Abstract
The aim of the present study was to investigate the effects of IL-1β and Escherichia coli on the expression and secretion of MIP-2, the mouse equivalent to human IL-8, MCP-1 and RANTES in the kidneys of mice with acute pyelonephritis. Female Bki NMRI, as well as IL-1β deficient mice and their wild-type littermates, were transurethrally infected with either E. coli CFT 073 or injected with NaCl 0·9% (w/v) and thereafter obstructed for 6 h. The Bki NMRI mice were killed at 0, 24, 48 h and 6 days and the IL-1β-deficient mice at 48 h. Chemokine mRNA and protein levels peaked at 24 h for the tested chemokines with the mRNA expression localized in the tubular epithelial cells and for MIP-2 also in neutrophils. Obstruction per se, also induced a chemokine expression similar to E. coli infection although at a lower level. Interestingly, MIP-2 levels were higher in the IL-1β deficient mice as compared with the wild-type littermates. Likewise, the inflammatory changes were more frequent and, when present, more widespread in the IL-1β-deficient mice than in the wild-type mice. Stimulation of a human renal tubular epithelial cell line (HREC), A498 and of primary human mesangial cells (HMC) with the same bacterial antigen depicted gene expression of the same chemokines. A rapid release of IL-8 and MCP-1 was observed from both cell types. RANTES response was delayed both in the HREC and the HMC. We conclude that acute E. coli pyelonephritis induces a MIP-2/IL-8, MCP-1 and RANTES expression and secretion localized primarily to the epithelial cells and that this production is confirmed after in vitro stimulation with the same bacterial antigen of human epithelial and mesangial cells. Blockade of induction of chemokine response may thus be an attractive target for possible therapeutic intervention.
Keywords: MCP-1, MIP-2/IL-8, RANTES, acute pyelonephritis, E. coli
Introduction
Acute pyelonephritis remains one of the most common serious infections in infancy and childhood. Without prompt and accurate treatment, it may lead to permanent damage to the kidneys. Renal scarring leads to loss of renal function and is still a worldwide major cause of end-stage renal failure. It has been speculated that a range of immunoactive peptides is involved in the pathogenesis of renal scarring. In concordance, treatment with anti-inflammatory drugs, such as ibuprofen combined with antibiotics or adjunctive oral corticosteroids suppresses renal scarring in animal models of acute pyelonephritis [1,2]. The scarring process is initiated by the influx of immunocompetent cells to the kidney tissue. In the acute stage of an infection, chemoattractant cytokines, referred to as chemokines, are released as a response to a wide range of bacterial virulence factors and cytokines. Chemokines are small peptides divided into four different groups of proteins – CXC, CC, C and CX3C, according to the position of conserved cystein residues. The CXC chemokines, to which IL-8 belongs, are mainly active on polymorphonuclear leucocytes, whereas the CC chemokines, including MCP-1 and RANTES, attract mainly mononuclear leucocytes. Both cell types are important mediators of inflammation; neutrophils by releasing cytotoxic factors like elastase, lysozyme and myeloperoxidase, and monocytes and macrophages by secreting different pro-inflammatory cytokines [3]. In the kidney chemokines are secreted by a variety of resident renal cells [4]. IL-8 is a potent chemoattractant responsible for leucocyte infiltration in the urinary tract [5] and has been widely reported in urinary tract infections [6–9] and in renal epithelial cells [10]. MCP-1 has been detected in the urine from patients with urosepsis [11] and is, together with RANTES and their respective receptors, reported to induce fibrosis in the kidney [12].
In pyelonephritis the primary target is the epithelial cell, not least that of the tubular system. Following the acute inflammation in the cortex, glomerular sclerosis [13] may appear and a substantial number of glomeruli may become atubular and non-filtering [14], as a result of fibrotic scarring. However, during the acute involvement of the cortex, other kidney cells like glomerular mesangial cells may also become activated. The mesangial cells are positioned to receive pro-inflammatory signals from capillary endothelium and infiltrating leucocytes, and have been shown to secrete chemokines in response to various pro-inflammatory stimuli like IL-1β[15]. Thus, it can not be ruled out that cytokines and chemokines secreted from pro-inflammatory stimulated mesangial cells may reach the tubular system via the urine flow and in such a way modulate the inflammatory reactions at the acute pyelonephritic sites [13,14].
