Abstract
Butyrate, a short-chain fatty acid released by colonic bacteria and administered therapeutically in inflammatory bowel diseases, exerts immunomodulatory properties. The aim of the study was to determine the functional consequences of butyrate exposure on the proinflammatory responsiveness of human intestinal epithelial cells (IEC). IL-8 promoter activity in IEC pretreated with butyrate then exposed to proinflammatory stimuli was assayed by transfection of luciferase constructs. IL-8 secretion was determined by ELISA and neutrophil migration by flow cytometry. Receptor mRNA was assessed by reverse transcriptase–polymerase chain reaction (RT-PCR). Butyrate modulated proinflammatory IL-8 secretion differentially in Caco-2 and HT-29 cells on the transcriptional level. Pointing to the potentially underlying mechanism of increased IL-1β-stimulated IL-8 secretion in HT-29 cells, butyrate up-regulated IL-1RI mRNA but not IL-1RII. Butyrate pretreatment of IEC lines stimulated by IL-1β modulated neutrophil migration significantly: reduction towards Caco-2 and enhancement towards HT-29/p cells. Pharmacological inhibition of protein tyrosine phosphatases or treatment with mesalamine or sulphasalazine diminished IL-1β-stimulated IL-8 secretion by butyrate-exposed HT-29 cells substantially. Immunomodulatory effects of butyrate on IEC are functionally relevant for neutrophil migration. Pharmacological inhibition of enhanced IL-1β-mediated IL-8 secretion in a subpopulation of IEC may improve the clinical efficacy of butyrate.
Keywords: cytokines, chemotaxis, intestinal epithelial cell, neutrophils, short-chain fatty acid
INTRODUCTION
The inflammatory bowel diseases, Crohn's disease and ulcerative colitis are thought to result from a perturbed immune response to luminal antigens in a genetically and environmentally disposed host [1]. Peptide regulatory factors such as cytokines, chemokines and growth factors contribute substantially to the initiation and perpetuation of intestinal inflammation leading to activation of mucosal cell populations and recruitment of circulating leucocytes. IL-1 is a proinflammatory cytokine of pivotal importance for the pathogenesis of inflammatory bowel diseases produced by activated monocytes, macrophages, fibroblasts, smooth muscle cells and endothelial cells [2]. Intestinal epithelial cells (IEC) form a physical barrier, but increasing evidence suggests that these cells have an active role in the initiation and perpetuation of intestinal inflammation. Human colonic carcinoma cell lines and primary intestinal epithelial cells have been shown to respond to IL-1 and other proinflammatory cytokines and to produce chemokines such as IL-8, a chemotactic and activating peptide for neutrophils [3]. Due to their exposed localization at the luminal surface and their involvement in mucosal immune homeostasis, IEC have become a potential target for anti-inflammatory treatment strategies comprising inhibition of proinflammatory cytokine production, receptor binding and signalling as well as induction, up-regulation or delivery of anti-inflammatory or immunoregulatory cytokines.
Butyrate, a short-chain fatty acid derived from bacterial fermentation of luminal carbohydrates, is the principal energy source of the colonic epithelium [4] and modulates enterocyte differentiation, proliferation and restitution [5,6]. Studies performed in vitro and in vivo support the assumption that butyrate has immunoregulatory effects on IEC and other mucosal cell population [7–10]. Typically, butyrate modulates gene expression induced by other factors through either stimulatory or inhibitory actions. Some of these activities are attributed to hyperacetylation of histones by inhibition of histone deacetylase [11]. Several pilot studies of administration of butyrate enemas to patients with ulcerative colitis have produced promising results. In the majority of larger trials, however, the results were unsatisfactory [12–14]. Insufficient clinical efficacy of butyrate enemas could be due to proinflammatory actions of butyrate on the target cell population, mainly IEC. In the present study we focused on the functional consequences of butyrate-modulated proinflammatory IL-8 secretion and pharmacological antagonism.
MATERIALS AND METHODS
Cell culture
Transformed human colonic HT-29/p epithelial cells (American Type Culture Collection, Bethesda, MD, USA; ATCC HTB 38) and HT-29/MTX cells, permanently differentiated upon transient exposure to methotrexate (generously provided by Dr Lesuffleur, INSERM, Villejuif, France) [15] were used between passages 20–40 and grown in Dulbecco's minimal essential medium (DMEM; Gibco, Long Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 2 mm l-glutamine (Gibco), and antibiotics/antimycotic (penicillin 100 U/ml, streptomycin 100 µg/ml, amphotericin B 250 ng/ml; Sigma, St Louis, MO, USA).
Caco-2 cells (ATCC HTB-37) at passages 40–60 were cultured in minimum essential medium (MEM, Gibco) containing 2 mm l-glutamine, 10% heat-inactivated fetal bovine serum, antibiotics and 100 µm nonessential amino acids (Gibco).
