Abstract
Vaginal candidosis represents a significant health problem to women of childbearing age worldwide. It has been postulated that localized T cells play a role in protection against vaginal candidosis. In an attempt to evaluate the role of vaginal T cells in protection against vaginal candidosis, T cell population kinetics was evaluated using an oestrogen-dependent vaginal candidosis murine model. Vaginal T lymphocytes were isolated at different time points post C. albicans inoculation, viable cells were enumerated, phenotypically analysed for the expression of CD3, CD4 and CD8 T cell markers and absolute numbers of T cell subsets were calculated. Oestrogen-induced persistence of vaginal candidosis resulted in a significant increase in the total number of vaginal lymphocytes within 24–48 h post infection; increased vaginal lymphocyte numbers persisted throughout the infection period. The number of CD3+ T cells dramatically increased following C. albicans administration and was maintained at high levels throughout the infection period. The majority of CD3+ T cells were of the CD8+ type; however, considerable numbers of both CD4+ T cells and CD4+CD8+ T cells were also observed throughout the infection period. The considerable and persistent increase in vaginal T cell numbers in general and that of CD8+ T cells in particular are evidence of the possible role played by localized T cells in protection against vaginal candidosis.
Keywords: Candida albicans, oestrogen, DTH responses, vaginal candidosis, T lymphocyte subsets
INTRODUCTION
Vaginal candidosis and recurrent vulvovaginal candidosis (RVVC) represent a major health problem to women of childbearing age in many countries [1,2]. The leading cause of vaginal candidosis, Candida albicans, resides as a commensal in the mucosa of the reproductive tract [3]. Vaginal candidosis and RVVC has been attributed to several predisposing factors including the presence of oestrogen in the reproductive tract and host compromised immunity [4]. Oestrogen is known to promote C. albicans adhesion by inducing hyphal growth [5,6]. Oestriol, pregnanediol, pregnanetrial and estradiol increase germination of C. albicans[7,8]. Moreover, the pleotropic effects of oestrogen on the epithelial surface of the reproductive tract are thought to facilitate and enhance C. albicans adhesion and colonization [6].
Studies which have examined anticandidal immune responses in women with RVVC and those carried out in murine models of oestrogen-dependent vaginal candidosis (EDVC) have collectively shown that systemic cell-mediated immunity (CMI) plays a minor role in protection against vaginal candidosis [9–13]. Although localized vaginal C. albicans infection induces systemic T cell responses [14–16], preinduced Candida-specific systemic CMI is only mildly protective against vaginal candidosis [9]. Circulating CD4+ and CD8+ T cells were shown to be of little significance in the defense against experimental vaginal candidosis [11]. This is further supported by the observation that a significant percentage of RVVC cases occur in women with competent immunity [4,17]. Several recent reports however, have indicated that CD8+ T cells play a significant role in protection against systemic and mucosal candidosis [18–20]. Studies which have addressed the role of polymorphonuclear cells [21,22] and humoral immunity [4] have shown that, though important, neither confers significant levels of protection against vaginal C. albicans infections. Based on these observations, it has been postulated that localized CMI may be responsible for the major immune response against vaginal C. albicans infections [13–16]. Significant numbers of vaginal T lymphocytes reside within the vaginal mucosa [23]. Previous studies which have evaluated the phenotypic properties of vaginal T cells have shown that the majority of these T cells are CD3+CD4+ T cells; a notably increased percentage of TCR-γδ+ T cells compared with peripheral T cells was also reported [24]. Recently, it has been reported that the percentage of the various T cell subsets does not significantly change during experimental vaginal candidosis even if vaginal T cells are involved in protection against C. albicans vaginal infection [25]. If we accept the premise that T lymphocytes do play a role in protection against vaginal candidosis [13–16,23,24], how can they do that without noticeably changing their kinetics as suggested by the report of Fidel et al.[25]. In other words, it is rather difficult to accept these findings as a plausible scenario to accommodate for possible T cell-mediated immune responses against vaginal candidosis. Therefore, the role of vaginal T cells in protection against vaginal candidosis at the vaginal mucosa level is still obscure. In this study, kinetics of vaginal T lymphocytes in terms of absolute numbers of the various T cell subsets during vaginal candidosis as means of assessing the role of T cells in protection against vaginal candidosis was addressed using the EDVC murine model. Concomitant with these studies, delayed type hypersensitivity (DTH) responses in correlation with the levels of C. albicans colonization were assayed at different time points during the course of infection in order to examine the level of immune competence of the host under the influence of oestrogen.
MATERIALS AND METHODS
Mice
Twelve to 14 week-old female Balb/c mice raised at the Hashemite University vivarium were used throughout the study.
