Abstract
Tick-Borne Encephalitis virus (TBEV) causes dangerous central nervous system diseases in humans. General infection leads to the development of meningitis or encephalitis, which is characterized by swelling of the brain due to inflammation. Tetracyclines may act locally to moderate inflammation in the CNS. In this study, we investigated the potential clinical benefits of administering tetracycline hydrochloride to patients hospitalized due to suspected TBEV infection presenting with fever and evidence of a recent tick bite. We also characterized an acute immune response to TBEV by profiling certain cytokines and soluble receptors in Tetracycline-treated and untreated patients. Increased serum levels of TNF-α, IL-1α and IL-6 were found in all patients at admission.
Soluble receptors presented in the serum of patients in a magnitude higher levels than the corresponding cytokines and were increasing during first weak of hospitalization. Levels of IL-10 were also rising during that period. In our study tetracycline hydrochloride acted as an immunomodulator, which was able to reduce manifestations of inflammation response during TBE course; this action led to quicker improvement of symptoms and, consequently, to a faster clinical recovery. The positive result of tetracycline hydrochloride treatment was accompanied by certain particularities in the dynamics of studyied cytokines and receptors: the concentrations of IL-6, IL-1β, TNF-α dropped quicker and reached lower levels, and the concentrations of sIL-6R, IL-1RA, sTNFR1 increased faster and reached higher maximum levels in the tetracycline-treated groups. Children had the highest levels of IL-6, which were not neurotoxic.
Keywords: tick-borne encephalitis, humans, cytokines, soluble receptors, tetracycline hydrochloride
INTRODUCTION
Tick-Borne Encephalitis virus (TBEV), member of the Flaviviridae family, causes dangerous central nervous system diseases in humans. Tick-Borne Encephalitis (TBE) is an increasing public health problem. Circulation of the subtype Siberian of TBEV (tick-borne encephalitis virus) in the Novosibirsk region of West Siberia (Russia) had been suggested by Ecker et al. [1] and confirmed by Bakhvalova et al. [2]. Lower neuroinvasiveness, especially for children, and lethality level of the Siberian strains of TBEV in comparison with Far Eastern Sofyin strain is known [2]. Annually, statistic data reveals more than 300–350 confirmed clinical cases of TBE in that part of West Siberia, with a mortality rate of no more than 2–3%.
General infection leads to the development of meningitis or encephalitis, which is characterized by swelling of the brain due to inflammation. The pathology observed subsequent to infection suggests that some components of the immune response contribute to the disease process [3–5]. Safety and efficacy of postexposure prophylaxis at any interval is controversial [6–10]. There is no specific therapy for TBE infection other than supportive measures. But it is of high importance to minimize the severity of the encephalitis, incidence of sequelae and case-fatality rate.
Most known data shows that the anti-inflammatory effect of tetracyclines is connected with the inhibition of synthesis of several inflammatory mediators [11–13]. Tetracyclines can rather easily cross the blood–brain barrier, especially during the inflammation in the CNS (central nervous system). Thus, tetracyclines may act locally to moderate inflammation in the CNS within the course of TBE. In our previous experimental studies, we have found that the administration of tetracycline hydrochloride during experimental TBEV infection in mice can significantly decrease the mortality rate (unpublished data).
In this study, we characterized an acute immune response to TBEV by profiling certain cytokines and soluble receptors in tetracycline-treated and untreated patients.
MATERIALS AND METHODS
Subjects
Patients (n = 29) from the predominantly rural region of Novosibirsk, Russia were hospitalized between May – June 2001 with suspected TBE. The SRC VB ‘Vector’ Ethical Committee (IRB00001360) approved the present study. Informed consent was obtained from all adult participants and from the parents of hospitalized children. All patients admitted within 5 days after the onset of fever, with evident symptoms of acute aseptic meningitis or meningoencephalitis and with the evidence of a recent tick bite were recruited to the study. No patients had a history of vaccination against TBE. The specific immunoglobulin against TBE was administered to all patients during the first two days after the tick bite (Omsk production facility of Bacterial Preparations, Russia). At admission adult patients were randomly stratified to one of two groups: with standard therapy against TBE or with combined therapy of standard care plus tetracycline hydrochloride. In the present manuscript, only patients with a confirmed diagnosis of TBE were included. Group 1 was comprised of 11 adult patients (5 M, 6 F) age 41–71 years (mean 50·7), who received standard therapy for the suspected TBEV infection. The standard treatment course of TBE includes only symptomatical and supportive care. Group 2 contained 14 adult patients (8 M, 6 F), age 41–65 years (mean 48·3), who received the same therapy as group 1 with the exception of tetracycline hydrochloride (Sumycin, Bristol-Myers Squib, USA). Group 3 was comprised of 4 children (3 M, 1 F), age 8–11 years (mean 9·75), who received standard therapy in addition to the Sumycin course, as antibiotic therapy is recommended for children until the serological confirmation of acute TBE is obtained.