Human intestinal primary epithelial cell (HIPEC) lines in long-term cultures were established as described previously [16]. Briefly, isolated crypt cells from surgically resected specimens were cultured in mucosal tissue derived autologous growth factor containing F12 medium supplemented with epidermal growth factor, insulin, transferin, retinoic acid and hydrocortisone (HIPEC medium). At the time of the experiments HIPECs were grown in HIPEC medium without autologous growth factor but supplemented with 5% dialysed fetal calf serum (Summit Biotech, Atlanta, GA, USA). HIPECs were derived from normal areas (at least 10 cm away from the tumour) of transverse colon (HIPEC-TDT), sigmoid (HIPEC-JHS) and rectum (HIPEC-MJR) from three different individuals with colon cancer.
The study was conducted in accordance with the second Helsinki Declaration.
Cells were cultured in a water-saturated atmosphere of 95% air/5% CO2. All cells were stimulated at subconfluence by human recombinant IL-1β (R&D Systems, Minneapolis, MN, USA). HT-29/p cells were also stimulated by human recombinant TNF-α (R&D Systems) or lipopolysaccharide (LPS, strain 0127: B8; Sigma). Sodium butyrate (Sigma) was added to the culture media at 5 mm. Neutralizing monoclonal anti-IL-8 antibody and the IgG goat control antibody (R&D Systems) were used at 10 µg/ml. The following agents were used (all Sigma): mesalamine at 0·1–10 mm, sulphasalazine at 0·1–2·5 mm, dexamethasone at 10-8-10-4 m, orthovanadate at 1 mm, staurosporine at 1 µm, genistein at 20 µm, wortmannin at 10 µm and the calcium ionophore A23187 at 10 µm.
RNA extraction and amplification by reverse-transcription–polymerase chain reaction (RT-PCR)
RNA was isolated using the Trizol method (Life Technologies, Long Island, NY, USA); 0·5 µg of total RNA was reverse transcribed in a volume of 25 µl containing 25 U RNasin (Promega, Madison, WI, USA), 0·5 mm dNTPs (dATP, dCTP, dGTP and dTTP; Pharmacia Biotech, Piscataway, NJ, USA), 5 nm of random hexamer primers and 125 U of Moloney murine leukaemia virus reverse transcriptase (Gibco). The reaction was carried out for 1 h at 39°C followed by 7 min at 93°C and 1 min at 1°C. PCR was carried out in a volume of 50 µl containing 1 µl of the reverse transcriptase mixture, 1 × PCR buffer (Perkin Elmer, Foster City, CA, USA), 5 pmol of each primer, 0·5 mm dNTPs and 1.25 U of Thermo aquaticus polymerase (Perkin Elmer). PCR was carried out in a 9600 Perkin-Elmer cycler set for various cycles to monitor the linearity of the amplification. The results presented were obtained at 32–36 cycles. The PCR temperatures used were 94°C for 45 s (denaturing), 56°C for 45 s (annealing) and 72°C for 2 min (polymerization) followed by a final extension at 72°C for 5 min. The PCR products (1/5 volume) were electrophoresed on 2% agarose gels containing ethidium bromide. A negative from the photographs of the gels (Polaroid 665 film, Polaroid Corp., Cambridge, MA, USA) was scanned with a silverscanner II PS v2·1a connected to a Power Mac 8100/80 computer and analysed with the Adobe Photoshop 2·5.1 software. As a negative control, tubes with no nucleic acid or with RNA only were used. The oligonucleotide primers used were: β-actin (GenBank submission X00351) (5′) 5′-CCAACCGCGAGAAGATGACC-3′ and (3′) 5′-GATCTTCATGAGGTAGTCAGT-3′ (product length 235 bp); hIL-1R I (GenBank submission NM000877) (5′) 5′-GATTCAG GACATTACTATTGCG-3′ and (3′) 5′-CTGGGATCCCAAG TCTACTTCC-3′ (product length 464 bp); hIL-1RII (GenBank submission M55646) (5′) 5′-GAAGAGACACGGATGTGGG CC-3′ and (3′) 5′-AAGCTGATATGGTCTTGAGGGGG-3′ (product length 493 bp).
Transfections and luciferase reporter assay
Caco-2 and HT-29/p cells were transfected using lipofectamine reagent (Gibco). The IL-8-promoter-luciferase constructs (wt)LUC, (mNFκB)LUC and (mAP-1)LUC were a generous gift from Dr G. Wu, Philadelphia [17]. Transfected cells were incubated overnight after which the DNA/lipofectamine media was replaced with the serum-containing media and the cells incubated for an additional 12 h. Cells were treated with or without IL-1β (1 ng/ml) for 12 h after which extracts were prepared using enhanced luciferase assay reagents (Berthold Detection Systems, Pforzheim, Germany). Luciferase assays were performed on a luminometer for 20 s (Berthold Detection System) and results were normalized for extract protein concentration measured with Bio-rad protein assay kit (Bio-rad, München, Germany).