Construction of the EDVC murine model
ATCC C. albicans strain 36082 used throughout the study was kindly provided by Dr MA Ghannoum (Mycology Reference Laboratory, University Hospital of Cleveland, OH, USA). The fungus was maintained on Sabouraud Dextrose Agar (SDA) (Difco, Detroit, MI, USA), stored at 4°C and subcultured at 3 month intervals. For inoculation, overnight cultures of C. albicans were grown at 37°C in SD broth as described previously [26]. Immediately before use, cells were harvested and washed twice in sterile physiological saline (SPS). Oestrogen was administered subcutaneously by injecting 0·5 mg of estradiol valerate (Schering AG, Germany) dissolved in 0·1 ml sesame oil 72 h prior to C. albicans inoculation and at weekly intervals thereafter. The vaginal C. albicans inoculate consisted of 50 µl containing 2 × 107 viable stationary-phase blastoconidia.
Examination of DTH responses
Testing for DTH responses was carried out as previously described [22,27,28]. Briefly, for each experimental group, separate groups of mice (4 mice per group) were right footpad-challenged with 2 × 107 heat-killed C. albicans in 50 µl pyrogen free saline at 2, 3, 4, 5 and 6 weeks post inoculation. The left footpad of all mice of all groups received 50 µl pyrogen free SPS. Thickness of the right and left footpads was measured 48 h later using a Schnelltaster caliper (H.T. Kroplin Hessen, Schluchtern, Germany). The reaction was considered as positive when the difference between the right versus left footpads was greater than 0·2 mm [29]. Data reported here is the average of three separate experiments.
Evaluation of C. albicans colonization
Mice were sacrificed at different time points post C. albicans inoculation; the vagina was isolated, examined for the presence of white lesions characteristic of C. albicans infection, trimmed and homogenized in 10 ml SPS in a sterile glass homogenizer (Ystral GmbH, Gottingen, Germany). Serial 10-fold dilutions were prepared from the homogenate; 1 ml aliquots of the appropriate dilution were added into culture plates containing 10 ml SDA and chloramphenicol at 50 mg/l, plates were left to solidify and then incubated at 37°C; each sample dilution was cultured in triplicate. Yeast colonies were counted 48 h after plating and colonization results were expressed as the mean colony-forming unit (CFU) per mouse based on data from three animals per group.
Isolation and phenotypic analysis of vaginal T lymphocytes
Isolation of vaginal T lymphocytes, staining and flow cytometric analysis was performed essentially as previously described [23–25,30]. Briefly, 5–6 mice were sacrificed per group per time point, the vagina was isolated and flushed with normal saline, opened up longitudinally and cut into 2 mm pieces. Tissue pieces were placed in 50 ml of warm RPMI-1640 (Sigma Chemicals, St Louis, MO, USA) solution containing 10 mm EDTA plus 1 mm DTT and stirred for 30 min at 37°C. The suspension was filtered through a 1-g nylon wool column moisturized with warm HBSS (Sigma Chemicals), filtrate was centrifuged and cell pellet was suspended in 1 ml HBSS. Cells were then counted, evaluated for viability using trypan blue vital stain and prepared for staining; 104 and 105 viable cells in 100 µl HBSS were used per sample for single- and dual-colour flow cytometric analysis, respectively.
Biotin-labelled anti-CD3 (KT3), PE-labelled anti-CD4 (YTS191·1) and FITC-labelled anti-CD8 (KT15) antibodies were obtained from Serotec Ltd. (Serotec Ltd., Oxford, UK). For single-colour analysis, biotin-labelled anti-CD3 was added at 1 µl per sample, left to react for 30 min on ice, cells were then centrifuged, washed with 100 µl HBSS, cell pellet was resuspended in 100 µl HBSS and reacted with PE-labelled streptavidin. For dual-colour analysis, cell samples were directly reacted with 1 µl PE-labelled anti-CD4 and 2 µl FITC-labelled anti-CD8 for 30 min on ice prior to washing. Bitmapping of vaginal T cells was based on cell size and granularity properties of control lymphocyte populations (thymocytes, spleen cells and lymph node cells). Flow cytometric analysis was performed on a Becton Dickinson flow cytometer (Mountain View, CA, USA); 1–5 × 104 cells were analysed per sample and data were collected and analysed using an Apple Macintosh computer equipped with Simulset software.
Statistical analysis
Using experimental mean values, analysis of variance (anova) test was employed to determine levels of significance within each experimental group (one-way anova) and between different experimental groups (two-way anova) regarding DTH responses and lymphocyte absolute numbers. Fisher's least significant difference (LSD) test was used to determine the difference between different means. Statistical analysis of T lymphocyte subpopulations within and between experimental groups was conducted using the student t-test at P < 0·005.