Sumycin was given just after admission to the hospital, and was administered orally to all patients until a clear resolution of symptoms was observed. Adult patients were administered 250 mg four times a day. Children were administered 25 mg/kg daily, divided into four doses. All patients from all groups received neither corticosteroids nor any other immunomodulatory drugs, nor plasma transfusion.
Diagnostic
The initial clinical diagnosis of TBE was confirmed by RT-PCR, as described by Godovikova et al. [14], with specific primers corresponding to the highly conservative protein E, its C-end domain (496 bp), of the TBE virus (strain Sofyin) kindly provided by N. Netesova [15]. Additional serological investigation using a commercial PCR test kit was made for the detection of Borrelia Burgdorferi (‘NEARMEDIC PLUS’, Mascow, Russia). The specific antibodies (IgG) against TBE virus were determined by commercial ELISA test kits (Vector-Best Ltd, Novosi Biesk region, Russia).
Cytokine assays
Blood samples from all patients were centrifuged to obtain serum and were stored at −70°C.Cytokine assays were performed blindly after the samples from all participants had been collected. Serum cytokine and cytokine receptor levels were determined using commercial ELISA test kits (R & D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The limits of detection for the various assays are as follows: tumour necrosis factor (TNF)-α, 4·4 pg/ml; soluble TNF receptor I (sTNFRI), 3·0 pg/ml; interleukin (IL)-1β, 1 pg/ml; IL-1 receptor antagonist (IL-1RA), 14 pg/ml; IL-2, less than 7 pg/ml; IL-6, 0·70 pg/ml; soluble IL-6 receptor (sIL-6R), 1·5 pg/ml; IL-10, 3·9 pg/ml. Results are expressed as the M ± SD.
Statistical analyses
Statistical analysis was performed with the Student t-test for paired or unpaired data. Spearman's rank correlation coefficient and single regression were used to correlate the data of cytokines and receptors levels. P-values <0·05 were considered statistically significant.
RESULTS
Clinical data
The initial clinical diagnosis of the TBE infection was confirmed in all patients. Only patients who appeared to be negative for Borrelia burgdorferi were included in the present paper. Antibodies (IgG) to TBEV were found to significantly increase in all patients at the time of discharge (group 1, 1:640; group 2, 1:640–1:2560; group 3, 1:1280) over the baseline levels seen upon initial hospitalization (group 1 and 2, 1:80–1:120; group 3, 1:120). Symptoms commonly associated with aseptic meningitis or meningoencephalitis were classified as mild to moderate in adults but were absent among children (Table 1). Disease severity was comparable among patients in groups 1 and 2. Transient leucocytosis (6·9–20·5 × 109/l) without neutrophiles shift to the left was noted in all patients. The blood sedimentation rate was not elevated in all patients (group 1, 63%; group 2, 56%; group 3, 50%) and appeared to be no higher than 29 mm/h. The platelet count as well as liver enzyme levels remained within the normal range. Serum concentrations of C-reactive protein were not elevated, but instead tended towards the high end of the normal range in most patients.
Table 1.
Clinical symptoms presented in TBE patients
| Data | Group 1·11 patients (%) | Group 2·14 patients (%) | Group 3·4 patients (%) |
|---|---|---|---|
| Headache | 100 | 100 | 100 |
| Fever (38–40°C) | 100 | 100 | 100 |
| General malaise | 18 | 28 | 100 |
| Muscle pains | 36 | 28 | 0 |
| Weakness of muscles of the upper body | 18 | 14 | 0 |
| Joints pains | 36 | 28 | 0 |
| Nausea and vomiting | 18 | 21 | 0 |
| Dizziness | 27 | 21 | 0 |
| Nuchal rigidity | 27 | 21 | 0 |
| Disturbances of concentration and memory | 9 | 0 | 0 |
| Mild disturbance of balance. | 18 | 21 | 0 |
All patients were discharged from the hospital with clinical improvement. By the term of clinical improvement we mean the following: absence of subjective symptoms, temperature normalization, normalization of nonspecific laboratory changes in blood, absence of meningeal irritation signs as well as of focal neurological deficits. The differences between group 1 and group 2 in the clinical outcomes were not statistically significant. Nevertheless the positive effect of Sumycin (Table 2) in patients from the ‘treated’ group 2 expressed in a quicker resolution of all clinical symptoms of the disease, and therefore in a significant decrease of the convalescence phase in comparison with the same data of group 1. Thus, the period of hospitalization in the Sumycin-treated group was statistically shorter (Table 2).