Chemotaxis assay
Chemotactic activity of cell culture supernatants was assayed by a modified Boyden chamber assay. Neutrophils from healthy donors were obtained by spontaneous sedimentation over Ficoll for 40 min at room temperature. Each well of a 24-well cell culture microplate (BD-Falcon) was filled with 350 µl phosphate buffered saline (PBS, negative control), the chemotactic peptide N-formylmethionylleucylphenylalanine (10−6m in PBS, positive control) or cell culture supernatants, respectively. Collagen precoated cell culture inserts were placed into each well and filled with 150 µl of the leucocyte-rich plasma containing approximately 750 000 neutrophils. After 20 min of incubation the inserts were removed and the migrated cells transferred from the well into 5-ml test polypropylene tubes (BD-Falcon). Cells were stained with 20 µl fluoresceinated monoclonal antibody against LECAM-1 (CD62L-FITC, BD-Pharmingen) for 15 min and counterstained with the vital DNA dye LDS-751 for a further 10 min on ice. Finally, 20 µl of a red-fluorescent bead suspension was added for counting purposes (all remaining components were obtained from ORPEGEN, Heidelberg, Germany). Samples were analysed on a bench-top flow cytometer (FACScan, BD, San José, CA, USA) equipped with an air-cooled argon laser emitting 15 mW at 488 nm adjusted for lymphocyte immunophenotyping with CalibriteTM beads (BD). To obtain relative cell concentrations data acquisition was stopped when 2000 beads were counted. List mode data were analysed for neutrophil counts as defined by a dual parameter dot plot of forward angle versus orthogonal light scatter, shape change in terms of forward scatter light intensity and fluorescence intensity of CD62 expression. N-formylmethionylleucylphenylalanine used as a positive control leads, on average, to a 100-fold migration of neutrophils into the lower compartment compared to PBS, accompanied by a shape change and CD62 down-regulation.
IL-8 enzyme-linked immunosorbent assay
A human IL-8 enzyme-linked immunosorbent assay (ELISA) of cell culture supernatants was performed in triplicate according to the manufacturer's specifications (R&D Systems).
Statistics
Data were expressed as mean ± standard error of the mean (s.e.). Differences between the groups were compared using analysis of variance and considered significant if P-values were <0·05.
RESULTS
Butyrate differentially modulates the proinflammatory responsiveness of intestinal epithelial cells: potential role of cytokine receptor expression
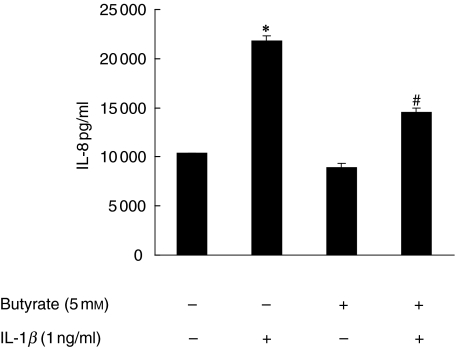
In accordance with findings by others, in our hands exposure of IEC lines to butyrate decreased cell proliferation without affecting cell viability, and instigated cellular differentiation as assessed by alkaline phosphatase activity (not shown). Furthermore, IL-1β-stimulated IL-8 secretion by Caco-2 cells previously exposed to butyrate was decreased (P < 0·05), while exposure of HT-29/p cells to butyrate prior to stimulation by IL-1β increased the IL-8 content in cell-free supernatant assayed by specific ELISA approximately four- to sixfold (not shown). In differentiated HT-29/MTX cells, enhancement of IL-1β-stimulated IL-8 secretion by butyrate was even stronger, ranging from a threefold increase at 0·001 ng/ml of IL-1β to a seven- and ninefold increase at 1 and 10 ng/ml of IL-1β, respectively (not shown). We further used primary intestinal epithelial cells, HIPEC lines from three different individuals with colon cancer to assess the effects of butyrate in non-transformed primary epithelial cells. Despite a high level of spontaneous secretion, IL-1β significantly up-regulated the IL-8 content in cell-free supernatants (P < 0·01) (Fig. 1). Butyrate significantly (P < 0·05) diminished this response, although basal levels were not reached (Fig. 1).
Fig. 1.
Butyrate-mediated modulation of IL-1β-stimulated IL-8 secretion by primary IEC. Semiconfluent HIPEC monolayers from three different patients (approximately 105 cells/ml) were cultured overnight in the presence or absence of 5 mm butyrate. Butyrate-treated and -untreated cells were then stimulated with IL-1β (1 ng/ml) for 24 h in triplicate. The IL-8 content of cell free supernatants was measured by ELISA (*P < 0·01 versus media only, #P < 0·05 versus IL-1β only).
