eosinophil number and activity in relation to lung function in patients with asthma and with eosinophilia. J Allergy Clin Immunol. 1991;87:548–57. doi: 10.1016/0091-6749(91)90014-f. [DOI] [PubMed] [Google Scholar]
- 3.Janson C, Herala M. Blood eosinophil count as risk factor for relapse in acute asthma. Respir Med. 1992;86:101–4. doi: 10.1016/s0954-6111(06)80223-4. [DOI] [PubMed] [Google Scholar]
- 4.Halldén G, Hellman C, Grönneberg R, Lundahl J. Increased levels of IL-5 positive peripheral blood eosinophils and lymphocytes in mild asthmatics after allergen inhalation provocation. Clin Exp Allergy. 1999;29:595–603. doi: 10.1046/j.1365-2222.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman B, Lanner A, Enander I, Zimmerman RS, Peterson CGB, Ahlstedt S. Total blood eosinophils, serum eosinophil cationic protein and eosinophil protein X in childhood asthma: relation to disease status and therapy. Clin Exp Allergy. 1993;23:564–70. doi: 10.1111/j.1365-2222.1993.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 6.Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137:62–9. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 7.Warner JO, Marguet C, Rao R, Roche WR, Pohunek P. Inflammatory mechanisms in childhood asthma. Clin Exp Allergy. 1998;28:71–5. doi: 10.1046/j.1365-2222.1998.028s5071.x. [DOI] [PubMed] [Google Scholar]
- 8.Kirby J, Hargreave F, Gleich G, O'Byrne P. Bronchoalveolar cell profiles of asthmatic and nonasthmatic subjects. Am Rev Respir Dis. 1987;136:379–83. doi: 10.1164/ajrccm/136.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Hargreave FE, Popov T, Kidney J, Dolovich J. Sputum measurements to assess airway inflammation in asthma. Allergy. 1993;48:81–3. doi: 10.1111/j.1398-9995.1993.tb04705.x. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth AE. Cell mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- 11.Liesveld JL, Abboud CN. State of the art; the hypereosinophilic syndromes. Blood Rev. 1991;5:29–37. doi: 10.1016/0268-960x(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Ejaz AA, Fitzpatrick PM, Durkin AJ, Wasiluk A, Haley WE, Goalen MJ, Ing TS, Zachariah PK. Pathophysiology of peritoneal fluid eosinophilia in peritoneal dialysis patients. Nephron. 1999;81:125–30. doi: 10.1159/000045266. [DOI] [PubMed] [Google Scholar]
- 13.Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–90. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- 14.Lowe D, Jorizzo J, Hutt MSR. Tumour associated eosinophilia, a review. J Clin Path. 1981;34:1343–8. doi: 10.1136/jcp.34.12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wardlaw AJ. Eosinophils in the 1990s. new perspectives on their role in health and disease. Postgrad Med J. 1994;70:536–52. doi: 10.1136/pgmj.70.826.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venge P. Epithelial injury by human eosinophils. Am Rev Respir Dis. 1988;138:54–7. doi: 10.1164/ajrccm/138.6_Pt_2.S54. [DOI] [PubMed] [Google Scholar]
- 17.Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–57. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker C, Virchow CJ, Bruijnzeel PLB, Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991;146:1829–35. [PubMed] [Google Scholar]
- 19.Qymar K, Elsayed S, Bjerknes R. Serum eosinophil cationic protein and interleukin-5 in children with bronchial asthma and acute bronchiolitis. Pediatr Allergy Immunol. 1996;7:180–6. doi: 10.1111/j.1399-3038.1996.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 20.Bentley AM, Meng Q, Robinson DS, Hamid Q, Kay AB, Durham SR. Increases in activated lymphocytes, eosinophils and cytokine mRNA expression for interleukin-5 and granulocyte/macrophages colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmatics. Am J Respir Cell Mol Biol. 1993;8:35–42. doi: 10.1165/ajrcmb/8.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocytes population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 22.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–8. [PubMed] [Google Scholar]
- 23.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan RW, Athwahl D, Chou CC, et al. Inhibition of pulmonary eosinophilia and hyperreactivity by antibodies to interleukin-5. Int Arch Allergy Immunol. 1995;107:321–2. doi: 10.1159/000237014. [DOI] [PubMed] [Google Scholar]
- 25.Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991;66:1175–84. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 26.Lopez AF, Elliott MJ, Woodcock J, Vadas MA. GM-CSF, IL-3 and IL-5: cross competition on human hematopoietic cells. Immunol Today. 1992;13:495–500. doi: 10.1016/0167-5699(92)90025-3. [DOI] [PubMed] [Google Scholar]
- 27.Miyajima A, Kitamura T, Harada N, Yokota T, Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- 28.Tavernier J, Tuypens T, Plaetinck G, Verhee A, Fiers W, Devos R. Molecular basis of the membrane-anchored and two soluble isoforms of the human interleukin 5 receptor alpha subunit. Proc Natl Acad Sci USA. 1992;89:7041–5. doi: 10.1073/pnas.89.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavernier J, Van der Heyden J, Verhee A, et al. Interleukin 5 regulates the isoform expression of its own receptor alpha-subunit. Blood. 2000;95:1600–7. [PubMed] [Google Scholar]
- 30.Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T, Kay AB, Hamid Q. CD34 (+) /interleukin-5Ralpha messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol. 1999;20:9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- 31.Sehmi R, Wood LJ, Watson R, Foley R, Hamid Q, O'Byrne PM, Denburg JA. Allergen-induced increases in IL-5 receptor alpha-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. J Clin Invest. 1997;100:2466–75. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellman C, Lundahl J, Hylander B, Halldén G. Phenotypic alterations of recruited eosinophils in ‘peritoneal fluid eosinophilia’. Perit Dial Int. 2000;20:295–300. [PubMed] [Google Scholar]
- 33.Hansel TT, De Vries IJ, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Meth. 1991;145:105–10. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Wu P, Cheewatrakoolpong B, Myers JG, Egan RW, Billah MM. Selective inhibition of IL-5 receptor alpha-chain gene transcription by IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor in human blood eosinophils. J Immunol. 1998;160:4427–32. [PubMed] [Google Scholar]
- 35.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology. 1993;80:281–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin-5 synthesis by eosinophils. association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–8. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kankaanranta H, Lindsay MA, Giembycz MA, Zhang X, Moilanen E, Barnes PJ. Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol. 2000;106:77–83. doi: 10.1067/mai.2000.107038. [DOI] [PubMed] [Google Scholar]
- 38.Tomaki M, Zhao LL, Lundahl J, Sjostrand M, Jordana M, Linden A, O'Byrne P, Lotvall J. Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL-5 and IL-5 receptor alpha. J Immunol. 2000;165:4040–50. doi: 10.4049/jimmunol.165.7.4040. [DOI] [PubMed] [Google Scholar]
- 39.Hellman C, Lönnkvist K, Hedlin G, Halldén G, Lundahl J. Down-regulated IL-5 receptor expression on peripheral blood eosinophils from budesonide-treated children with asthma. Allergy. 2002;57:323–8. doi: 10.1034/j.1398-9995.2002.1o3482.x. [DOI] [PubMed] [Google Scholar]