Table 2.
Comparative data of different groups of patients with TBE
| Group | Day of the start of medical care, relative to the onset of temperature | Course of Sumycin (days) | Day of symptom improvement | Duration of hospitalization (days) |
|---|---|---|---|---|
| 1 | 1–4 | – | 5–23 (14) | 9–28 (18·5) |
| 2 | 1–5 | 6–10 | 2–6 (4) | 6–12 (9) |
| 3 | 1–4 | 6–10 | 4–8 (6) | 9–16 (12·5) |
minimum–maximum duration (median)
Cytokines levels in the serum of Group 1 patients
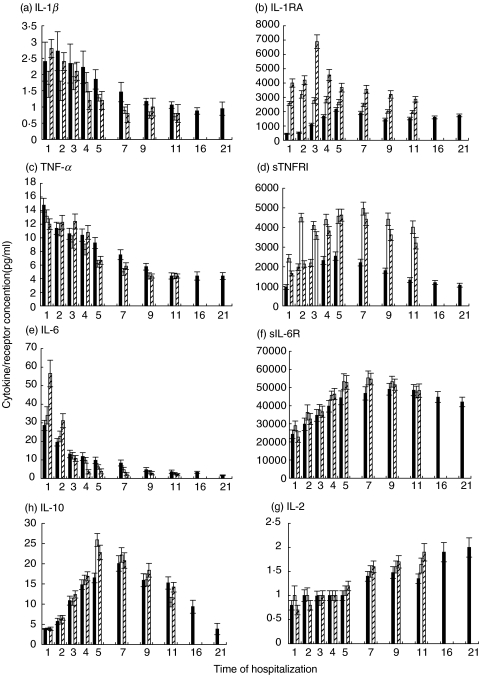
In general, increased serum levels of TNF-α, IL-1α and IL-6 were demonstrated in all patients from group 1 when admitted to the hospital. This picture indicates that the peak of concentration of these cytokines may have occurred before admission to the hospital (Fig. 1). The maximum value of TNF-α, noted on day 1, was 14·8 ± 1.0 pg/ml and of IL-6 was 28·6 ± 2·8 pg/ml. The maximum value of IL-1β, noted on day 2, was 2·72 ± 0·6 pg/ml (Fig. 1). Minimum values were detected before the patients were discharged from the hospital. In general, the concentration of IL-6 decreased during the clinical course of TBE about 6-fold, TNF-α– about 4-fold, IL-1β– about 2-fold. There was strong positive correlation between these cytokines (TNF/IL-6, r = 0·95; P= 0·0001; TNF/IL-1, r = 0·93; P= 0·0001; IL-6/IL-1, r = 0·87, P= 0·0001).
Fig. 1.
Serum levels of cytokines and receptors in humans with TBE virus infection. a, IL-1β; b, IL-1RA; c, TNF-α; d, sTNFRI; e, IL-6; f, sIL-6R; g, IL-10; IL-2. ▪ group 1, □ group 2,  group 3. All examined parameters of group 1 are shown by day 21, as the hospitalization period in that group was longer in comparison with that in the groups 2 and 3. Data are mean ± SD.
group 3. All examined parameters of group 1 are shown by day 21, as the hospitalization period in that group was longer in comparison with that in the groups 2 and 3. Data are mean ± SD.
The lowest values of the corresponding receptors (sTNFRI, sIL-6R, IL-1RA) were detected at admission (Fig. 1). A gradual enlargement of the concentrations of these receptors was observed within the first 9 days of hospitalization. The predominance of the value of sIL-6R within the clinical course of TBE was very impressive (Fig. 1).
A peak was reached on day 5 by sTNFRI (2540 ± 214 pg/ml) and IL-1RA (2158 ± 126 pg/ml), and on day 9 by sIL-6R (49060 ± 3240 pg/ml). Concentrations of IL-1RA (r = −0·71, P= 0.0001) and sIL-6R (r = −0·91, P= 0·0001) had strong negative correlation with the concentrations of the corresponding cytokines. Concentrations of TNF-α and sTNFRI showed weak correlation.