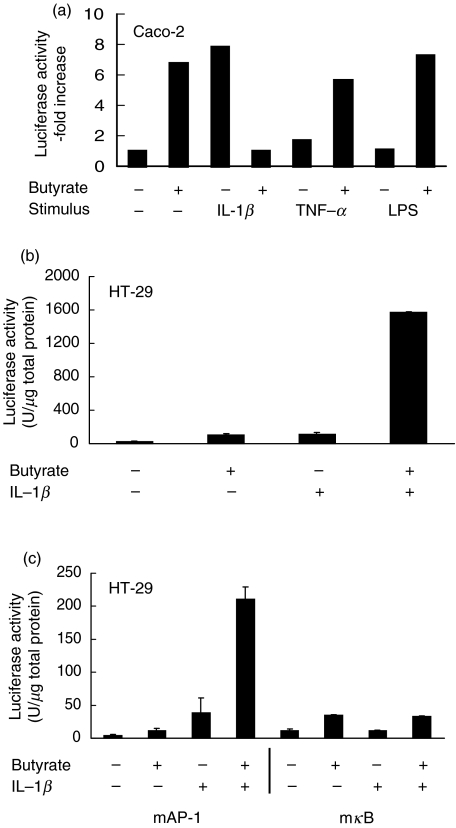
Transfection studies with promoter–luciferase constructs were performed to investigate whether effects of butyrate were mediated at the transcriptional or translational level. Induction of IL-8 gene expression by proinflammatory stimuli has been shown to comprise activation, nuclear translocation and sequence-specific binding of transcription factors such as AP-1 and NF-κB. HT-29/p and Caco-2 cells were transiently transfected with the full-length IL-8 promoter–luciferase construct or with similar constructs containing a mutated (non-fractionated) κB (mκB) or AP-1 (mAP-1) binding sequence, and stimulated by IL-1β. In Caco-2 cells, butyrate exposure specifically decreased the IL-1β-stimulated IL-8 promoter activity compared to TNF-α and LPS-stimulation (Fig. 2a). By contrast, up-regulated secretion of IL-8 protein by butyrate in IL-1β-stimulated HT-29/p cells was paralleled by substantially increased IL-8 full-length promoter activity (P < 0·01) (Fig. 2b). Further analysis with the mutant constructs showed that enhancement of IL-1β-stimulated IL-8 promoter activity by butyrate was dependent on κB, but independent of AP-1 binding (Fig. 2c); however, butyrate alone induced the IL-8 promoter by a mechanism that was only partially dependent on AP-1 and κB and not paralleled by IL-8 secretion.
Fig. 2.
Involvement of transcription factor activation in butyrate-modulated proinflammatory IL-8 secretion. (a) Caco-2 cells were transfected transiently with a full-length IL-8 promoter–luciferase construct and exposed to 5 mm butyrate for 12 h and to proinflammatory stimuli for additional 12 h as indicated. Three additional experiments gave similar results. (b) HT-29/p cells were transfected transiently with a full-length IL-8 promoter–luciferase construct and exposed to 5 mm butyrate for 12 h and to IL-1β (1 ng/ml) for an additional 12 h. (c) HT-29/p cells were transfected with an IL-8 promoter–luciferase construct with a mutant (defective) AP-1 (mAP-1) or κB (mκB) binding site. Data are means of triplicates. Four additional experiments gave similar results.
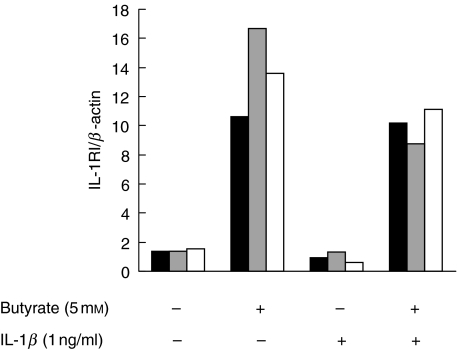
IL-1 effects are transduced after binding of the ligand to the signalling receptor IL-1RI, while the IL-1RII does not contain an intracellular domain and functions as a decoy receptor [18]. To assess the possibility that butyrate-mediated modulation of cytokine receptors was responsible for the observed up-regulation of IL-1β-induced secretion of IL-8 in HT-29 cells, expression of IL-1RI and IL-1RII mRNA in butyrate-exposed HT-29/p cells was determined by RT-PCR and assessed semiquantitatively by densitometry compared to expression of the housekeeping gene β-actin. Upon exposure of HT-29/p cells to butyrate IL-1RI steady-state transcript levels were significantly up-regulated (Fig. 3), while IL-1RII levels remained unchanged (not shown). In contrast, no increase was seen in Caco-2 cells (not shown).
Fig. 3.
Butyrate modulates receptor mRNA expression in HT-29/p cells. Cells were treated with 5 mm butyrate for 24 h prior to extraction of RNA and RT-PCR analysis. After pretreatment with butyrate, cells were treated with IL-1β for various time-points before RT-PCR for IL-1RI. Band intensity was analysed semiquantitatively by densitometry and related to the expression of β-actin. Two more experiments gave similar results. ▪, 0 h;  , 4 h; □, 12 h.
, 4 h; □, 12 h.