In this study we examined the expression, secretion and localization of the CXC chemokine MIP-2, the mouse equivalent to human IL-8 and the CC-chemokines MCP-1 and RANTES in response to Escherichia coli exposure in an experimental model of acute pyelonephritis in normal as well as IL-1β deficient mice. The reason to use IL-1β deficient mice is that IL-1β expression has been shown to be essential to survive severe bacterial infections [16]. Also, the macrophage-derived IL-1β is important in mediating/activating the Th1 responses. Thus, lack of IL-1β may in particular affect the outcome of acute E. coli pyelonephritis by lesser Th1 activation [17]. In addition, we studied the same chemokines expressed and secreted in a human renal epithelial cell line, as well as primary human mesangial cells, in response to the same bacterial strain and to IL-1β. Moreover, we investigated the effect of IL-1β on inflammation and renal scarring. We hypothesize that E. coli, as well as IL-1β, up-regulates the chemokine response in mesangial and tubular epithelial cells.
Materials And Methods
Bacteria
E. coli CFT 073 was obtained from the blood of a patient with acute pyelonephritis (kindly provided by David E. Johnson, VA Medical Center, Baltimore, Maryland, USA). It expresses P, S and type 1 fimbriae, haemolysin and induces pyelonephritis in experimentally challenged normal mice. The bacteria were grown on CLED agar at 37°C, harvested and cultured in Luria-Bertani broth overnight. Prior to stimulation, the bacteria were washed twice in PBS and centrifuged at 2500 g for 10 min and then resuspended in PBS, for animal experiment and cell culture medium for cell experiment, to a final concentration of 1 × 109 CFU/ml and 1 × 108 CFU/ml, respectively, using spectrophotometer at 600 nm. Bacterial concentration was confirmed by viable count.
Animals
Female Bki NMRI mice (Charles River, Uppsala, Sweden) as well as female IL-1β deficient mice [18] and their wild-type counterparts 8–13 weeks old, about 30 g weight, were used in this study. The IL-1β deficient mice were backcrossed to produce F3 generation in the C57/black background that was used as wild-type control through out all experiments [18]. Four animals were housed in each cage, with food and water ad libitum. The study was approved by the animal ethics committee.
Experimental pyelonephritis
The different groups of mice were each divided into two groups. One subgroup was infected with a bacterial suspension of E. coli CFT 073 (n = 6, Bki NMRI; n = 3–5, IL-1β knock out; n = 4, wild type) and the other subgroup, serving as a control-group, was inoculated with 0·9% NaCl (w/v) (n = 6). Bki NMRI mice were killed at 0, 24, 48 h and 6 days, respectively, while IL-1β deficient mice and their wild-type littermates were killed only at 48 h. This time point was chosen because we found more pronounced inflammatory reactions at 48 h compared with 24 h in the Bki NMRI mice. Unmanipulated animals, i.e. mice that were neither inoculated with E. coli nor with NaCl (n = 3, Bki NMRI), were killed at 0 h. In all other respects, the animals were treated identically. Inoculation was performed by urethral catheterization as previously described [19] under anaesthesia with Diazepam® 30 µl/30 g and Hypnorm® 10 µl/30 g given intraperitoneally. Fifty microlitres bacterial suspension or 0·9% NaCl (w/v) was deposited in the bladder through a soft sterile polyethylene catheter (outer diameter 0·61 mm and inner diameter 0·28 mm) (Clay Adams, Becton Dickinson, NJ, USA). Immediately after the catheter was withdrawn, an obstruction with collodion (Sves 46, Swedish Pharmacopia, National Corporation of Swedish Pharmacies, Stockholm, Sweden) was applied for 6 h. India ink was added to monitor integrity of the collodion and detection of urine leakage on the absorbent paper. Each mouse was housed separately during the first 6 h. All mice were killed at pre-set time points. Kidneys were removed aseptically and placed on dry ice and isopentone. Survival was monitored during the first 6 h and then daily.