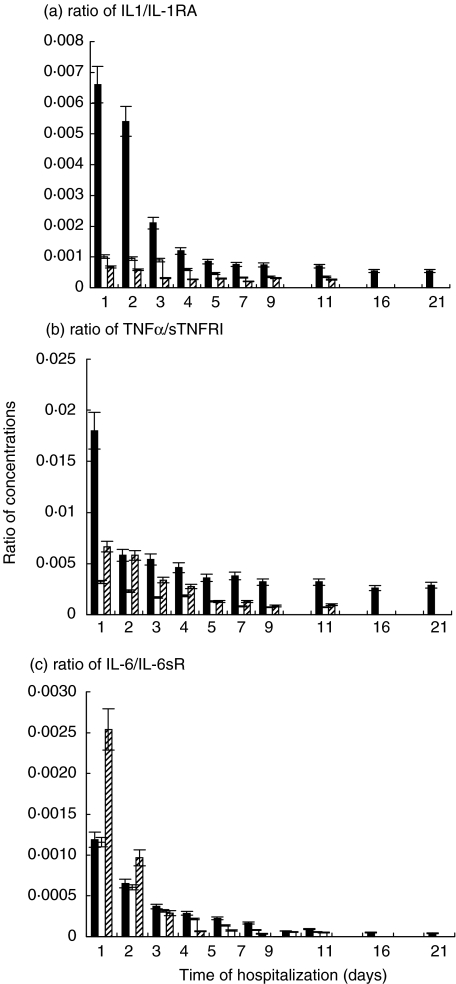
As soluble receptors presented in the serum of patients in a magnitude higher levels than the corresponding cytokines, the ratio of cytokine to its soluble receptor was affected (Fig. 2). The highest levels of the calculated ratios were observed at admission to the hospital. The profile of changes was similar to those of the corresponding cytokines.
Fig. 2.
Ratios of certain cytokines to their receptors in serum of humans with TBE virus infection. ▪ group 1, □ group 2,  group 3. a, Ratio of IL-1/IL-1RA; b, Ratio of TNF-α/sTNFRI; c, IL-6/IL-6sR. All calculated ratios of group 1 are shown by day 21, as the hospitalization period in that group was longer in comparison with that in the groups 2 and 3. Data are mean ± SD.
group 3. a, Ratio of IL-1/IL-1RA; b, Ratio of TNF-α/sTNFRI; c, IL-6/IL-6sR. All calculated ratios of group 1 are shown by day 21, as the hospitalization period in that group was longer in comparison with that in the groups 2 and 3. Data are mean ± SD.
The minimum value of IL-10 was detected on the first day of hospitalization (less 3·9 pg/ml). The level of IL-10 enlarged about 5·2-fold and reached its maximum of 20·1 ± 1·9 pg/ml on day 7 of hospitalization. It showed a positive correlation with soluble receptors of TNF-α (r = 0·66, P= 0·001) and IL-6 (r = 0·75, P= 0·001), as well as with IL-1RA (r = 0·70, P= 0·0001).
The dynamic of IL-2 serum concentration (Fig. 1) showed a gradual growth with the maximum level (2·0 ± 0·2 pg/ml) reached on the day of discharge. This picture indicates that the peak of concentration of this cytokine may have occurred after discharge from the hospital. The concentration of IL-2 in the serum of patients enlarged 2·5-fold during hospitalization. It had a strong negative correlation with: IL-1β (r = −0·88, P= 0·001), TNF-α (r = −0·90, P= 0·0001) and IL-6 (r = −0·80, P= 0·0001).
Cytokines' and receptors' levels in relation to treatment
We have made statistical comparison of the cytokines' and receptors' profile between adult patients from group 1 (standard medical care) and the profile of group 2 (additional Sumycin course of treatment) for the evaluation of the effect of Sumycin on the development of TBE infection. Cytokine levels in the serum of children with TBE have been described here without statistical comparison with adults, as it would be incorrect. Throughout the clinical course of TBE infection, patients from the ‘treated’ group 2 had statistically lower levels of IL-1β (P < 0·001), as well as of TNF-α (P < 0·001) than patients from group 1. Children from group 3 showed the high levels of IL-1β comparable within the first three days with those in patients from group 1. TNF-α concentrations in the children's group were rather stable within the first three days (Fig. 1).
Comparing the concentrations of IL-1RA, patients from group 2 had statistically higher levels (P < 0·01), and the maximum level was reached earlier in comparison with patients from group 1. The highest levels of IL-1RA were found in the group of children. Considerably higher levels of sTNFRI were noticed in the ‘treated’ group of adult patients in comparison to those in group 1 (P < 0·001). Children from group 3 demonstrated the high concentrations of sTNFRI comparable with those of patients from group 2. Weak correlation between TNF-α and sTNFRI in group 1 (r = 0·29, P= 0·2) became moderate during the Sumycin treatment course in group 2 (r = 0·59, P= 0·002)
The remarkable difference between the ‘treated’ and ‘untreated’ groups of adults was shown by the ratio of IL-1β/IL-1RA (P < 0·005) and ratio of TNFα/sTNFRI (P < 0·01) (Fig. 2). The lowest values of IL-1β/IL-1RA ratio were observed in children. With regard to the ratio of TNFα/sTNFRI, children had higher levels than adults from the ‘treated’ group 2, but lower than adults from the ‘untreated’ group 1.