RESULTS
Oestrogen treatment results in persistence of colonization and suppression of DTH responses
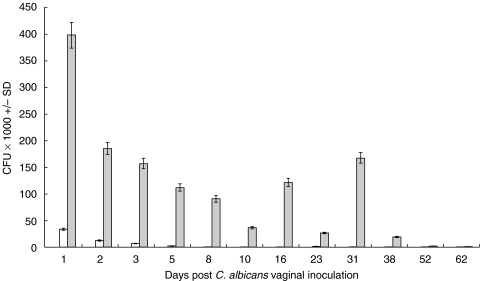
Levels of vaginal C. albicans colonization were evaluated at different time points following inoculation. Consistent with previous reports [31], treated infected mice exhibited significantly higher levels of C. albicans burden compared with that in infected untreated mice. Furthermore, C. albicans colonization was far more persistent in the treated infected group compared with that in the untreated infected group (Fig. 1). Though persistent, levels of colonization in treated infected mice precipitously decreased until the infection was completely cleared off by week eight of inoculation. No detectable levels of C. albicans colonization were observed in mice from the naive untreated control group.
Fig. 1.
Vaginal C. albicans burden during experimental vaginal candidosis. Levels of C. albicans colonization in naïve untreated mice, untreated infected mice and treated infected mice were assessed at days 1, 2, 3, 5, 8, 10, 16, 23, 31, 38, 52 and 62 post infection. Mean C. albicans CFU count per vagina ± SD was calculated based on three separate experiments per group. □ infected untreated mice;  infected treated mice.
infected treated mice.
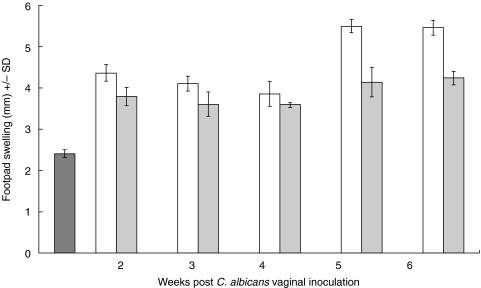
Footpad swelling, as a measure of the DTH response, was evaluated in untreated infected mice, treated infected mice and naïve untreated mice. As shown in Fig. 2, there were significant differences between untreated naïve mice and infected untreated mice (P < 0·00000005) or infected treated mice (P < 0·0000005) throughout the study period. Consistent with previous reports [31], footpad swelling was significantly greater in untreated infected mice compared with that in treated infected mice especially at week 5 (P < 0·000002) and week 6 (P < 0·00002). This suggests that oestrogen has a suppressive effect on the capacity of treated infected mice to mount effective cell-mediated immune responses against vaginal candidosis. Notwithstanding the pleotropic effects of oestrogen [5,6], this may partially explain the persistence of C. albicans colonization in the EDVC model.
Fig 2.
DTH responses at different time points during experimental vaginal candidosis. DTH responses were assayed by measuring the footpad swelling 48 h after right footpad challenge with 2 × 107 heat-killed C. albicans in naïve untreated mice untreated infected mice and treated infected mice. In infected mice, DTH was measured at weeks 2, 3, 4, 5 and 6 post infection. Data presented represents the average ± SD of three separate experiments per group. □ infected untreated mice;  infected treated mice,
infected treated mice,  naïve untreated mice.
naïve untreated mice.
Kinetics of vaginal lymphocytes during experimental vaginal candidosis
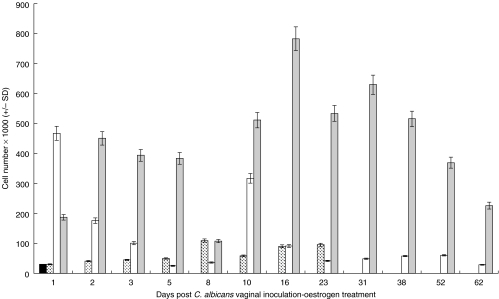
The absolute numbers of vaginal lymphocytes isolated from naïve untreated mice, treated noninfected mice, untreated infected mice and treated infected mice were evaluated at different time points following the start of the experiment. As shown in Fig. 3, the number of vaginal lymphocytes was less than 3 × 104 cells per naïve mouse. Consistent with previous findings [25], oestrogen treatment alone resulted in a minor increase in the absolute numbers of vaginal lymphocytes, which was statistically insignificant when compared with that observed in naive untreated mice. The kinetics of vaginal lymphocytes in the treated noninfected group was such that absolute lymphocyte numbers gradually but insignificantly increased within the first week; the numbers remained basically unchanged for the rest of the treatment period (24 days) in this