Tissue processing for histo-pathology, in situ hybridization, enzyme immunosorbent assay and bacterial culture
The kidneys were cut into 1·1 mm slices using a device with parallel razor blades. Every second tissue slice was systematically and randomly sampled into two separate tissue blocks for histological evaluation and in situ hybridization, respectively. The latter were snap frozen and cut in 10-µm thick sections in a cryostat. The frozen sections were placed on ProbeOn microscope slide (Fisher, Biotech, Pittsburgh, PA, USA), and stored in − 20°C until assayed. For histological evaluation, the tissue blocks were embedded in paraffin after fixation in 4% buffered paraformaldehyde. From the tissue blocks, 4-µm thick sections were cut and stained with haematoxylin and eosin. The inflammatory changes in each kidney were summed following a semi-quantitative scoring (0–3: 0 = no inflammatory changes; 1 = pelvic inflammation; 2 = parenchymal inflammation adjacent to the fornices; 3 = inflammation involving medulla and cortex). The mean of the scoring sum was calculated for each group of animals. The sections were evaluated by the same investigator (GJ) without knowledge of other laboratory data. For EIA and bacterial cultures, kidneys were homogenized in a ratio of 50 mg kidney/ml PBS. The homogenate was centrifuged at 300 g. for 10 min and the supernatant was stored at − 70°C until EIA assay was performed.
Cell cultures
The A498 cell line (ATCC, Rockville, MD, USA) is a human renal epithelial cell line (HREC). Cells were cultured in Eagle's minimal essential medium with 0·1 mm non-essential amino acid, 1·0 mm sodium pyruvate and 10% foetal bovine serum (GIBCO BRL, Gaithersburg, MD, USA) in cell culture flasks. The primary human mesangial cells (HMC) (Clonetics, Walkersville, MD, USA) expressed fibronectin and cytokeratin 18 but not von Willebrand factor or cytokeratin 19. They were cultured in mesangial cell basal medium (Clonetics) supplemented with 5% heat inactivated foetal bovine serum, 50 µg/ml of gentamicin and 50 ng/ml of amphotericin B (Clonetics) for detection of protein levels or in RPMI-1640 supplemented with l-glutamine, insulin-transferrin-selenium (Life Technologies) and 50 µg/ml gentamicin and 50 ng/ml amphotericin (Clonetics) for mRNA detection. All cells were cultured at 37°C in 5% CO2. When they reached confluency, cells were trypsinized in 0·25% trypsin (Sigma Chemical Co., St Louis, MO, USA) until detached and then resuspended in medium and seeded into 24-well plates (Nunc, Roskilde, Denmark).
Stimulation
When the cells were confluent, the medium was aspirated and the cells were washed once with PBS in order to clear them from any non-adhering cells or debris. Fresh, serum-free medium was added, including E. coli CFT 073 (108 CFU/ml) or IL-1β (10 ng/ml) or a combination of both. Incubation with medium alone served as negative controls. To avoid bacterial overgrowth and cell detachment, the cell experiments were carried out in the presence of endotoxin free gentamicin 50 µg/ml [20]. At this concentration, cell viability was 90% at 24 h as determined by trypan blue staining. Supernatants were collected at 0 h, 2 h, 6 h and 24 h and centrifuged at 300 g for 10 min and stored in −20°C pending ELISA analysis.
Detection of protein levels by ELISA
Human IL-8, MCP-1 and RANTES or mouse MIP-2, MCP-1 and RANTES analysis was performed using an enzyme-linked immunosorbent assay, ELISA (R & D Systems, Abingdon, UK). The limits of detection for human IL-8, MCP-1 and RANTES were 10·0, 5·0, 8·0 pg/ml, respectively, and for mouse MIP-2, MCP-1 and RANTES, 2·0, 1·5 and 2·0 pg/ml, respectively.