Butyrate-mediated modulation of IL-8 secretion by intestinal epithelial cells is functionally relevant for neutrophil migration
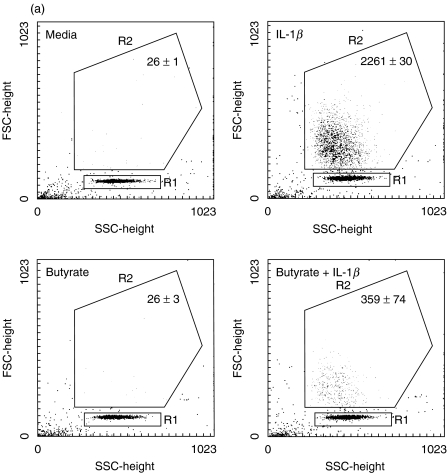
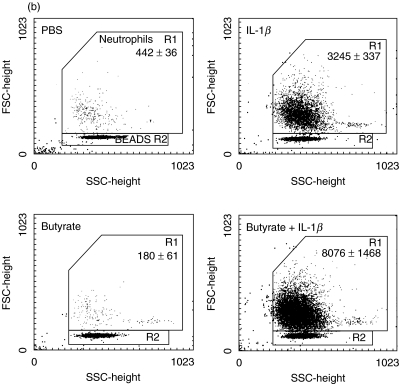
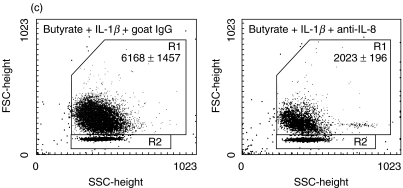
While proinflammatory chemokine induction in IEC has been the focus of many studies, its functional significance in comparison to chemokine production by lamina propria immune cells has been debated. We performed migration assays to evaluate the impact of IL-1β-mediated IEC-derived IL-8 secretion on neutrophil chemokinesis and activation. Neutrophils were exposed to conditioned media of Caco-2 and HT-29/p cells. As a positive control, cells were stimulated by N-formylmethionylleucylphenylalanine at 10−6m. Butyrate and the IL-1β protein had no intrinsic chemotactic effect on neutrophils in the absence of conditioned media. Supernatants from both IL-1β-stimulated Caco-2 and HT-29/p cells induced migration of neutrophils. This process was diminished significantly by exposure of Caco-2 cells to butyrate (Fig. 4a) but enhanced significantly by butyrate preincubation exposure of HT-29/p cells (Fig. 4b). Emphasizing the major role of IL-8 for neutrophil chemokinesis, neutralization of IL-8 by a monoclonal antibody diminished neutrophil migration significantly (P < 0·05) in response to butyrate-treated HT-29/p cells, while the control antibody had no significant effect (Fig. 4c).
Fig. 4.
Butyrate pretreatment modulates differently chemokinesis of neutrophils in response to conditioned medium of Caco-2 and HT-29/p cells stimulated by 1 ng/ml IL-1β for 12 h. Neutrophils obtained by spontaneous sedimentation were transferred onto precoated cell culture inserts and exposed to conditioned media. Migrated cells were quantified (counts related to 2000 beads given in figure) and characterized by flow cytometry.(a) Caco-2 cells: media, negative control; IL-1β, IEC stimulation by IL-1β; butyrate, IEC pretreatment with 5 mm butyrate in culture media; butyrate + IL-1β, IEC pretreatment with 5 mm butyrate followed by IL-1β stimulation.(b) HT-29/p cells: PBS, negative control; IL-1β, IEC stimulation by IL-1β; butyrate, IEC pretreatment with 5 mm butyrate; butyrate + IL-1β, IEC pretreatment with 5 mm butyrate followed by IL-1β stimulation (P < 0·05 versus butyrate, P < 0·01 versus butyrate + IL-1β).(c) HT-29/p cells: butyrate + IL-1β+ goat IgG, IEC pretreatment with 5 mm butyrate followed by IL-1β stimulation in the presence of the IgG goat control antibody (P < 0·05 versus butyrate + IL-1β+ anti-IL-8); butyrate + IL-1β+ anti-IL-8, IEC pretreatment with 5 mm butyrate followed by IL-1β stimulation in the presence of the monoclonal anti-IL-8 antibody (P < 0·05 versus butyrate + IL-1β).Data are means of triplicates ± s.d. Two more experiments gave similar results.
Pharmacological abrogation of butyrate-mediated enhancement of IL-8 secretion in HT-29/p cells
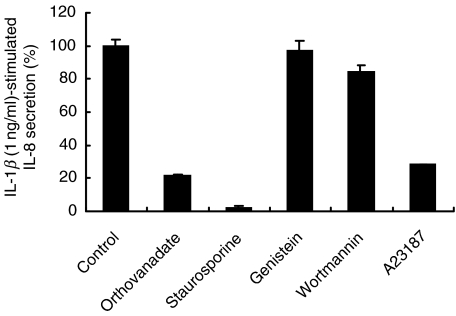
To abrogate the enhanced proinflammatory IL-1β-mediated IL-8 secretion pharmacologically, we applied specific inhibitors of signal transduction pathways and also clinically relevant medications used in the treatment of inflammatory bowel diseases that have been shown to possess in vitro inhibitory effects on proinflammatory transcription factor activation. Levels of IL-1β-stimulated IL-8 (100%) remained unchanged upon exposure to genistein, an inhibitor of tyrosine kinases, and to wortmannin, an inhibitor of the phosphatidyl inositol-3′-kinase (Fig. 5). Release of calcium by the ionophore A23187 and blockade of tyrosine phosphatase activity by orthovanadate reduced the IL-8 content in the supernatant by more than 75% in HT-29/p cells (Fig. 5). Staurosporine, an inhibitor of protein kinase C activation, abrogated IL-8 secretion down to baseline levels (Fig. 5); however, cell proliferation and vitality were markedly reduced. In contrast, action of orthovanadate was not due to impaired cell viability as assessed by trypan blue exclusion and by LDH levels in supernatants. However, calcium release and blockade of tyrosine phosphatase activity did not specifically abrogate butyrate-mediated effects (data not shown).