group. Consistent with the transient pattern of C. albicans colonization in untreated infected mice, the absolute number of vaginal lymphocytes precipitously decreased to background levels within about two weeks post infection. In that, absolute numbers at day one were significantly higher than that at all subsequent time points (P = 3·86 × 10−13 for day 1 versus day 8; P = 1·94 × 10−12 for day 1 versus day 16 and P = 7·61 × 10−13 for day 1 versus day 52). In the treated infected group, the number of vaginal lymphocytes continued to increase following infection; a peak was consistently noted between the third and fourth week of infection (day 16 to day 31). Statistically significant differences in absolute numbers were observed when comparing the early versus middle phases of the infection period. In that statistical comparison of absolute lymphocyte numbers obtained at day 1 versus those obtained at day 16 yielded a P-value of 1·97 × 10−7, day 2 versus day 16 yielded a P-value of 0·000072 and day 8 versus day 16 yielded a P-value of 4·9 × 10−8. Furthermore, statistically significant differences in absolute numbers were also observed when comparing the middle versus late phases of the infection period. In that statistical comparison of absolute lymphocyte numbers obtained at day 16 versus those obtained at day 52 yielded a P-value of 8·79 × 10−6 and day 16 versus day 62 yielded a P-value of 4·02 × 10−7. However, no significant differences were observed when absolute numbers at day 16 were compared with those observed at day 31 (P = 0·018862) or when those observed at day 23 were compared with those observed at day 31 (P = 0·111129). Absolute lymphocyte numbers observed in untreated infected mice were statistically different from those observed in treated infected mice at various time points (P < 0·001) and from those observed in untreated infected mice during the early phase of infection (P < 0·001). Overall, increased numbers of vaginal lymphocytes were maintained throughout the infection period in the treated infected group compared with those in the untreated infected group or the naïve untreated control group. These results suggest that either a massive influx of peripheral lymphocytes occurs immediately following C. albicans infection or that significant levels of in situ lymphocyte proliferation proportional to the levels of colonization occur during the course of infection or perhaps a combination of both events.
Fig 3.
Quantitative kinetics of vaginal lymphocytes during experimental vaginal candidosis. Absolute numbers of total vaginal lymphocytes isolated from the naïve untreated mice, treated noninfected mice, untreated infected mice and treated infected mice were evaluated at different time points post infection and or oestrogen treatment. At each time point, 5–6 mice per group were sacrificed, pooled cell preparations were enumerated and the average number of cells per vagina was calculated. Data shown represent the average ± SD of three separate experiments. ▪ naïve untreated mice,  naïve treated mice, □ infected untreated mice;
naïve treated mice, □ infected untreated mice;  infected treated mice.
infected treated mice.
Vaginal T lymphocyte subset distribution during experimental vaginal candidosis
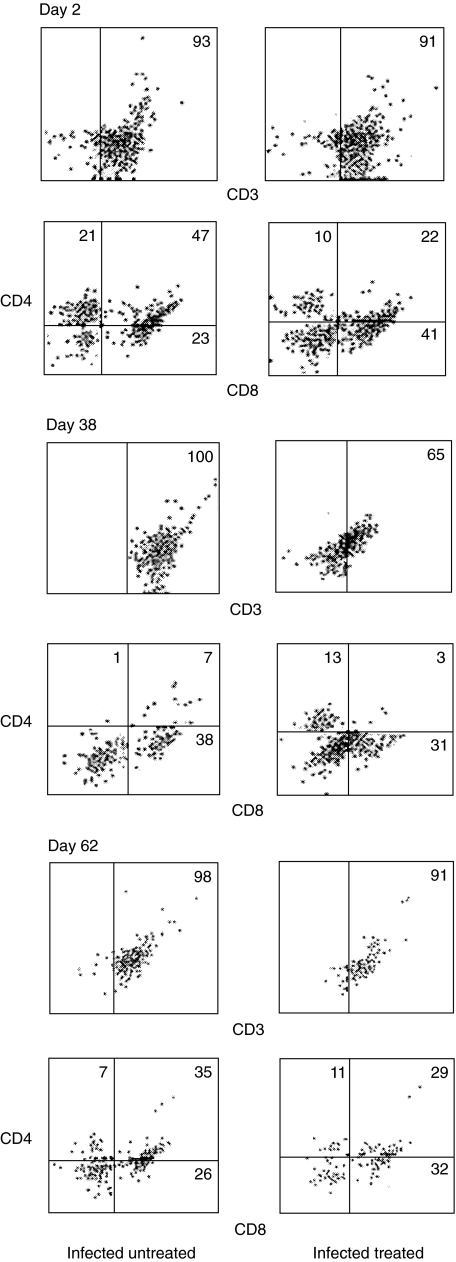
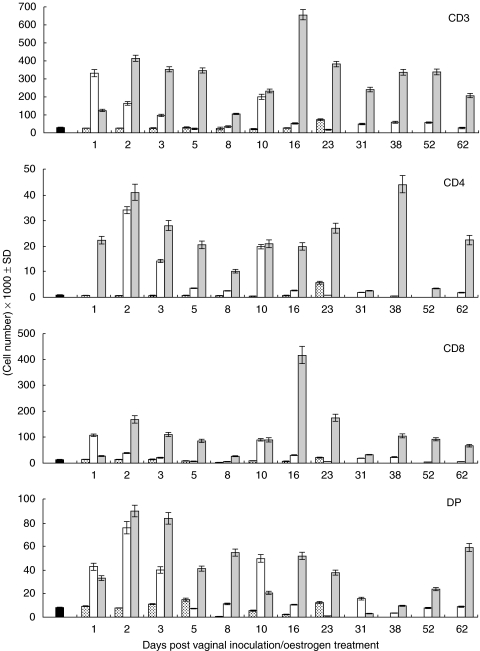