During the first two days after admission patients from group 2 (P < 0·01), as well as children from group 3, demonstrated higher values of IL-6 (Fig. 1) in comparison to patients from the ‘untreated’ group 1, but the decline occurred more rapidly in the ‘treated’ groups. Patients from group 2 had higher levels of sIL-6R (P < 0·001), and the maximum level was reached earlier, in comparison to the patients from the ‘untreated’ group 1 (Fig. 2). Children from group 3 had the lowest concentrations of sIL-6R on day one, but it grew rapidly, and became similar to those in adults from group 2. From the very beginning, the ratio of IL-6/sIL-6R was lower in group 2 (P < 0·01) than in group 1 (Fig. 2). Children demonstrated the highest value of that ratio on the day of admission, but it dropped very rapidly.
The maximum levels of IL-10 (Fig. 1) were reached earlier in the ‘treated’ groups 2 and 3 in comparison to the levels reached later in group 1. The maximum value of IL-10 was statistically higher in the ‘treated’ group 2, than in the ‘untreated’ group 1 (P < 0·001). The correlation between TNF- α and IL-10 was weak in group 1, but was moderate in group 2 during the Sumycin course of treatment (r = 0·64, P= 0·001). The correlation between IL-6 and IL-10 was moderate in group1 (r = 0·49, P < 0·02), but was strong in group 2 (r = 0·76, P= 0·001).
There was a quicker increase of IL-2 (Fig. 1) in the ‘treated’ groups, especially in the children's group, and higher concentrations were reached earlier in the ‘treated’ groups.
DISCUSSION
IL-1α and TNF-α acting synergistically initiate the cascade of inflammatory mediators by targeting endothelium. Patients with TBE showed the highest levels of TNF-α, IL-1α and IL-6 right at the admission to the hospital. Concentrations of TNF-α, IL-1β and IL-6 were changing in parallel with strong positive correlation. Increased levels of these cytokines gradually dropped during the clinical course with a positive outcome, as it was found in our study. The most consistent correlations of clinical severity in inflammatory, autoimmune, or infectious diseases [16] and lethality in patients with sepsis [17–19] with plasma cytokine levels are clearly those with IL-6, not IL-1α or TNF-α. and of great interest was to study the role of IL-6 in the TBE patients, especially taking into consideration recent reports about the participation of cytokines of the IL-6 family in regulatory and inflammatory processes within the nervous system [20]. In patients with TBE, concentrations of IL-6 were dominant within the first week of hospitalization. Earlier was found that high concentrations of IL-6 could be neurotoxic [21], and elevated levels of IL-6 were associated with many neurological disorders and diseases [22–24]. It was an unexpected finding: children had the highest levels of IL-6 at admission, but did not have manifested symptoms of meningitis or meningoencephalitis as adults had. Adults from the Sumycin-treated group also had higher levels of IL-6 in comparison to the ‘untreated’ group. Like many other cytokines, IL-6 has both proinflammatory and anti-inflammatory properties. The covalent complex of IL6 + sIL-6R inhibited significantly the production of TNF-α in a gp130-dependent manner, whereas IL-6 and sIL-6R alone were not effective [25,26]. Thus, IL-6 requires the soluble IL-6R to stimulate cells response and neuronal survival as well as regeneration [27–29]. Also the stability of the IL-6/sIL-6R or IL-6/sIL-6R/gp130 complex determines whether gp130 on cells will be stimulated, and depending on the nature of the target cells, this may lead to neurotoxic or neuroprotective effects [20]. In the light of the data mentioned above, we have calculated the ratio of IL-6/sIL-6R, and have used it as a surrogate marker of the complex of IL-6/sIL-6R. Adults from the Sumycin-treated group had lower levels of the ratio of IL-6/sIL-6R when compared to the Sumycin-untreated patients. Concerning the highest levels of the ratio of IL-6/sIL-6R, found in children at admission, one could suppose children have other target cells in the CNS and action of the IL-6/sIL-6R complex was not neurotoxic. Another possibility is that the effect of such high levels of the IL-6/sIL-6R complex in children could be neuroprotective. Future studies of levels of IL-6 and sIL-6R and correlation of their levels in the CSF with those in the serum will help in the elucidation of not only the exact roles of IL-6 and its soluble receptor in the CNS, but also it's role in the pathogenesis of TBE.
