Abstract
The acquired loss of CR1 (CD35) on erythrocytes in specific autoimmune diseases and chronic infections may be due to autoAb against CR1. An ELISA using rCR1 was established to measure antiCR1 IgG autoAb. Plasma containing alloAb to polymorphism on CR1 (Knops blood group Ab) reacted strongly against rCR1 and were used as positive controls. AntiCR1 Ab was found in 3/90 (3·5%) plasma samples from healthy blood donors. The binding of these Ab was not inhibited by high salt concentrations. AntiCR1 Ab were present in the IgG fractions of plasma, and they bound to rCR1 on Western Blot. Affinity chromatography on rCR1-sepharose depleted the plasma of antiCR1, and the acid-eluted fractions contained the antiCR1 Ab. An increased frequency of antiCR1 autoAb was found in patients with SLE (36/78; 46%), liver cirrhosis (15/41; 36%), HIV infection (23/76; 30%) (all P < 0·0001), and in patients with anticardiolipin Ab (4/21; 19%, P < 0·01) multiple sclerosis (7/50; 14%, P < 0·02), and myeloma (autoAb (8/56; 14%, P < 0·02), but not in those with acute poststreptococcal glomerulonephritis (1:32; 3%). Because C1q binds to CR1, antiC1q Ab were analysed in the same patients. There was no correlation between levels of antiC1q and antiCR1 autoAb. In HIV patients, levels of antiCR1 did not correlate with low CR1 levels expressed on erythrocytes or soluble CR1 in plasma.
The binding of antiCR1 autoAb to rCR1 fixed on ELISA plates was not inhibited by soluble rCR1 or by human erythrocyte CR1, in contrast to alloAb and one SLE serum, which induced partial blockade. Thus, antiCR1 autoAb recognize mostly CR1 epitope(s) not present on the native molecule, suggesting that they are not directly involved in the loss of CR1. Rather antiCR1 autoAb might indicate a specific immune response to denatured CR1.
Keywords: antiCR1, auto-antibody, antiC1q, SLE, HIV
INTRODUCTION
Complement receptor 1 (CR1, CD35) was first described in 1953 by Nelson [1] as the immune adherence receptor of erythrocytes (E). Nelson showed that E bound complement opsonized bacteria and delivered them in an appropriate way to phagocytes. Over the years, the characteristics of CR1 have been defined. This rod-shaped glycoprotein of 210 kD has 2/3 binding sites for C3b and structurally comprises a series of short consensus repeats which have a high homology [2]. It is expressed on most circulating cells including E, monocytes, neutrophils, eosinophils, B lymphocytes, some T lymphocytes, as well as on glomerular epithelial cells [3–5]. Although the number of CR1 is low on E (100–1000), the unique distribution of the molecule in clusters allows a high avidity binding of opsonized immune complexes [6].
In vivo data over the last 15 years have established that CR1 is lost on E of patients with specific autoimmune diseases, in particular SLE, and also in chronic infections such as HIV, lepromatous leprosy and HCV [5,7–9]. Today the general hypothesis is that CR1 loss on E is acquired during the lifespan of E as indicated by genetic studies, experiments producing acute and high immune complex loads, and analysis of the loss of CR1 from reticulocytes to erythrocytes [10–12]. It is thought that CR1 is lost during downloading of ICs from E to fixed macrophages in liver and spleen [13].
However the loss of CR1 in vivo might be due to a different mechanism. Autoantibodies (autoAb) against CR1 have been described in 3 patients with SLE, one of whom had almost no CR1 on E during active disease [14,15]. In this latter patient the authors reported a disappearance of the autoAb concomitant with the reappearance of CR1 following treatment and remission. Whether the presence of autoAb against CR1 might be involved in the loss of CR1 in SLE and in chronic infections has not been studied [14].
In this study we have analysed for the presence of antiCR1 Ab in normal individuals, and in patients with SLE, other autoimmune diseases, HIV infection and some other diseases.
PATIENTS AND METHODS
Study population
The study population included the following; 90 healthy blood donors (BD), 6 blood donors containing alloAb to CR1 Knops blood group (plasma kindly provided by Dr Hustinx, BSD SRK Bern, Switzerland) [16,17], 78 SLE patients (satisfying the ARA criteria), 50 patients with multiple sclerosis (MS) patients, 21 patients with anticardiolipin Ab (aCL) but without 4 criteria for SLE, 32 patients with acute post-streptococcal glomerulonephritis (APGN) all exhibiting haematuria, proteinuria and low C3, 76 patients with HIV infection, 41 patients with liver cirrhosis Ci (alcoholic n= 21; HCV n= 14; others n= 6), 56 patients with myeloma, and 3 patients with factor I deficiency [18]. The aliquots of plasma of the healthy blood donors were obtained after informed consent and with the local ethical approval. All plasma samples were aliquoted and maintained at −80°C until tested.