Distribution of the various vaginal T lymphocyte subsets at different time points during experimental vaginal candidosis was phenotypically evaluated. Figure 4 depicts the percentage distribution of vaginal CD3+, CD4+ and CD8+ T lymphocytes isolated from untreated infected mice and treated infected mice at representative time points. In the untreated infected group, the percentage of CD3+ remained at greater than 90% throughout the study period; however, it fluctuated between 60 and 90% in the treated infected group. Additionally, the percentage of CD8+ T cells in both groups was higher than that of CD4+ T cells at all time points tested. To gain more insight into the vaginal T lymphocyte population kinetics during experimental vaginal candidosis, absolute numbers per T cell subset in naive untreated mice, treated noninfected mice, untreated infected mice and treated infected mice were evaluated at different time points following the start of the experiment (Fig. 5). CD3+, CD8+ and CD4+ T cell subsets were generally present at significantly higher numbers in the treated infected group compared with that in the untreated infected group (P < 0·000282 for CD3, P < 0·014006 for CD8 and P < 0·004637 for CD4). Though low, the absolute numbers of CD3+, CD4+ and CD8+ T cell subsets were significantly higher in the untreated infected group compared with those in the naïve untreated group throughout the infection period (P < 0·0005) and with those in the treated noninfected group (P < 0·01). No statistically significant differences were observed when absolute numbers of CD3+, CD4+ or CD8+ T cell subsets in naive untreated mice were compared with those in treated noninfected mice at any time point following the star of the experiment. This suggests that oestrogen, independent of infection, may not be sufficient to induce significant changes in the number or subset distribution of the vaginal T cell population. The majority of CD3+ T cells isolated from the infected treated group were CD8+ this was consistent throughout the study period. Absolute numbers of CD4+ T cells consistently remained at less than 10–15% that of the total CD3+ T cell numbers. Similar to the pattern of total lymphocyte numbers shown in Fig. 3, a peak in CD3+ T cells and CD8+ T cells was repeatedly noted at around week three-four of infection. Prior to and during this period, levels of C. albicans colonization showed a precipitous decrease followed by an increase at about week 5 post infection; this was consistently observed in three separate experiments.
Fig 4.
Expression of CD3, CD4 and CD8 T cell markers on vaginal T cells during experimental vaginal candidosis. Vaginal T cells were isolated from infected untreated mice and from infected treated mice at days 2, 38 and 62 post infection, cells were pooled from 5 to 6 mice per group. Cells were reacted with biotin-labelled anti-CD3 for single colour analysis or with FITC-labelled anti-CD8 and PE-labelled anti-CD4 for dual colour analysis. Data presented is representative of three separate experiments per group.
Fig 5.
Absolute numbers of the various vaginal T cell subsets during experimental vaginal candidosis. Mean absolute numbers of CD3+, CD8+, CD4+ and CD4+8+ (DP) vaginal T cells isolated from naïve untreated mice, treated noninfected mice, untreated infected mice and treated infected mice were calculated at different time points following the start of the experiment. Mean cell number per vagina was obtained from pooled cell preparations isolated from 5 to 6 mice per group ± SD, data reported is based on three separate experiments per group. □ infected untreated mice;  infected treated mice,
infected treated mice,  naïve treated mice; ▪ naïve untreated mice.
naïve treated mice; ▪ naïve untreated mice.
Interestingly, a population of CD4+CD8+ (DP) T cells was detected in naïve mice, which expanded considerably in the untreated infected and treated infected groups during the course of infection (Figs 4 and 5). Numbers of DP T cells were statistically not different in the untreated infected versus that in the treated infected group (P < 0·0738).