Fig. 5.
Inhibition of IL-8 protein secretion by blockade of early signalling events. HT-29/p cells were exposed to orthovanadate (1 mm), staurosporine (1 µm), genistein (20 µm), wortmannin (10 µm) and the calcium ionophore A23187 (10 µm) for 30 min prior to addition of 5 mm butyrate to the culture media for 24 h. Cells were then stimulated by 1 ng/ml of IL-1β for 12 h, and supernatants assessed for IL-8 content. IL-8 levels of controls treated with butyrate alone and then stimulated by IL-1β are considered to be 100%. Data are means of triplicates. Two other experiments gave similar results.
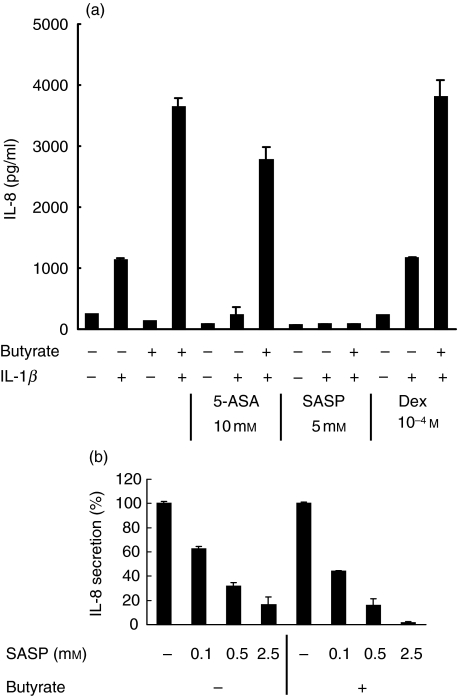
Surprisingly, dexamethasone at 10−9−10−4m did not reduce IL-8 levels induced by IL-1β (Fig. 6a), either in the presence or absence of butyrate. However, the dexamethasone used was biologically active, as it inhibited LPS-stimulated TNF-α production by mouse splenocytes (data not shown). Interestingly, 5-aminosalicylic acid (mesalamine) substantially reduced IL-1β stimulated IL-8 secretion (Fig. 6a), while sulphasalazine abrogated IL-8 secretion to levels comparable to unstimulated controls in a dose-dependent fashion (Fig. 6a,b). Sulphasalazine did not appear to be toxic at the doses investigated by cell morphological analyses and assessment of LDH release into the culture supernatant. These results demonstrate that 5-ASA products can abrogate the up-regulation of IL-1β-induced chemokine expression by butyrate.
Fig. 6.
Pharmacological abrogation of IL-1β-stimulated and butyrate-enhanced IL-8 protein secretion. (a) HT-29/p cells were treated with butyrate or media alone and stimulated by IL-1β (1 ng/ml) for 24 h in the presence or absence of mesalamine (5-ASA), sulphasalazine (SASP) or dexamethasone (Dex) prior to assessment of supernatants for IL-8 protein content by specific ELISA. (b) Increasing doses of sulphasalazine (SASP) were used to modulate IL-8 secretion induced by IL-1β in the presence or absence of butyrate. Data are presented as percentage of control level obtained in the absence of SASP. Data are means of triplicates. Two more experiments gave similar results.
DISCUSSION
This study was designed to explore modulation of IL-8 secretion in IEC by the short-chain fatty acid butyrate, to analyse functional consequences of exposing IEC to butyrate and to provide examples of pharmacological antagonism. This investigation provides evidence that alteration of IL-8 release – enhancement in HT-29 and inhibition in Caco-2 cells – is functionally relevant for neutrophil migration. Furthermore, IL-1β stimulated IL-8 secretion in HT-29/p cells is completely abrogated in the presence of sulphasalazine, a clinically efficacious drug in the treatment of ulcerative colitis, thus providing a potential tool to synergistically enhance the protective properties of butyrate on IEC metabolism and restitution, while blocking any proinflammatory consequences of butyrate administration.