ELISA for antiCR1 autoAb
ELISA wells (MaxisorpNunc Immuno plates, Life Technologies, Basel, Switzerland) were coated overnight with 1 µg or 10 µg/well of soluble recombinant complement receptor 1 (rCR1, a gift from Dr Ryan, AVANT Immunotherapeutics, Inc., Needham, MA, USA) in carbonate buffer, pH 9·6, at room temperature. After 4 washes of ELISA plates with PBS Tween 0·05%, 100 µl of plasma diluted at 1:50 in PBS- Tween 0·05% containing 1% BSA were added to wells and incubated for 1 h at 37°C. In some cases (specified in the Results sections), NaCl at final concentrations of 0·3, 0·5, or 1 m was included in the PBS-Tween-BSA buffer for the first incubation step with the plasma. Bound IgG was detected with biotinylated monoclonal mouse antihuman IgG (1:10000: Southern Biotechnology Associates). After washing the plates, 100 µl of peroxidase-conjugated streptavidin (1:3000: Jackson Immuno Research Laboratories Inc., West Grove, PA, USA) was added to each well and the incubation continued for 1 h at 37°C. After washing, 100 µl of substrate at a concentration of 0·4 mg/ml (orthophenyldiamine, in 50 ml citrate-phosphate buffer pH 5 plus 20 µl of H2O2) was added to each well. The reaction was stopped with H2SO4 and the absorbance was measured with a microplate reader (MR 600, Dynatech, 8423 Embrach-Embraport, Switzerland) at 492 nm. Results are expressed as absorbance at 492 nm. All assays were performed in duplicate.
The plasma containing alloAb to polymorphisms on CR1, i.e. anti-Knops Ab, reacted strongly in the ELISA [16,17]. Dilutions of these plasma samples up to 1:800 were used as standards. Where stated, positive plasma samples were incubated with an excess of rCR1 (5 µg of rCR1/10 µl plasma) for 30 min at 37°C before being analysed by ELISA. Similarly, positive plasma samples were incubated either with human E or sheep E for 30 min at 37°C [19]. Thereafter samples were centrifuged for 10 min at 3000 ×g and the supernatants analysed for the presence of antiCR1 by ELISA.
Gel filtration, IgG purification, Western blot and immunoaffinity
For IgG filtration, aliquots of one positive antiCR1 autoAb sample and one negative plasma sample were diluted 1:1 with PBS and applied to FPLC-Superdex 200 HR 10/30 (Amersham Pharmacia Biotech, Piscataway, NJ, USA); collected fractions were loaded on the rCR1 ELISA. Plasma samples were mixed 1:1 with PBS and applied onto protein G-Sepharose CL-4B (Pharmacia). IgG was eluted from the column using 0·1 m glycine HCl, pH 2·8, and the pH of eluted fractions was immediately neutralized with 1 m Tris buffer, pH 9. Fractions containing IgG were pooled, dialysed against PBS, concentrated with Microsep 30 K (Pall Filtron, Bioconcept, Allschwill, Switzerland) and analysed by ELISA and Western blot.
rCR1 was diluted into 1% fetal calf serum, the mixture was denatured by boiling and adding Laemmli buffer and electrophoresed on SDS-PAGE containing 5% acrylamide. Resolved proteins were electroblotted onto nitrocellulose membranes and blots were incubated overnight at 4°C with the purified concentrated IgG preparations from the antiCR1 positive- or the antiCR1 negative-blood donors. Monoclonal Ab antiCR1 (3D9) was used as a positive control. The blots were then washed, and bound IgG or mAb were revealed using biotinylated mouse antihuman IgG or biotinylated sheep antimouse IgG, respectively. Streptavidin-horseradish peroxidase at 1:2000 was added and the blots were revealed with Enhanced Chemiluminescence detection reagents (Amersham, UK) [20]. rCR1 was coupled to Epoxy-activated Sepharose 6B (Sigma) according to the manufacturer's instructions. Typically, coupling efficiencies in the range of 3–5 mg rCR1 per ml of affinity gel slurry were obtained. After extensive washes of the beads with PBS, plasma diluted 1:1 in PBS was loaded. Following further extensive PBS washes, the proteins bound to the rCR1 column were eluted with 0·1 m glycine HCl, pH 2·8, and the pH of eluted fractions was immediately neutralized with 1 m Tris buffer, pH 9. Eluted fractions were analysed by ELISA.
ELISA for antiC1q autoAb
AntiC1q autoAb were determined using the method described by Siegert, as modified by Trendelenburg [21,22]. Briefly, ELISA wells (MaxisorpNunc Immuno plates) were coated overnight with 1 µg/well of C1q (Calbiochem, La Jolla, CA, USA) in sodium hydrogen carbonate buffer, pH 9·6, at room temperature. After washing of plates, 100 µl of the plasma diluted 1:25 in PBS –0·05% Tween containing 1% FCS (PBSTwFCS) and 1 m NaCl were incubated for 1 h at 37°C. Bound IgG was detected using biotinylated mouse monoclonal antihuman IgG (1:10000) (Southern Biotechnology Associates, Bioreba AG, Reinach, Switzerland) diluted in PBSTwFCS and 1 m NaCl, and revealed with streptavidin-HRP (Jackson ImmunoResearch).
Measurements of CR1 on E and in plasma
E were solubilized as previously described [12]. Briefly, 5 × 108 E/ml were washed 3–4 times with saline, haemolysed in buffer (5 mm phosphate, 1 mm EDTA, 1 mm PMSF), and the membranes solubilized at 4°C for 20 min with 1% Triton X-100, in the presence of 5 mm EDTA and 1 mm PMSF. After centrifugation at 20 000 g for 20 min, solubilized E were applied on ELISA for the determination of CR1 number [23]. Briefly, a mAb against CR1 (3D9) was coated onto the wells. After incubation of the sample to be tested, CR1 bound to the primary CR1 mAb was revealed using a biotinylated secondary mAb (J3D3) [24] that recognizes another epitope of CR1. Recombinant soluble human CR1 was used as the standard. The absorbance values obtained in the ELISA using solubilized E or plasma samples were compared with those obtained using known amounts of rCR1. The limit of detection was 0·75 ng CR1/ml.