Decreasing levels of IL-1β, TNF-α and IL-6 within the first week of hospitalization of patients with TBE were accompanied by the growth of IL-10, known as a cytokines' synthesis inhibition factor [30]. It has been determined that patients, who preferentially express high levels of IL-10 and reduced levels of TNF-α were more likely to die from meningococcemia and a variety of other infections [31,32]. The concentrations of IL-10 in the serum of TBE patients from all groups were not detected as much greater than those of proinflammatory TNF-α and IL-1β. The picture of the dynamic of IL-10 found in patients with TBE also concords with suggestions [33] that for maintaining a normal balance between TH1 and TH2 cells, the effect of IL-10 on TH polarization must be short-lived, in order not to be harmful. In the Sumycin-treated group 2, the concentration of IL-10 decreased quicker than in patients from the group taking only standard medical care. At the same time, earlier and higher peaks of IL-10 in the Sumycin-treated groups of patients may explain the higher levels of the specific antibodies (IgG), which were reached earlier in comparison to the ‘untreated’ patients.
IL-2 cytokine did not show demonstrable activity in the serum of patients with TBE within the first week of hospitalization, probably under the inhibition of growing concentrations of IL-10 [34]. The highest levels of IL-2 in the serum of TBE patients, probably, occurring after discharge from the hospital restored the balance TH1/TH2 through IFNα.
The concentrations of circulating IL-1RA and sTNFRI in the serum of TBE patients were at a 100-fold molar excess to the corresponding IL-1β and TNF-α, but sIL-6R was at a 1000-fold in comparison to IL-6. Our data have been concurred with previously described [20,35]. It is known that the ratio of IL-1 to IL-1RA is more significant for the outcome of the infection, than the concentration of IL-1 itself [36,37]. and it was showed earlier in several bacterial [37,38] and viral [39,40] infections. Just like the ratio of IL-1 to IL-1RA, the ratio of TNF to its soluble receptor could be a prognostic marker of the outcome of infectious diseases [40,41]. The increase of these ratios was the negative prognostic characteristic. In our study, the dropping dynamics of all calculated ratios characterized a positive clinical outcome of TBE. In our opinion, the ratio of a cytokine to its receptor gives a clearer impression of the balance between cytokine and its receptor. For example, levels of IL-1β in children looked more prevalent within first three days in comparison with those in the ‘treated’ group of adults. But at the same, time children had higher concentrations of the corresponding receptor antagonist. Also calculated ratios of IL-1α/IL-1RA showed that children had a better balance between the cytokine and its receptor. With regard to TNF-α, children on day 1 had lower levels of TNF-α and lower levels of sTNFRI, but the corresponding ratio was considerably higher in the children's group than in the adults from group 2. The ratio of IL-6/sIL6R also shows the state of the system cytokine- soluble receptor more efficiently than their concentrations alone. Patients from the Sumycin-treated group had higher levels of IL-6 than patients untreated with Sumycin, but the ratio of IL-6/sIL-6R was higher in the last group of patients.
In our study, Sumycin showed itself as an immunomodulator, which has been able to reduce manifestations of inflammation response during TBE viral infection; this action led to an earlier symptoms improvement and, consequently, a quicker clinical recovery. The positive result of Sumycin treatment was accompanied by certain particularities in the dynamic of studied cytokines and receptors, which expressed in the specific dynamic of the calculated ratios. The results of our study let us surmise that Sumycin might be effectively added to the treatment protocol of not only mild and moderate cases, but also of the more severe clinical course of TBE.
Acknowledgments
We thank to Dr Vladimir Orlovskiy, Chief of the Regional Center on HIV and Infectious Diseases, Novosibirsk region, Russia, for providing clinical data of patients.