Detection of cytokine mRNA by in situ hybridization
In situ hybridization was performed as described previously [21]. In short, synthetic oligonucleotide probes (Scandinavian Gene Synthesis AB, Köping, Sweden) were labelled using 35S deoxyadenosine-5-α-(thio)-triphosphate (New England Nuclear, Cambridge, MA, USA) with terminal deoxynucleotide transferase (Amersham, Little Chalfont, UK). To increase the sensitivity of the method a mixture of two to three different, approximately 30-base-pair-long oligonucleotide probes were used. The oligonucleotide sequences for rat MIP-2, rat MCP-1 and rat RANTES were obtained from GenBank and probes were designed using Mac Vector software [Table 1]. The homology between rat and mice is 100% for MIP-2, 93% for MCP-1 and 96% for RANTES, respectively. Controls were obtained by parallel hybridization of adjacent sections with the 35S-labelled sense probe for each chemokine. These control probes produced a uniformly weak background without revealing any positive cells. Emulsion autoradiography was performed and the slides were coded. Cells expressing more than 15 grains over the cytoplasm were counted by dark field microscopy at 100× magnification. Accuracy was checked at higher magnification and light microscopy [Fig. 1]. The tissue section areas were measured by graded mesh and results were expressed as numbers of mRNA expressing cells per 100 mm2 tissue section.
Table 1.
Probes used for detection of chemokine mRNA expression by in situ hybridization
| Target (accession no.) Probe designation | Sequence |
|---|---|
| MIP-2 (S77604) | |
| MIP-2–48L30 | 5′-GGAGGAGCAGGACCAGTACAGCATTGAGGA-3′ |
| Control | 5′-TCCTCAATGGCAGGACCAGTACAGCATTGAGGA-3′ |
| MIP-2–205L30 | 5′-GTGGCTATGACTTCTGTCTGGGCGCAGTGG-3′ |
| Control | 5′-CCACTACGCCCAGACAGAAGTCATAGCCAC-3′ |
| MIP-2–268L30 | 5′-TGGACGATCCTCTGAACCAAGGGGGCTTCA-3′ |
| Control | 5′-TGAAGCCCCCTTGGTTCAGAGGATCGTCCA-3′ |
| MCP-1 (M57441) | |
| MCP-1–44l30 | 5′-ACAGGCCCAGAAGCGTGACAGAGACCTGCA-3′ |
| Control | 5′-TGCAGGTCTCTGTCACGCTTCTGGGCCTGT-3′ |
| MCP-1159L29 | 5′-CCAGCCGACTCATTGGGATCATCTTGCCA-3′ |
| Control | 5′-TGGCAAGATCCCAATGAGTCGGCTGG-3′ |
| RANTES (U06436) | |
| RANTES-21L30 | 5′-GCTGCAACGAGGATGACGGTGAGGGTAGCA-3′ |
| RANTES-83L30 | 5′-CAAAGCAGCAGGGAGTGGTGTCCGAGCCAT-3′ |
| RANTES- 128L30 | 5′-AATACTCCTTCACGTGGGCACGGGGCAGTG-3′ |
| Control | 5′-CGTCTTTGTCACTCGAAGGAACCGCCAAGT-3′ |
Fig. 1.
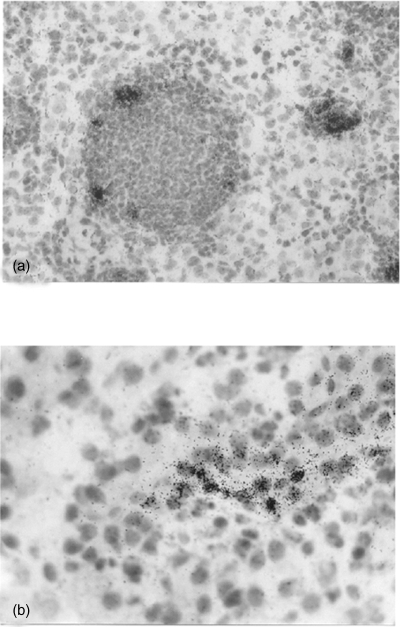
The juxtamedullary portion of the renal cortex of a mouse kidney. In situ hybridization of (a) MIP-2 mRNA localized in dilated tubules with granulocytic casts, magnification ×250. (b) RANTES overlying a tubular epithelial cell, magnification ×400.