Statistical analysis
The Fischer's test, the two-sample t-test, and the Spearman rank correlation coefficient (rs) were applied where appropriate using the StatViewTM SE + Graphics program.
RESULTS
AntiCR1 autoAb in the normal population
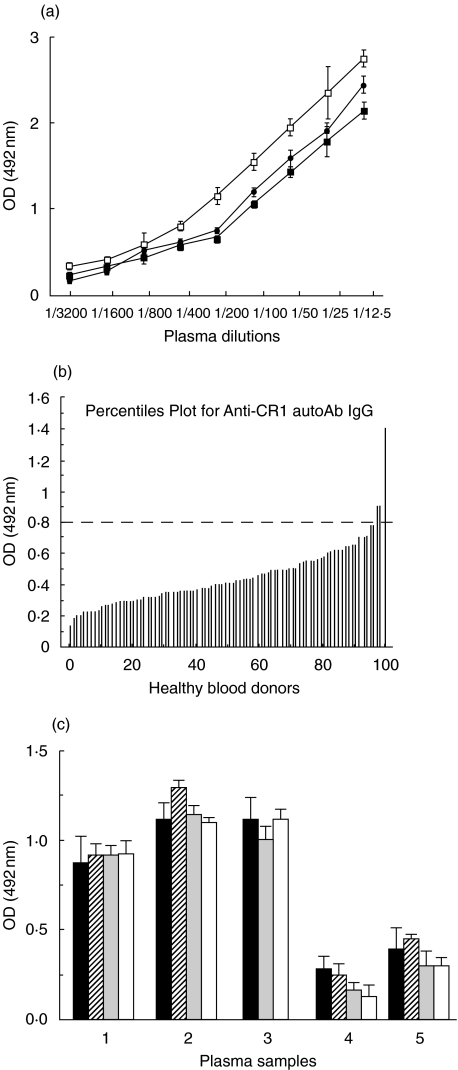
The antiCR1 ELISA was established to detect plasma IgG autoAb against rCR1. Plasma samples containing anti-Knops blood group Ab, which are known to be alloAb to polymorphisms on CR1, served as positive controls. Figure 1a illustrates the binding of antiCR1 IgG alloAb in the ELISA. The detection of the alloAb was dose dependent. Increasing the ionic strength during incubation with the plasma to eliminate weak interactions produced a small decrease in the signal, which remained however, dose dependent. We then tested 90 plasma from healthy blood donors (Fig. 1b). The mean OD was: 0·45 ± 0·36 (±2SD) ranging from 0·14 to 1·5. The OD of plasma samples from 3 subjects was above the mean +2SD. These 3 plasma samples were negative by serological tests for anti-Knops Ab. They were tested under high ionic strength conditions, but the signals were not significantly modified (Fig. 1c). When the plates were coated with 10 µg of rCR1 instead of 1 µg, the signals of these 3 plasma samples were only minimally increased similarly to observations with alloAb (data not shown), indicating that at 1 µg of CR1 the density of Ag on the plate was sufficient.
Fig. 1.
(a) Binding of antiCR1 alloAb (anti-Knops). The binding increased with the amount of plasma. The 3 ionic strengths tested provided similar results, with the highest ionic strength producing only a small drop in the binding of alloAb. □ 0.15m; • 0.5 m; ▪ 1.0 m. The Ab binding is expressed in OD (optical density) units. Data are given as mean values ± S.E.M. (b) AntiCR1 autoAb in 90 healthy blood donors. The dashed line represents the mean OD signal of the 90 plasma samples + 2SD. (c) Effect of the ionic strength on binding of antiCR1 autoAb. A high ionic strength did not significantly modify the binding in the 3 positive (1–3) and 2 negative (4 and 5) blood donors. ▪ 0.15m;  0.3 m;
0.3 m;  0.5m; □ 1.0m.
0.5m; □ 1.0m.