DISCUSSION
Several important points can be concluded based on the results of this study. First, the total number of vaginal lymphocytes significantly increases and vaginal lymphocyte subset distribution significantly changes during experimental vaginal candidosis. This is consistent with previous reports, which have shown that the number of T cells especially those expressing the CD4 T cell marker significantly increase in a rat model of vaginal candidosis [32–34]. Increased T cell numbers following C. albicans infection may result from peripheral T cell homing or from in situ T cell proliferation or both. This is indicative of a possible involvement of T cells in the defense against vaginal C. albicans infection. Previously, it has been suggested that effective immune responses against vaginal candidosis occur mainly through the cellular arm of the immune system [35]. Several reports have documented the indirect involvement of T cells in fighting off localized C. albicans infections through different mechanisms [9–16]. Furthermore, persistence of localized C. albicans infection in immunocompromized individuals and in T cell-deficient animal models is very well established [4,36,37]. Homing of T lymphocytes from the periphery to the vagina as means of initiating or consolidating cell-mediated immune responses against vaginal infections is well documented [38,39]. Inconsistent with these findings however, is the recent observation that, notwithstanding the possible role of T cells in fighting off C. albicans infection at the vaginal mucosa level, T cells may operate without showing significant changes in their subset percentage distribution [25]. This discrepancy can be reconciled by observing that, where in the current study we have evaluated T cell subsets in terms of absolute numbers, the previous study has evaluated T cells in terms of subset percentage distribution. Changes in the number of total vaginal lymphocytes, let alone changes in numbers of the various T cell subsets, observed during the course of infection as reported here is clear evidence that subset percentage distribution analyses alone is not enough to address the role of T cells in vaginal candidosis. It should be mentioned that similar to the findings reported by Fidel et al. [25], little changes in T cell percentage distribution were noted when subset percentage analyses were carried out on results obtained by this study (data not shown).
Second, results presented here indicate that most changes in T cell number are attributable to the increase in the number of CD3+CD8+ T cells suggesting that this subset is involved in the elimination of vaginal C. albicans infections. In agreement with this finding, previous studies have shown that CD8+ T cells are involved in the elimination of C. albicans from the livers of mice [18] and in protection against gastrointestinal mucosa C. albicans infections [20]. Additionally, activated CD8+ T cells were shown to inhibit the growth of C. albicans hyphae [19]. Of interest is the observation that changes in CD4+ vaginal T cell numbers during experimental vaginal candidosis were much less pronounced compared with those of CD8+ T cells. This is slightly different from previous findings however, which have indicated that CD4+ and not CD8+ vaginal T cells are the subject of considerable change in a rat model of vaginal candidosis [33,34]. Previous studies have shown that vaginal T cells partially express a unique CD4 molecule which may not be picked up by antibodies against the conventional CD4 molecule [40], which may partially explain the low numbers of CD4+ T cell compared to that of CD8+ T cells. It is worth noting that at any time point during the course of infection, the combined number of CD4+, DP and CD8+ T cells is less than that of the number of CD3+ T cells. Therefore, it is possible that this difference can be accounted for by undetected CD4+ vaginal T cells. While the results implicate the CD8+ vaginal T cell subset as a potential player in protection against vaginal candidosis, one should not loose sight of the possible role of CD4+ T cells in this process which have been recently reported to play a major immunological role against vaginal candidosis [34].
Third, the patterns of C. albicans colonization, DTH responses and T cell subset population kinetics suggest that, under the influence of oestrogen, there is continuous interaction between the pathogen and T cells. Coupled with the oestrogen-induced colonization-enhancing conditions [5,6], suppressed immunity in the EDVC mouse model may be partially responsible for the prolonged persistence of C. albicans vaginal infection. Not withstanding the fact that it takes more than 8 weeks for the infection to resolve in infected mice in the presence of oestrogen compared with about 1 week in the absence of oestrogen, the infection do ultimately resolve. Instead of viewing the dramatic increase in T cell numbers during vaginal candidosis and suppressed immunity as contradictory findings, they may be two aspects of the same scenario, in that immunosuppression may be qualitative rather than quantitative. Should this hold true, T cells are likely to be undergoing continuous interaction with the pathogen during active vaginal candidosis until they clear off the infection. The alternative to this possibility is to view immunosuppression in this case as a periphery restricted phenomenon. This is highly unlikely based on the observation that vaginal candidosis in the EDVC model is persistent [31] and the observation that systemic T cells play a minor role in protection against vaginal candidosis [9]. Therefore, irrespective of the state of immunocompetence at the systemic level, immunocompetence at the local level is the significant component in the fight against vaginal candidosis.
Fourth, CD4+CD8+ (DP) T cells seen in the vaginal mucosa meliu before and during the course of infection, though may not have functional role, may be in situ T cell precursors involved in some sort of a local (extrathymic) T cell development event within the vaginal mucosa. As a precedent for this proposition, the occurrence of extrathymic T cell development within the murine small intestinal mucosa has been extensively studied and documented [30,41]. It is worth noting that parallels between vaginal T cells and intestinal intraepithelial lymphocytes (IEL) can be drawn. Both populations are localized, they both contain much higher percentages of TCR-γδ+ T cells and CD3− T cells compared with peripheral T cells [23,24]. Parallels between the vaginal mucosa and that of the small intestine in terms of continuous exposure to diverse repretoires of pathogens and the need for localized, rapid and efficient immune response of relative-independence of the periphery are readily clear. In this context, it is of interest to evaluate the possibility of localized extrathymic T cell development and functional maturation within the vaginal mucosa meliu.