Detection of mRNA by RT-PCR
Total RNA was extracted from adherent HREC, A498 and HMC cells using RNAzol B™ (Biotecx Laboratories, Houston, TX, USA) according to the manufacturer's instructions. Reverse transcription of 1 µg RNA was performed using 100 pmol random hexamer primers pd(N)6 (Amersham Pharmacia Biotech, Uppsala, Sweden) and 300 U SuperScript RNAse H− (GIBCO BRL) with the mixture of 5× buffer, 10 µmol dNTP, 0·1 mmol DTT and 60 U RNAsin (Boehringer Mannheim, Mannheim, Germany) at 45°C for 2 h. PCR was performed using the following primers; IL-8: sense-TCTCTTGGCAGCCTTCCT; antisense-AATTCTCAGCCTCTTCAAAAACTT; MCP-1: sense-CTCTGCCGCCCTTCTGTGCC, antisense-GTCTTCGG AGTTTGGGTTTGC; RANTES: sense-CGGCACGCCTCGCT GTCATC, antisense-TGTACTCCCGAACCCATTT [22]. All chemokine primers were obtained from Innovagen (Lund, Sweden). G3PDH primer: sense-TGAAGGTCGGAGTCAACG GATTTGGT, antisense-CATGTGGGCCATGAGGTCCACC AC [23] (Cybergene AB, Huddinge, Sweden). PCR of 2 µl of the cDNA was performed with 0·025 U/µl Taq polymerase (GIBCO BRL), 2 mm MgCl2, 0·2 mm dNTP, PCR buffer and 0·5 µm of each primer in a final volume of 25 µl in a DNA Thermocycler 480 (Perkin Elmer, Norwalk, CT, USA). After 1 min at 95°C, PCR was conducted for 26 (MCP-1), 28 (IL-8), 30 (RANTES) and 28 (G3PDH) cycles using the following conditions: 1 min of denaturation at 94°C, 1 min of annealing at 62, 62, 61 and 60°C, respectively, and 1 min of extension at 72°C, followed by a final extension for 5 min at 72°C and cooling to 4°C. Repeated experiments were performed to ensure that the reaction was within the linear phase.
The PCR products were separated by electrophoresis on a 1·5% agarose gel (GIBCO BRL). Following staining with ethidium bromide, the gels were photographed and the intensity of the bands were measured under UV-light using Gel Doc 2000 (Bio-Rad Laboratories, Hercules, CA, USA). A ratio of the intensity between the chemokine and G3PDH was calculated.
Statistical analysis
Mann–Whitney U-test was used to evaluate statistical significance between infected and control groups.
Results
Histo-pathological kidney changes more severe in IL-1β deficient than in wild-type animals
In the Bki NMRI, IL-1β deficient as well as their wild-type littermates, the inflammatory response was most often confined to the pelvis, i.e. pyelitis. A varying degree of inflammatory exudate made up of neutrophils was seen in and under the pelvic and calyceal epithelium which were thickened due to reactive cellular changes. In kidneys with more severe pyelitis, infiltrates of neutrophils were also seen in the fornices. From here the inflammation tended to spread into the adjacent parenchyma. In some kidneys (4/10 of IL-1β-deficient mice and 1/8 their wild-type littermates), destructive inflammation with intratubular casts, containing large number of neutrophils and cell debris, as well as abscesses, was seen in the medulla and in the cortex. The cortical inflammation was most often localized in the polar regions. Inflammatory changes were observed after 48 h in 6/10 kidneys from IL-1β-deficient mice and in 1/8 wild types with an inflammatory mean score of 1·30 and 0·37, respectively. In conclusion, the inflammatory changes were more frequent and, when present, more widespread in the IL-1β-deficient mice compared with the wild-type animals.
Accumulation of MIP-2, MCP-1 and RANTES in tubular epithelial cells
All tested chemokines were detected in tubular epithelial cells in tubules localized within areas with inflammatory infiltrates. In these areas, MIP-2 was also observed in tubular casts, composed of neutrophils and macrophages (Figs 1a and b). In situ hybridization of sectioned kidneys from Bki NMRI mice with E. coli pyelonephritis showed a time-dependent increase in chemokine mRNA induction. The highest detected mRNA values for MIP-2, MCP-1 and RANTES were observed after 24 h (P < 0·001, respectively, vs. unmanipulated mice) with a decrease at 48 h and for RANTES further at 6 days (P < 0·01 vs. 48 h) [Fig. 2]. MIP-2 on the other hand remained on a similar level on day 6 (P < 0·001 versus unmanipulated mice). Mice injected with NaCl and obstructed also revealed increased mRNA expression, although at a lower level but with similar kinetics as after E. coli infection.