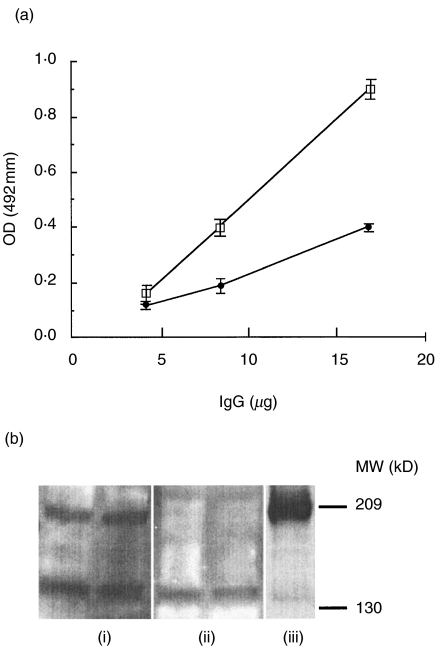
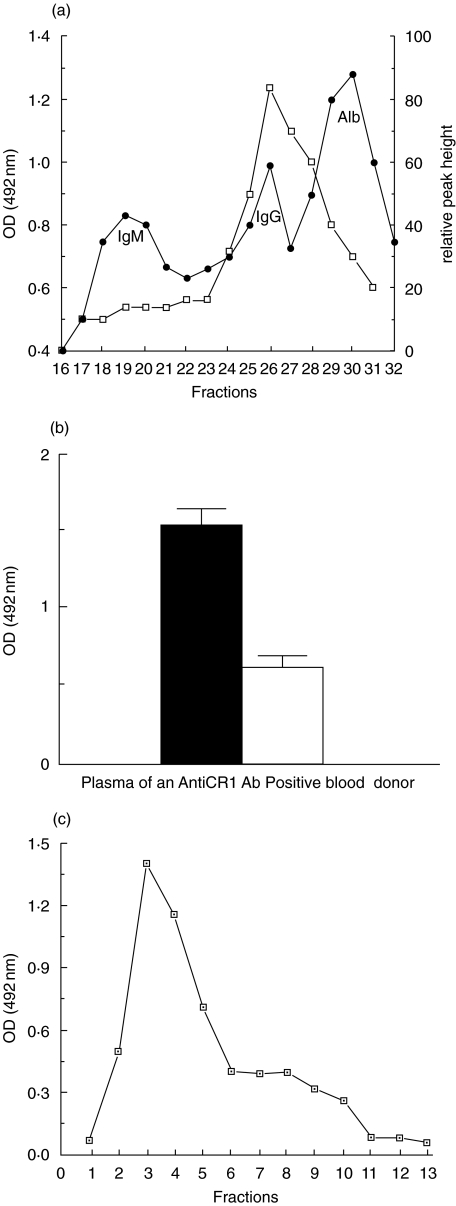
The IgG from one antiCR1-positive plasma sample was purified by Protein G chromatography, and was shown to react in the rCR1 ELISA (Fig. 2a). This IgG also reacted against rCR1 by Western blot, whereas IgG purified from a negative control did not (Fig. 2b). To determine whether the signal was due to monomeric IgG, an aliquot of the same plasma sample was separated by gel filtration on Superdex 200 HR 10/30 and the eluted fractions applied to the ELISA. The positive signal clearly corresponded to the IgG peak, and no signal was seen in larger-sized fractions corresponding to immune complexes (Fig. 3a). A further aliquot of the same plasma sample was then used to purify antiCR1 autoAb by affinity chromatography on rCR1 covalently attached to Sepharose. Correcting for dilution, plasma that passed through the column was depleted of autoAb (Fig. 3b), whereas the fractions recovered by acid elution retained the specific binding activity against CR1 (Fig. 3c).
Fig. 2.
Binding of purified IgG from a healthy blood donor to rsCR1 in ELISA and Western blot (a) Purified IgG from a positive plasma and the plasma itself (not shown) had similar positive OD signals when tested at an equal IgG concentration. The IgG from a plasma without antiCR1 Ab remained negative. □ positive IgG; • IgG control. (b) AntiCR1 IgG reacted with rCR1 (0·5 and 1 µg) blotted onto nitrocellulose. Protein G purified IgG from (i) a positive plasma and (ii) a negative plasma. (iii) Monoclonal antiCR1 Ab 3D9 as a positive control. Molecular weight markers in kD.
Fig. 3.
Gel filtration and affinity chromatography of antiCR1 positive plasma (a) The fractions positive for antiCR1 corresponded to the expected position of IgG (fractions 25–27) of the FPLC profile. The left vertical axis indicates the OD of the signal of these fractions after being applied to the antiCR1 ELISA (□). The right vertical axis indicates protein concentration (•). (b) Plasma after versus before affinity chromatography on rCR1 showed depletion of antiCR1 autoAb. The sample volume applied was corrected for dilution. (c) AntiCR1 autoAb were recovered in the acid eluate (fractions 3–5).
The presence of IgM antiCR1 autoAb was tested in comparable assays. Only 2 of 90 blood donors had such autoAb. The affinity of IgM was much lower than IgG Ab, since high ionic strength buffers significantly reduced the OD signals. After gel filtration, the reactivity in the ELISA corresponded to the IgM peak, although this did not allow a differentiation from immune complexes. The biology of IgM antiCR1 autoAb was not further explored.
AntiCR1 autoAb in different diseases
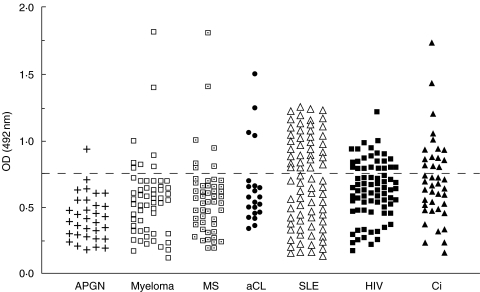
The cut-off for the detection of antiCR1 autoAb was defined as the normal range + 2SD. This value corresponded to the 1:400 dilution of the plasma containing alloAb to CR1 shown in Fig. 1, so that this plasma was used as a control in the ELISA. An increased frequency of antiCR1 autoAb was found in patients with SLE (36/78; 46%, P < 0·0001), liver cirrhosis (15/41; 36%, P < 0·0001), HIV infection (23/76, 30%, P < 0·0001), the patients with anticardiolipin Ab (4/21, 19%, P < 0·01) and multiple sclerosis (7/50; 14%, P < 0·02), but not in those with APGN (1/32; 3%) (Fig. 4). The myeloma patients analysed also showed an increased frequency of antiCR1 Ab compared with healthy blood donors (8/56, 14%, P < 0·02). Although the 3 patients with factor I deficiency had very low CR1/E (28, 32 and 52, respectively), none had detectable levels of antiCR1 autoAb.