In conclusion, results obtained by this study suggest that changes in the number of vaginal T cell subsets do indeed occur during experimental vaginal candidosis; most significant changes occur in the number of CD8+ T cells. This is indicative of the potential role of T cells in the defense against vaginal candidosis. In light of these findings, persistence of vaginal candidosis can be interpreted in immunological terms as a window of time required for the immune system to muster enough strength to clear off the infection; the length of which varies depending on the degree of immunocompetence of the host. In healthy women of childbearing age, the concentration of oestrogen in the reproductive tract environment might be a factor of great significance in this regard.
Acknowledgments
This work was supported by research grant MH-99/02, The Hashemite University, Jordan. The authors wish to thank Dr Ali Elkarmi for fruitful insights and Dr Basma Hasan for technical help with flow cytometry.
References
- 1.Ferrer J. Vaginal candidosis. epidemiological and etiological factors. Intl J Gynecol Obstet. 2000;71:521–7. doi: 10.1016/s0020-7292(00)00350-7. [DOI] [PubMed] [Google Scholar]
- 2.Sobel JD. Pathogenesis and epidemiology of vulvovaginal candidosis. Ann NY Acad Sci. 1988;544:547–57. doi: 10.1111/j.1749-6632.1988.tb40450.x. [DOI] [PubMed] [Google Scholar]
- 3.Arendorf TM, Walker DM. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol. 1980;25:1–15. doi: 10.1016/0003-9969(80)90147-8. [DOI] [PubMed] [Google Scholar]
- 4.Odds FC. Candida and Candidosis. 2. London: Bailliere Tindall; 1988. [Google Scholar]
- 5.Larsen B, Galask RP. Influence of estrogen and normal flora on vaginal candidiasis in the rat. J Reprod Med. 1984;29:863–8. [PubMed] [Google Scholar]
- 6.Kinsman OS, Collard AE. Hormonal factors in vaginal candidiasis in rats. Infect Immun. 1986;53:498–504. doi: 10.1128/iai.53.3.498-504.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinsman OS, Pitblado K, Coulson CJ. Effect of mammalian steroid hormones and luteinizing hormone on the germination of Candida albicans and implications for vaginal candidosis. Mycoses. 1988;31:617–24. doi: 10.1111/j.1439-0507.1988.tb04416.x. [DOI] [PubMed] [Google Scholar]
- 8.White S, Larsen B. Candida albicans morphogenesis is influenced by estrogen. Cell Mol Life Sci. 1997;53:744–9. doi: 10.1007/s000180050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fidel PL, Lynch ME, Sobel JD. Effects of pre-induced Candida-specific systemic cell-mediated immunity on experimental vaginal candidiasis. Infect Immun. 1994;62:1032–8. doi: 10.1128/iai.62.3.1032-1038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fidel PL, Lynch ME, Conaway DH, et al. Mice immunized by primary vaginal Candida albicans infection develop acquired vaginal mucosal immunity. Infect Immun. 1995;63:547–53. doi: 10.1128/iai.63.2.547-553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidel PL, Lynch ME, Sobel JD. Circulating CD4 and CD8 T cells have little impact on host defense against experimental vaginal candidiasis. Infect Immun. 1995;63:2403–8. doi: 10.1128/iai.63.7.2403-2408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidel PL, Cutright JL, Sobel JD. Effects of systemic cell-mediated immunity on vaginal candidiasis in mice resistant and susceptible to Candida albicans infections. Infect Immun. 1995;63:4191–4. doi: 10.1128/iai.63.10.4191-4194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steele C, Ozenci H, Luo W, et al. Growth inhibition of Candida albicans by vaginal cells from naive mice. Med Myco. 1999;37:251–9. [PubMed] [Google Scholar]
- 14.Fidel PL, Lynch ME, Sobel JD. Candida-specific cell-mediated immunity is demonstrable in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:1990–5. doi: 10.1128/iai.61.5.1990-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidel PL, Lynch ME, Sobel JD. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun. 1993;61:4202–7. doi: 10.1128/iai.61.10.4202-4207.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidel PL, Ginsburg KA, Cutright JL, et al. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J Infect Dis. 1997;176:728–39. doi: 10.1086/514097. [DOI] [PubMed] [Google Scholar]
- 17.Giraldo P, von Nowaskonsk A, Gomes FA, et al. Vaginal colonization by Candida in symptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obst Gyn. 2000;95:413–6. doi: 10.1016/s0029-7844(99)00577-3. [DOI] [PubMed] [Google Scholar]
- 18.Kretchmar M, Jung E, Fontagnier B, et al. Activation of CD8+ T cells are involved in the elimination of Candida albicans from the livers of mice. Mycoses. 1997;11:41–6. doi: 10.1111/j.1439-0507.1997.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 19.Beno D, Stover AG, Herbert L, et al. Growth inhibition of Candida albicans Hyphae by CD8+ Lymphocytes. J Immunol. 1995;154:5273–81. [PubMed] [Google Scholar]