We focused on IL-1β-mediated chemokine induction in IEC for two reasons. First, IL-1 has been shown to be important in the pathogenesis of intestinal inflammation. Secondly, as opposed to other proinflammatory mediators such as TNF-α[19], both transformed and non-transformed IEC express the IL-1R and respond to IL-1β. Upon binding of IL-1β to the IL-1R, signal transduction is activated through the IL-1R associated kinase and its adaptor proteins through the NF-κB and Jun–N-terminal kinase pathways followed by nuclear translocation of the transcription factors NF-κB and AP-1 [20–24]. Binding of transcription factors to the corresponding promoter sequence initiates chemokine gene expression. Our migration assay for neutrophils emphasizes clearly the functional importance of this IL-1β-mediated gene activation and chemokine release by IEC. It illustrates further the potential to modulate this particular effector function of IEC significantly. Consistant with previous ELISA results, the significantly opposing functional effects of butyrate on two colon-derived IEC lines in our study are remarkable: enhancement of neutrophil migration in response to HT-29/p cells, and reduction in response to Caco-2 cells. Evidence has been provided that cellular differentiation alters the immunoregulatory potential of IEC, leading to decreased IL-1 responsiveness of differentiated surface-like cells [25,26]. Our data suggest that butyrate reverses this phenomenon at least partially: when comparing moderately differentiated parental HT-29/p to methotrexate-differentiated HT-29 cells, the relative augmentation of the IL-1β-stimulated IL-8 secretion by butyrate increased with the degree of cellular differentiation. This assumption appears to contradict the potential of butyrate to differentiate IEC and to induce the icIL-1RaI which antagonizes IL-1β-mediated IEC effector functions [25]. However, we focused in our study on early events after short-term exposure to butyrate, and cellular differentiation was not completed at this time. Caco-2 cells have been the target of studies on butyrate effects performed by others generating conflicting data [8,27]. We confirm that butyrate diminishes IL-1β-stimulated IL-8 secretion by Caco-2 cells. Ongoing investigations are designed to elucidate the molecular mechanisms responsible for the cell-line specific differences in butyrate responses, and we hypothesize at this point only that cellular differentiation may be a principal factor. It is noteworthy that butyrate-mediated modulation of the proinflammatory responsiveness of primary IEC was similar to that seen in Caco-2 cells, indicating that Caco-2 cells might be more representative for the in vivo situation than HT-29/p cells. Furthermore, RT-PCR for specific receptor mRNA expression suggested that butyrate affects the receptor concentration. Conspicuously, butyrate substantially increased steady-state transcript levels of the signalling receptor IL-1RI, while mRNA expression of the decoy receptor IL-1RII remained unchanged.
Pharmacological agents were applied to substantially decrease or to abrogate IL-8 secretion stimulated by IL-1β. We chose two classes of anti-inflammatory drugs used widely as first-line treatment of the inflammatory bowel diseases. Corticosteroids, used to treat a variety of inflammatory diseases, inhibit various proinflammatory pathways and interfere with nuclear translocation of NF-κB by up-regulating expression of the cytoplasmic inhibitor IκB [28,29]. The corticosteroid dexamethasone inhibited IL-8 gene expression induced by Entamoeba histolytica in Caco-2 and T84 cells [30]. Considering the data presented by others and by us demonstrating dependence of NF-κB for proinflammatory induction of IL-8 in IEC, dexamethasone surprisingly had no inhibitory effect in the IEC lines used in this investigation – neither in the absence nor in the presence of butyrate – yet was biologically active in unfractionated stimulated murine splenocytes. Mesalamine, 5-aminosalicylic acid, is a free aminosalicylate, while sulphasalazine is an azo-conjugated aminosalicylate. Both agents inhibit gut inflammation in ulcerative colitis and to a lesser degree in Crohn's disease. Mesalamine appears to regulate NF-κB activity by modulating the phosphorylation of one of its transcriptionally active proteins [31]. The anti-inflammatory action of sulphasalazine is attributed to two functions. It is a prodrug for mesalamine, but the parent compound seems to have additional activities. It was shown that sulphasalazine, but not its components mesalamine or sulphapyridine, inhibited NF-κB activation by TNF-α[32]. We now demonstrate that IL-1β-stimulated IL-8 secretion is inhibited dose-dependently by sulphasalazine as well, and that sulphasalazine was superior to mesalamine and to dexamethasone. This adds in vitro credit to a compound that in clinical therapies has been frequently replaced by mesalamine.
Therapeutic application of butyrate in inflammatory bowel diseases is based on the beneficial effects of this short-chain fatty acid on IEC proliferation and restitution. However, the present study demonstrates potentially counterbalancing proinflammatory effects of butyrate on a subpopulation of IEC in the presence of IL-1β, with stimulation of cytokines with functional chemotactic activity. These discordant activities of butyrate may account for the inconsistent clinical results of butyrate administration. Pharmaceutical agents used in the treatment of intestinal inflammation antagonize this effect, providing a potential means to improve clinical efficacy of butyrate. Clinical trials comparing combined treatment of ulcerative colitis with butyrate and sulphasalazine versus application of either component alone would address the clinical implications of our in vitro observations.