Fig. 4.
AntiCR1 autoAb in different diseases The dotted line indicates the normal mean + 2SD.
The specificity of the antiCR1 autoAb was confirmed in control experiments using plasma samples from several patients. Firstly, the binding in the ELISA was not significantly modified at high ionic strength buffer (8 plasma tested). Secondly, by gel filtration antiCR1 corresponded to the IgG peak in the plasma of a patient with anticardiolipin Ab. Thirdly, IgG purified by protein G from 3 positive antiCR1 plasma samples (2 cirrhosis and one myeloma) reacted positively in the ELISA. Finally, plasma from one patient with cirrhosis was depleted of antiCR1 autoAb after passage on a rCR1-immunoaffinity column and the specific Ab could be recovered in the acid eluate.
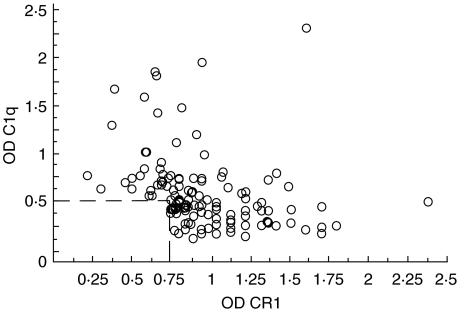
Lack of correlation between levels of anti-CR1 and antiC1q autoAb
Because C1q has been described to bind specifically to CR1 [25], it was important to exclude a possible crossreactivity between antiCR1 and antiC1q autoAb. Plasma samples from 288 patients with either SLE, cirrhosis, HIV, aCL Ab, MS, myeloma, or APGN were tested additionally for the presence of antiC1q. Of these patients, 43% (124/288) were positive for one or both autoAb. Taking into account all samples positive in one or both autoAb, we could find no positive correlation between the levels of the two types of autoAb. In fact the Spearman rank correlation coefficient was negative rs = −0·5 (n = 124, P= 0·001), almost excluding a cross-reactivity in the two ELISA (Fig. 5). Finally, no positive correlation was found when the diseases were evaluated separately.
Fig. 5.
AntiCR1 versus antiC1q autoAb The level of the two types of autoAb correlated inversely (n = 124, rs = −0·5, P= 0·0001). The dotted line represents the cut off for the two autoAb.
No correlation between antiCR1 autoAb and erythrocytes CR1 in HIV
The next issue was whether antiCR1 autoAb could explain the loss of CR1 on E in vivo. Acquired loss of CR1 is best established in HIV infection [8]. Thus, the presence and levels of antiCR1 were correlated with the number of CR1 on E in 76 patients with HIV infection at different stages of the disease. The CR1 on E ranged from 29 to 800. The levels of E CR1 in patients with antiCR1 autoAb were not significantly different from those without autoAb (respectively 365 ± 224 and 280 ± 188, P = 0·1). Plasma levels of sCR1 in patients with antiCR1 autoAb were not significantly different from those without autoAb as well ((34·9 ± 10·7 and 32·9 ± 11·07 ng/ml, P= 0·3).
AntiCR1 auto- versus allo-Ab
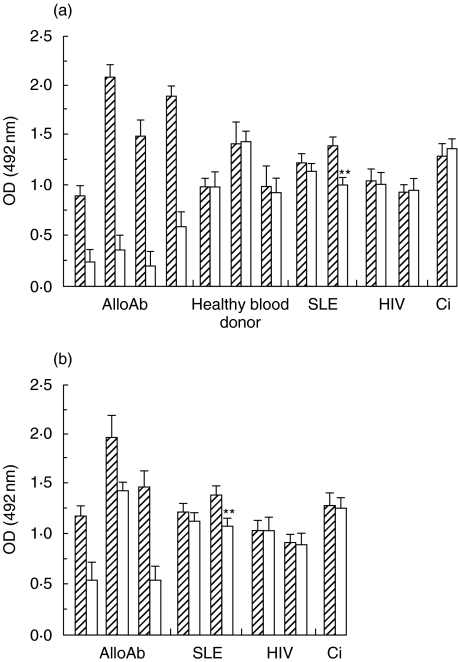
To define whether autoAb reacted against rCR1 in the fluid phase or CR1 on E, competitive assays were performed. In the standard ELISA plate, an excess of rsCR1 added to alloAb positive plasma blocked the binding of the alloAb (Fig. 6a). Binding was also significantly inhibited when plasma samples were preincubated with human E (Fig. 6b), but not with sheep E (data not shown). In contrast, detection of autoAb in the plasma of normal blood donors and patients was not significantly modified by either rCR1 or following preincubation with human E with the exception of one SLE plasma which produced a small inhibition in both assays (illustrated in Fig. 6). Total number of patients tested: 6 SLE, 4 HIV, 1 cirrhosis, 1 myeloma, all the patients are not shown. These results suggested that the epitopes of CR1 recognized by the autoAb were different from those recognized by alloAb and not present on the molecule in its soluble/native conformation.
Fig. 6.