- 20.Jones-Carson J, Vazquez-Torez A, Balish E. B cell-independent selection of memory T cells after immunization with Candida albicans. J Immunol. 1997;158:4328–35. [PubMed] [Google Scholar]
- 21.Black AC, Fiona ME, Russel A, et al. Acute neutropenia decreases inflammation associated with murine vaginal candidosis but has no effect on the course of infection. Infect Immun. 1998;66:1273–85. doi: 10.1128/iai.66.3.1273-1275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romani L, Mencacci A, Cenci E, et al. Natural killer cells do not play a dominant role in the CD4+ subset differentiation in Candida albicans-infected mice. Infect Immun. 1993;61:3769–74. doi: 10.1128/iai.61.9.3769-3774.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibraghimov A, Sacco RE, Sandor M, et al. Resident CD4+αβ T cells in the murine female genital tract: a phenotypically distinct T cell lineage that rapidly proliferates in response to systemic T cell activation stimuli. Intl Immunol. 1995;7:1763–9. doi: 10.1093/intimm/7.11.1763. [DOI] [PubMed] [Google Scholar]
- 24.Fidel PL, Wolf NA, Kukuruga MA. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun. 1996;64:3793–9. doi: 10.1128/iai.64.9.3793-3799.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fidel PL, Luo W, Steele C, et al. Analysis of vaginal cell populations during experimental vaginal candidosis. Infect Immun. 1999;67:3135–40. doi: 10.1128/iai.67.6.3135-3140.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Elteen KH, Abdul-Malek AMM, Abdul-Wahid NA. Prevalence and susceptibility of vaginal yeast isolates in Jordan. Mycoses. 1997;40:179–85. doi: 10.1111/j.1439-0507.1997.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 27.Cenci E, Romani L, Vecchiarelli A, et al. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989;57:3581–7. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romani L, Mocci S, Bietta L, et al. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–54. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagaya K, Shinoda T, Fukazawa Y. Murine defense mechanism against Candida albicans. In collaboration of cell-mediated and humoral immunities in protection against systemic C. albicans infection. Microbio Immunol. 1981;25:647–54. doi: 10.1111/j.1348-0421.1981.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 30.Hamad M, Wetsell M, Klein JR. T cell precursors in the spleen give rise to complex T cell repertoires in the thymus and the intestine. J Immunol. 1995;155:2866–76. [PubMed] [Google Scholar]
- 31.Hamad M, Abu-Elteen KH, Ghaleb MM. Persistent colonization and transient suppression of DTH responses in an estrogen-dependent vaginal candidosis murine model. The New Microbiologica. 2002;25:65–73. [PubMed] [Google Scholar]
- 32.De Bernardis F, Boccnera M, Adriani D, et al. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginits in rats. Infect Immun. 1997;65:3399–405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Bernardis F, Santoni G, Boccnera M, et al. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68:3297–304. doi: 10.1128/iai.68.6.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santoni G, Boccanera M, Adriani D, et al. Immune cell-mediated protection against vaginal candidiasis. evidence for a major role of vaginal CD4 (+) T cells and possible participation of other local lymphocyte effectors. Infect Immun. 2002;70:4791–7. doi: 10.1128/IAI.70.9.4791-4797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidel PL, Sobel JD. Protective immunity in experimental Candida vaginitis. Res Immunol. 1998;149:361–73. doi: 10.1016/s0923-2494(98)80760-8. [DOI] [PubMed] [Google Scholar]
- 36.Balish E, Wagner RD, Vazquez-Torres A, et al. Mucosal and systemic candidiasis in IL-8Rh-\-Balb\c mice. J Leuko Biol. 1999;66:144–50. doi: 10.1002/jlb.66.1.144. [DOI] [PubMed] [Google Scholar]
- 37.Balish E, Wagner RD, Vazquez-Torres A, et al. Candidiasis in interferon γ knockout (IFN-γ–\–) mice. J Infect Dis. 1998;178:478–87. doi: 10.1086/515645. [DOI] [PubMed] [Google Scholar]
- 38.McDermott MR, Goldsmith CH, Rosenthal KL, et al. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J Infect Dis. 1989;159:460–4. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]
- 39.King NJC, Parr EL, Parr MB. Migration of lymphoid cells from the vaginal epithelium to Iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. 1998;160:1173–80. [PubMed] [Google Scholar]
- 40.Wormley FL, Scott M, Luo W, et al. Evidence for a unique expression of CD4 on murine vaginal CD4+ cells. Immunology. 2000;100:300–8. doi: 10.1046/j.1365-2567.2000.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poussier P, Edouard P, Lee C, et al. Thymus-independent develop-ment and negative selection of T cells expressing T cell receptor α/β in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J Exp Med. 1992;176:187–99. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]