References
- 1.Ecker M, Allison SL, Meixner T, et al. Sequence analysis and genetic classification of tick-borne viruses from Europe and Asia. J General Virol. 1999;80:179–85. doi: 10.1099/0022-1317-80-1-179. [DOI] [PubMed] [Google Scholar]
- 2.Bakhvalova VN, Rar VA, Tkachev SE, et al. Tick-borne encephalitis virus strains of Western Siberia. Virus Res. 2000;70:1–12. doi: 10.1016/s0168-1702(00)00174-x. [DOI] [PubMed] [Google Scholar]
- 3.Kondrusik M, Hermanowska-Szpakowicz T, Jaroszewicz E. Concentrations of tumor necrosis factor alpha and interleukin-1 beta in cerebrospinal fluid in the course of tick-borne encephalitis (in Polish) Pol Merkuriusz Lek. 1998;21:126–9. [PubMed] [Google Scholar]
- 4.Kondrusik M, Pancewicz S, Zajkowska J, Hermanowska-Szpakowicz T. Tumor necrosis factor alpha and interleukin 1-beta in serum of patients with tick-borne encephalitis (in Polish) Pol Merkuriusz Lek. 2001;61:26–8. [PubMed] [Google Scholar]
- 5.Kreil TR, Eibl MM. Viral infection of macrophages profoundly alters requirements for induction of nitric oxide synthesis. Virology. 1995;212:174–8. doi: 10.1006/viro.1995.1465. [DOI] [PubMed] [Google Scholar]
- 6.Arras C, Fescharek R, Gregersen JP. Do specific hyperimmunoglobulins aggravata clinical course of tick-borne encephalitis? Lancet. 1996;347:1331. doi: 10.1016/s0140-6736(96)90977-0. [DOI] [PubMed] [Google Scholar]
- 7.Leonova GN, Isachkova LM, Borisevich VG, Fisenko AI. Experimental tick-borne encephalitis in golden hamsters treated with specific immunotherapy (in Russian) Vopr Virusol. 2000;45:28–33. [PubMed] [Google Scholar]
- 8.Kaizer R. The clinical and epidemiological profile of tick-borne encephalitis in southern Germany 1994–98: a prospective study of 656 patients. Brain. 1999;122:2067–78. doi: 10.1093/brain/122.11.2067. [DOI] [PubMed] [Google Scholar]
- 9.Waldvogel K, Bossart W, Huisman T, Boltshauser E, Nadal D. Severe tick-borne encephalitis following passive immunization. Eur J Pediatr. 1996;155:775–9. doi: 10.1007/BF02002905. [DOI] [PubMed] [Google Scholar]
- 10.Dumpis U, Crook D, Oksi J. Tick-borne encephalitis. Clin Inf Dis. 1999;28:882–90. doi: 10.1086/515195. [DOI] [PubMed] [Google Scholar]
- 11.Kloppenberg M, Brinkman BM, de Rooij-Dijk HH, Miltenburg AM, Daha MR, Breedveld FC, Dijkmans BA, Verweij C. The Tetracycline derivative minocycline differentially affects cytokine production by monocytes and T lymphocytes. Antimicrob Agents Chemother. 1996;40:934–40. doi: 10.1128/aac.40.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapira L, Soskolne WA, Houri Y, Barak V, Halabi A, Stabholz A. Protection against endotoxic shock and lipopolysaccharide-induced local inflammation by tetracycline: correlation with inhibition of cytokine secretion. Infect Immun. 1996;64:825–8. doi: 10.1128/iai.64.3.825-828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapira L, Barak V, Soskolne WA, Halabi A, Stabholz A. Effects of tetracyclines on the pathologic activity of endotoxin: in vitro and in vivo studies. Adv Dent Res. 1998;12:119–22. doi: 10.1177/08959374980120010401. [DOI] [PubMed] [Google Scholar]
- 14.Godovikova TS, Orlova TN, Dobrikova EY. High sensitive nonradioactive detection of tick-borne encephalitis virus (in Russian) Bioorganicheskaya Chim. 1994;20:1196–205. [PubMed] [Google Scholar]
- 15.Netesova NA, Belavin PA, Rykavishnikov MU, Maligin EG. A recombinant plasmid DNA pGSDE1 coding protein E of tick-borne encephalitis virus (TBEV) and E.coli strain, producent of protein Å. 1997. TBE //Patent Russian Federation, N 97114729 02.09.97.