Effects of soluble rCR1 on autoAb versus alloAb detection in the ELISA. (a) Pre-incubation of plasma with an excess of rsCR1 inhibited the binding of alloAb (4 different plasma: columns 1–4), had no effects on autoAb in 3 blood donors (columns 5–7), partially inhibited the binding of autoAb in one SLE patient (column 9), but had no effect on, another SLE patient (column 8), 2 HIV patients (columns 10, 11), and a liver cirrhosis patient (column 12).  Before rsCR1; □ After rsCR1. (b) Pre-incubation of plasma with human erythrocytes (HE) inhibited the binding of alloAb (3 different plasma: column 1–3), partially inhibited the binding of autoAb in the same SLE patient (column 5), but had no effect on the other same plasma tested (SLE: column 4; 2 HIV patients: column 6,7 and the cirrhosis: column 8).
Before rsCR1; □ After rsCR1. (b) Pre-incubation of plasma with human erythrocytes (HE) inhibited the binding of alloAb (3 different plasma: column 1–3), partially inhibited the binding of autoAb in the same SLE patient (column 5), but had no effect on the other same plasma tested (SLE: column 4; 2 HIV patients: column 6,7 and the cirrhosis: column 8).  Before HE; □ After HE.
Before HE; □ After HE.
DISCUSSION
The data presented herein establishes that antiCR1 autoAb are found in some normal individuals, and with an increased frequency in individuals with SLE, other autoimmune diseases, HIV infection and liver cirrhosis. The autoAb characterized here were of IgG class, reacted specifically with rCR1 fixed onto a support, and could be purified by immunoaffinity chromatography. However they did not react with rCR1 in solution or with E bearing native CR1, and were, in that respect, different from the well described alloAb against polymorphism of CR1. The presence of these Ab appeared not to influence the number of CR1 on E or soluble CR1 of plasma, as suggested by the results obtained in HIV patients.
The ELISA used in this study allowed quantitative measurement of the binding of plasma IgG to rCR1. The specificity of this binding was tested by increasing buffer ionic strength, which is known to reduce non–specific ionic interactions and low affinity binding. Indeed, rCR1 fixed onto the plate would be expected to bind C3H2O or C3b/bi present in plasma, and these molecules might in turn bind natural occurring Ab against C3/C3b or immunoconglutinins [26]. However binding of C3 fragments to rCR1 is abolished by high salt concentrations [27], and thus the presence of IgG bound to rCR1 could not be due to such interactions. Indirect binding might also have occurred via C1q. Recent evidence indicates that C1q binds CR1 [25], so that our assay might have detected antiC1q autoAb. Several results make this possibility unlikely. Firstly, whole plasma and purified plasma IgG binding to rCR1 was identical. Secondly, fractions of plasma obtained by gel filtration that bound to rCR1 corresponded to the size of IgG and were very unlikely to contain C1q, which has a higher molecular weight. Thirdly, there was no positive correlation between plasma levels of antiCR1 and antiC1q autoAb.
Almost half of the patients with SLE had antiCR1 autoAb, which is at a much higher prevalence than that reported by Wilson et al.[14], and Cook et al.[15]. These authors in fact reported only on the few patients having very high levels of autoAb. The 2 patients with SLE described by Cook et al. had Ab with properties similar to those described here, namely binding to CR1 on ELISA plate but not interfering with native CR1. In contrast, the SLE patient described by Wilson et al. appears unique since the Ab reacted with both ELISA-bound and native CR1. Wilson et al. did not exclude the possibility that this Ab was an alloreactive (McCoy/Knops) Ab. Of the 6 patients with SLE we could analyse, one had a small fraction of his autoAb which reacted with native CR1. He had no alloAb according to standard assays although we could not test his E.
Similarly to other autoAb such as antiC1q [28], or antiCRP [29], antiCR1 reacted with its antigen only when bound to a plastic or nitrocellulose support, whereas the native antigen was not recognized (with the 2 exceptions mentioned above). This clearly differentiates antiCR1 autoAb from alloAb which reacted with soluble rCR1 and was adsorbed by E-CR1. Indeed such alloAb produce agglutination of human E in vitro[16,30]. A conclusion to be drawn from these observations is that antiCR1 autoAb do not modify CR1 in vivo. Support of this conclusion may be obtained from our findings in HIV patients in whom the presence of antiCR1 Ab did not correlate with either a reduction of CR1 number on E or a low soluble CR1 in plasma. A corollary of these results is that the antiCR1 autoAb described here will not interfere with rCR1 administered to patients, as now done in phase 1 studies [31]. However, the strong reactivity of alloAb against the rCR1 cautions that such alloAb should be determined before administering rCR1.
Another major issue is to understand the origin of the antiCR1 autoAb. CR1 may become antigenic only under specific circumstances, when exposing new epitopes not present on the native molecule. CR1 is degraded when immune complexes are transferred from E to fixed macrophages [13]. SLE and HIV infections are both characterized by the presence of circulating immune complexes, and by a selective loss of E CR1 that occurs in vivo[7,12]. Indeed, in many experimental models, large loads of immune complexes in blood have been shown to acutely and chronically induce a loss of CR1 [32]. Most authors consider this loss to be related to the biological function of E CR1 to transport immune complexes [33]. Macrophages removing large amounts of ICs and CR1 from E [13] might function as an antigen presenting cell for fragments of CR1, thus inducing the formation of autoAb against linear epitopes of the molecule. These autoAb would not be expected to react against the native molecule, nor against the fragment remaining on the E cell surface after removal of ICs. All these characteristics would be consistent with the increased prevalence of antiCR1 Ab in SLE, HIV infection, and liver cirrhosis (including HCV) described in this study. Alternatively, the expression of cryptic epitopes present on altered CR1 molecules remaining on E might be recognized as foreign. Interestingly, the 3 patients with Factor I deficiency had undetectable levels of antiCR1 autoAb. Although the number of patients is low, this deficiency is however, characterized by a continuous generation of C3b which is known to attach to CR1, and also by a severe in vivo acquired loss of E CR1 [12,18]. Thus, even maximal complement activation and CR1 loss appear not to automatically induce antiCR1 autoAb. Furthermore, in patients with APGN and low C3, the frequency of autoAb was also not increased. Thus it is not complement alone, but possibly the transport and removal of immune complexes by E CR1 which plays a major role in the induction of autoAb.