Acknowledgments
This study was supported by grants of the Deutsche Forschungsgemeinschaft (Bo 1340/2–1) and the Medical Faculty of Mannheim to U.B. and NIH DK47700 to R.B.S. We thank Karin Hartmann, Department of Clinical Chemistry, University of Heidelberg, Mannheim, Germany for technical assistance in the chemotaxis assay.
References
- 1.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 2.Dinarello CA, Wolff SM. The role of interleukin-1 in disease. N Engl J Med. 1993;328:106–13. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- 3.Eckmann L, Jung HC, Schurer-Maly C, et al. Differential cytokine expression by human intestinal epithelial cell lines. regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 4.Roediger WE. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–8. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44:3961–9. [PubMed] [Google Scholar]
- 6.Tsao D, Morita A, Bella A, Jr, et al. Differential effects of sodium butyrate, dimethyl sulfoxide, and retinoic acid on membrane-associated antigen, enzymes, and glycoproteins of human rectal adenocarcinoma cells. Cancer Res. 1982;42:1052–8. [PubMed] [Google Scholar]
- 7.Fusunyan RD, Quinn JJ, Fujimoto M, et al. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med. 1999;5:631–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Huang N, Katz JP, Martin DR, et al. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine. 1997;9:27–36. doi: 10.1006/cyto.1996.0132. [DOI] [PubMed] [Google Scholar]
- 9.Kamitani H, Ikawa H, Hsi LC, et al. Regulation of 12-lipoxygenase in rat intestinal epithelial cells during differentiation and apoptosis induced by sodium butyrate. Arch Biochem Biophys. 1999;368:45–55. doi: 10.1006/abbi.1999.1284. [DOI] [PubMed] [Google Scholar]
- 10.Ohno Y, Lee J, Fusunyan RD, et al. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci USA. 1997;94:10279–84. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–21. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 12.Breuer RI, Soergel KH, Lashner BA, et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40:485–91. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig Dis Sci. 1996;41:2254–9. doi: 10.1007/BF02071409. [DOI] [PubMed] [Google Scholar]
- 14.Steinhart AH, Hiruki T, Brzezinski A, et al. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729–36. doi: 10.1046/j.1365-2036.1996.d01-509.x. [DOI] [PubMed] [Google Scholar]
- 15.Lesuffleur T, Barbat A, Dussaulx E, et al. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50:6334–43. [PubMed] [Google Scholar]
- 16.Panja A. A novel method for the establishment of a pure population of nontransformed human intestinal primary epithelial cell (HIPEC) lines in long term culture. Lab Invest. 2000;80:1473–5. doi: 10.1038/labinvest.3780154. [DOI] [PubMed] [Google Scholar]
- 17.Wu GD, Lai EJ, Huang N, et al. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem. 1997;272:2396–403. [PubMed] [Google Scholar]
- 18.Colotta F, Re F, Muzio M, et al. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–5. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- 19.Panja A, Goldberg S, Eckmann L, et al. The regulation and functional consequence of proinflammatory cytokine binding on human intestinal epithelial cells. J Immunol. 1998;161:3675–84. [PubMed] [Google Scholar]
- 20.Burns K, Martinon F, Esslinger C, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–9. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 21.Cao Z, Henzel WJ, Gao X. IRAK: a kinase associated with the interleukin-1 receptor. Science. 1996;271:1128–31. doi: 10.1126/science.271.5252.1128. [DOI] [PubMed] [Google Scholar]
- 22.Cao Z, Xiong J, Takeuchi M, et al. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–6. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 23.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, et al. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–6. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 24.Wesche H, Henzel WJ, Shillinglaw W, et al. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–47. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 25.Böcker U, Damiao A, Holt L, et al. Differential expression of interleukin 1 receptor antagonist isoforms in human intestinal epithelial cells. Gastroenterology. 1998;115:1426–38. doi: 10.1016/s0016-5085(98)70021-6. [DOI] [PubMed] [Google Scholar]
- 26.Böcker U, Schottelius A, Watson JM, et al. Cellular differentiation causes a selective down-regulation of interleukin (IL)-1beta-mediated NF-kappaB activation and IL-8 gene expression in intestinal epithelial cells. J Biol Chem. 2000;275:12207–13. doi: 10.1074/jbc.275.16.12207. [DOI] [PubMed] [Google Scholar]
- 27.Fusunyan RD, Quinn JJ, Ohno Y, et al. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1beta and lipopolysaccharide. Pediatr Res. 1998;43:84–90. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Auphan N, DiDonato JA, Rosette C, et al. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 29.Scheinman RI, Cogswell PC, Lofquist AK, et al. Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–6. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 30.Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite–enterocyte contact. Gastroenterology. 1997;112:1536–47. doi: 10.1016/s0016-5085(97)70035-0. [DOI] [PubMed] [Google Scholar]
- 31.Egan LJ, Mays DC, Huntoon CJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–53. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 32.Wahl C, Liptay S, Adler G, et al. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–74. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]