- 16.Dinarello CA. Cytokines as Mediators in the Pathogenesis of Septic Shock. In: Rietschel ET, Wagner H, editors. Pathology of Septic ShockBerlin Heidelberg. New York: Springer; 1996. pp. 131–65. [Google Scholar]
- 17.Hack CE, de Groot ER, Felt-Bersma RJF, et al. Increased plasma levels of interleukin in sepsis. Blood. 1989;74:1704–10. [PubMed] [Google Scholar]
- 18.Lowry SF, Moldawer LL, Calvano SE. Cytokine markers of the human responses to sepsis. In: Vincent J-L, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin Heidelberg. New York: Springer; 1994. pp. 14–23. [Google Scholar]
- 19.Fisher CJJ, Opal SM, Dhainaut J-F, et al. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. Crit Care Med. 1993;21:318–27. doi: 10.1097/00003246-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Marz P, Otten U, Rose-John S. Neuronal activities of IL-6type cytokines often depend on soluble cytokine receptors. Eur J Neurosci. 1999;11:2995–3004. doi: 10.1046/j.1460-9568.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 21.Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol. 1997;15:307–39. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- 22.Klein MA, Moller JC, Jones LL, Bluethmann H, Kreitzberg GW, Raivich G. Impaired neurological activation in interleukin-6 deficient mice. Glia. 1997;19:227–33. doi: 10.1002/(sici)1098-1136(199703)19:3<227::aid-glia5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–5. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntec H, Riederer P. Interleukin-1 and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 25.Tilg H, Vannier E, Vachino G, Dinarello CA, Mier JW. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin-1 (IL-1) receptor antagonist over interleukin-1β synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993;178:1629–36. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Igaz P, Horvath A, Horvath B, et al. Soluble interleukin-6 receptor (sIL-6R) makes IL-6R negative T cell line respond to IL-6; it inhibits TNF production. Immunol Lett. 2000;71:143–8. doi: 10.1016/s0165-2478(00)00157-7. [DOI] [PubMed] [Google Scholar]
- 27.Marz P, Cherng J-C, Gadient RA, Patterson P, Stoyan T, Otten U, Rose-John S. Sympathetic neurons can produce and respond to Interleukin-6. Proc Natl Acad Sci USA. 1998;95:3251–6. doi: 10.1073/pnas.95.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Their M, Marz P, Otten U, Weis J, Rose-John S. Interleukin-6 (IL-6) supports survival of sensory neurons: autocrine trophic effects of IL-6 and soluble IL-6 receptor and enhanced activity of an IL-6 designer cytokine. J Neurosci Res. 1996;55:411–22. doi: 10.1002/(SICI)1097-4547(19990215)55:4<411::AID-JNR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 29.Hirota H, Kiyama H, Kishimoto T, Taga T. Accelerated Nerve Regeneration in Mice by upregulating espression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med. 1996;183:2627–34. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opal SM, Wherry JC, Grint P. Interleukin-10. potential benefits and possible risks in clinical infectious diseases. Clin Infect Dis. 1998;27:1497–507. doi: 10.1086/515032. [DOI] [PubMed] [Google Scholar]
- 31.Lehmann AK, Halstensen A, Sornes S, et al. High levels of interleukin-10 in serum are associated with fatality in meningococcal disease. Infect Immun. 1995;63:2109–12. doi: 10.1128/iai.63.6.2109-2112.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dissel JT, van Langevelde P, Westerndorp RGJ, et al. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–3. doi: 10.1016/S0140-6736(05)60606-X. [DOI] [PubMed] [Google Scholar]
- 33.Pellegrini P, Contasta I, Berghella AM, Del Beato T, Casciani CU, Adorno D. The TH1 and TH2 cytokine network in healthy subjects: suggestions for experimental studies to create prognostic and diagnostic indices for biotherapeutic treatments. Cancer Biother Radiopharmaceuticals. 2000;15:267–78. doi: 10.1089/108497800414365. [DOI] [PubMed] [Google Scholar]
- 34.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA. Interleukin-1, interleukin receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–99. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. The biological properties of interleukin-1. Eur Cytokine Netw. 1994;5:517–31. [PubMed] [Google Scholar]
- 37.Pruitt JH, Welborn MB, Edwards PD. Increased soluble interleukin-1 type II receptor concentrations in postoperative patients and in patients with sepsis syndrome. Blood. 1996;87:3282–8. [PubMed] [Google Scholar]
- 38.Lennard AC. Interleukin-1 receptor antagonist. Critic Rev Immunol. 1995;15:77–105. doi: 10.1615/critrevimmunol.v15.i1.30. [DOI] [PubMed] [Google Scholar]
- 39.Ignatyev GM, Kaliberov SA, Godneva AT, Tverdokhlebov AV, Pereboeva LA, Patrusheva IV, Kashentceva EA. Immunobiol Virol Inf Proc Third Congress Europ Soc Virol. Lyon: Fondation Marcel Merieux; 1995. Immunity parameters in mice of different lines infected with Machupo or Lassa viruses; pp. 250–3. [Google Scholar]
- 40.Ignatyev G, Steinkasserer A, Atrasheuskaya A, Streltsova M, Agafonov A, Lubitz W. Experimental study of possibility of treatment of some hemorrhagic fevers. J Biotech. 2000;81:67–76. doi: 10.1016/s0168-1656(00)00300-x. [DOI] [PubMed] [Google Scholar]
- 41.Girardin E, Roux-Lomberd P, Grau GE, et al. Imbalance between tumor necrosis factor-alpha and soluble TNF receptor concentration in severe meningococcaemia. Immunology. 1992;76:20–3. [PMC free article] [PubMed] [Google Scholar]