An alternative explanation would be that antiCR1 are naturally occurring autoAb which increase when the B-cells are chronically stimulated. Autoimmunity and chronic infections would satisfy these criteria. However, IgM antiCR1 autoAb were not more frequent than IgG autoAb in the normal population, and the affinity of the IgG autoAb was sufficient to resist high ionic strength, suggesting a high affinity. Natural Ab are generally recognized as being of IgM class and of low affinity.
The high prevalence of antiCR1 autoAb in SLE and HIV infection may be a marker of a specific pathological process. More studies are required to define this relationship. In particular it will be important to define the specific epitopes recognized, since CR1 belongs to a family of complement regulators and other proteins having a similar structure of short consensus repeats. Whether the antiCR1 autoAb described here recognize primarily a more general structure remains to be defined.
Acknowledgments
This work was supported by the Swiss National Science Fondation 0032·49446·96/1 and 0032·66708·01. We thank Dr Hustinx, BSD SRK Bern for providing plasma containing antiCR1 alloAb. We thank Thérèse Resink and Jameel Inal for their helpful comments.
References
- 1.Nelson RA. The immune adherence phenomenon. an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953;118:733. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- 2.Krych M, Hauhart R, Atkinson JP. Structure-function analysis of the active sites of complement receptor type 1. J Biol Chem. 1998;273:8623–9. doi: 10.1074/jbc.273.15.8623. [DOI] [PubMed] [Google Scholar]
- 3.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leucocyte,B lymphocyte, and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon D, Ahearn JM. Complement receptor type 1 (C3b/C4b receptor; CD 35) and complement receptor type 2 (C3d/ Epstein-Barr virus receptor; CD21) In: Lambris JD, editor. Current Topics in Microbiology and Immunology (the Third Component of Complement) Vol. 153. Berlin: Springer Verlag; 1989. pp. 83–98. [DOI] [PubMed] [Google Scholar]
- 5.Kazatchkine MD, Fearon DT. Deficiencies of human complement receptors type 1 (CR1, CD35) and type 2 (CR2, CD21) Immunodef Rev. 1990;2:17–41. [PubMed] [Google Scholar]
- 6.Paccaud JP, Carpentier JL, Schifferli JA. Direct evidence for the clustered nature of complement receptors type 1 on the erythrocyte membrane. J Immunol. 1988;141:3889–94. [PubMed] [Google Scholar]
- 7.Iida K, Mornaghi R, Nussenzweig V. Complement receptor (CR) deficiency in erythrocytes from the patients with systemic lupus erythematosus. J Exp Med. 1981;155:1427–38. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tausk FA, McCutchan A, Spechko P, Schreiber RD, Gigli I. Altered erythrocyte C3b receptor expression, immune complexes, and complement activation in homosexual men in varying risk groups for acquired immune deficiency syndrome. J Clin Invest. 1986;78:977–82. doi: 10.1172/JCI112688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanto T, Hayashi N, Takehara T, et al. Low expression of erythrocyte complement receptor type 1 in chronic hepatitis C patients. J Med Virol. 1996;50:126–34. doi: 10.1002/(SICI)1096-9071(199610)50:2<126::AID-JMV5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 10.Moldenhauer F, Botto M, Walport MJ. The rate of loss of CR1 from ageing erythrocytes in vivo in normal subjects and SLE patients: no correlation with structural or numerical polymorphisms. Clin Exp Immunol. 1988;72:74–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Walport MJ, Ross GD, Mackworth-Young C, Watson JV, Hogg N, Lachmann PJ. Family studies of erythrocyte complement receptor type 1 levels: reduced levels in patients with SLE are acquired, not inherited. Clin Exp Immunol. 1985;59:547–54. [PMC free article] [PubMed] [Google Scholar]
- 12.Lach-Trifilieff E, Marfurt J, Schwarz S, Sadallah S, Schifferli JA. Complement receptor 1 (CD35) on human reticulocytes: normal expression in systemic lupus erythematosus and HIV-infected patients. J Immunol. 1999;162:7549–54. [PubMed] [Google Scholar]
- 13.Davies KA, Hird V, Stewart S, et al. A study of in vivo immune complex formation and clearance in man. J Immunol. 1990;144:4613–20. [PubMed] [Google Scholar]
- 14.Wilson JG, Jack RM, Wong WW, Schur PH, Fearon DT. Autoantibody to the C3b/C4b receptor and absence of this receptor from erythrocytes of a patient with systemic lupus erythematosus. J Clin Invest. 1985;76:182–90. doi: 10.1172/JCI111944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook JM, Kazatchkine MD, Bourgeois P, Mignon F, Mery JP, Kahn MF. Anti-C3b-receptor (CR1) antibodies in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1986;38:135–8. doi: 10.1016/0090-1229(86)90131-5. [DOI] [PubMed] [Google Scholar]
- 16.Moulds JM, Nickells MW, Moulds JJ, Brown MC, Atkinson JP. The C3b/C4b receptor is recognized by the Knops, McCoy, Swain-langley, and York blood group antisera. J Exp Med. 1991;173:1159–63. doi: 10.1084/jem.173.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulds JM, Zimmerman PA, Doumbo OK, et al. Molecular identification of Knops blood group polymorphisms found in long homologous region D of complement receptor 1. Blood. 2001;97:2879–85. doi: 10.1182/blood.v97.9.2879. [DOI] [PubMed] [Google Scholar]
- 18.Sadallah S, Gudat F, Laissue JA, Spath PJ, Schifferli JA. Glomerulonephritis in a patient with complement factor I deficiency. Am J Kidney Dis. 1999;33:1153–7. doi: 10.1016/S0272-6386(99)70155-1. [DOI] [PubMed] [Google Scholar]
- 19.Yazdanbakhsh K, Oyen R, Yu Q, et al. High-level, stable expression of blood group antigens in a heterologous system. Am J Hematol. 2000;63:114–24. doi: 10.1002/(sici)1096-8652(200003)63:3<114::aid-ajh2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Sadallah S, Lach E, Lutz HU, Schwarz S, Guerne PA, Schifferli JA. CR1, CD35 in synovial fluid from patients with inflammatory joint diseases. Arthritis Rheum. 1997;40:520–6. doi: 10.1002/art.1780400318. [DOI] [PubMed] [Google Scholar]
- 21.Trendelenburg M, Marfurt J, Gerber I, Tyndall A, Schifferli JA. Lack of occurrence of severe lupus nephritis among anti-C1q autoantibody-negative patients. Arthritis Rheum. 1999;42:187–8. doi: 10.1002/1529-0131(199901)42:1<187::AID-ANR24>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Siegert C, Daha M, Westedt ML, van der Voort E, Breedveld F. IgG autoantibodies against C1q are correlated with nephritis, hypocomplementemia, and dsDNA antibodies in systemic lupus erythematosus. J Rheumatol. 1991;18:230–4. [PubMed] [Google Scholar]
- 23.Pascual M, Duchosal MA, Steiger G, et al. Circulating soluble CR1 (CD35). serum levels in diseases and evidence for its release by human leukocytes. J Immunol. 1993;151:1702–11. [PubMed] [Google Scholar]
- 24.Nickells M, Hauhart R, Krych M, et al. Mapping epitopes for 20 monoclonal antibodies to CR1. Clin Exp Immunol. 1998;112:27–33. doi: 10.1046/j.1365-2249.1998.00549.x. [published erratum appears in: Clin Exp Immunol 1998; 113: 315] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tas SW, Klickstein LB, Barbashov SF, Nicholson-Weller A. C1q and C4b bind simultaneously to CR1 and additively support erythrocyte adhesion. J Immunol. 1999;163:5056–63. [PubMed] [Google Scholar]
- 26.Nilsson B, Ekdahl KN, Svarvare M, Bjelle A, Nilsson UR. Purification and characterization of IgG immunoconglutinins from patients with systemic lupus erythematosus: implications for a regulatory function. Clin Exp Immunol. 1990;82:262–7. doi: 10.1111/j.1365-2249.1990.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fries LF, Prince GM, Gaither TA, Frank MM. Factor I co-factor activity of CR1 overcomes the protective effect of IgG on covalently bound C3b residues. J Immunol. 1985;135:2673–9. [PubMed] [Google Scholar]
- 28.Martensson U, Sjoholm AG, Sturfelt G, Truedsson L, Laurell AB. Western blot analysis of human IgG reactive with the collagenous portion of C1q: evidence of distinct binding specificities. Scand J Immunol. 1992;35:735–44. doi: 10.1111/j.1365-3083.1992.tb02982.x. [DOI] [PubMed] [Google Scholar]
- 29.Bell SA, Faust H, Schmid A, Meurer M. Autoantibodies to C-reactive protein (CRP) and other acute-phase proteins in systemic autoimmune diseases. Clin Exp Immunol. 1998;113:327–32. doi: 10.1046/j.1365-2249.1998.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulds JM, Moulds JJ, Brown M, Atkinson JP. Antiglobulin testing for CR1-related (Knops/McCoy/Swain-Langley/York) blood group antigens. negative and weak reactions are caused by variable expression of CR1. Vox Sang. 1992;62:230–5. doi: 10.1111/j.1423-0410.1992.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 31.Zimmerman J, Dellinger R, Straube R, Levin J. Phase I trial of the recombinant soluble complement receptor 1 in acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2000;28:3149–54. doi: 10.1097/00003246-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Cosio FG, Shen XP, Birmingham DJ, Van Aman M, Hebert LA. Evaluation of the mechanisms responsible for the reduction in erythrocyte complement receptors when immune complexes form in vivo in primates. J Immunol. 1990;145:4198–206. [PubMed] [Google Scholar]
- 33.Tausk F, Gigli I. The human C3b receptor. function and role in human diseases. J Invest Dermatol. 1990;94(Suppl.):141S–5S. doi: 10.1111/1523-1747.ep12876125. [DOI] [PubMed] [Google Scholar]