Fig. 2.
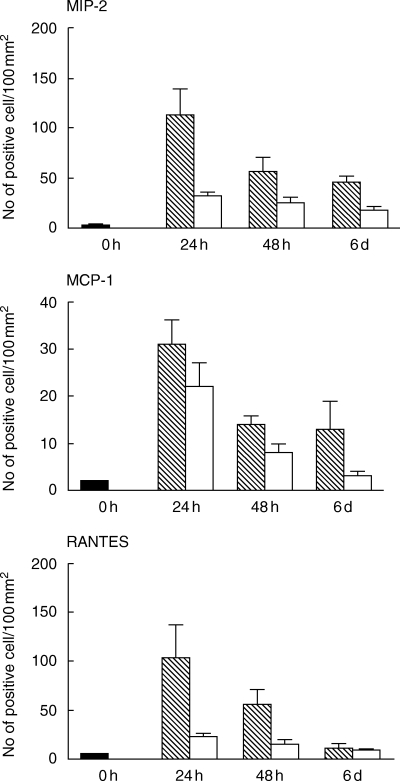
MIP-2, MCP-1 and RANTES mRNA expression in transurethrally E. coli-infected (hatched bars) versus NaCl (open bars) injected female Bki NMRI mice assessed by in situ hybridizatition expressed in means with s.e.m. (n = 6). Untouched mice, i.e. mice that were neither inoculated with E. coli nor with NaCl (n = 3) were killed at 0 h (filled bar). Cells expressing more than 15 grains over the cytoplasm were regarded as positive. The results are expressed as mean numbers of mRNA expressing cells + s.e.m. per 100 mm2 tissue section.
E. coli, but not obstruction per se, induces rapid chemokine release
A rapid MIP-2, MCP-1 and RANTES response was observed in supernatants from the E. coli infected homogenized kidneys at 24 h (P < 0·01, P < 0·001 and P < 0·001, respectively, versus unmanipulated mice). MCP-1 returned to normal values over the 6-day study period, while both MIP-2 and RANTES declined more slowly and remained elevated after 6 days in the E. coli infected group compared with unmanipulated mice (P < 0·01, and P < 0·001, respectively). Obstruction per se did not produce any detectable MIP-2 or MCP-1 levels and low levels as for RANTES (P < 0·01 at 24 h) [Fig. 3].
Fig. 3.

Box plot with median and percentiles (10–90%) for MIP-2, MCP-1 and RANTES as determined by EIA in mice kidney homogenate at 0 h (untouched), 24 h, 48 h and 6 days (infected and NaCl injected). The limits of detection for mouse MIP-2, MCP-1 and RANTES were 2·0, 1·5 and 2·0 pg/ml, respectively. For each time-point 5–6 mice were used, both kidneys were analysed individually.
Increased MIP-2 levels in IL-1β-deficient mice
MIP-2 protein levels were significantly higher in the IL-1β-deficient mice compared with the wild-type mice (P < 0·001) [Fig. 4]. Likewise, the mRNA expression for MIP-2 was increased, but as only a few cells were positive, no definitive conclusion can be drawn from these results. For MCP-1 or RANTES, neither mRNA expression nor protein levels (data not shown) showed any differences.
Fig. 4.

Box plot with median and percentiles (10–90%) for MIP-2 as determined by EIA in mice kidney homogenate at 48 h in E. coli-infected IL-1β-deficient (n = 3) and wild-type mice (n = 4), both kidneys were analysed individually. The limit of detection for mouse MIP-2 was 2·0 pg/ml.
Chemokine mRNA expression in HREC and HMC
The IL-8/G3PDH mRNA ratio increased 2 h after E. coli stimulation and in the HREC, A498 epithelial cell-line, further at 6 h, while it remained stationary in HMC. For MCP-1 the mRNA ratio in HREC, A498 increased at 2 h with a further increase at 6 h, after which a decrease was observed. In the HMC, however, the MCP-1 ratio did not increase until at 6 h. The gene expression for RANTES/G3PDH ratio was elevated at 6 h in the HREC, A498, while an increase was seen already after 2 h in the HMC and thereafter further during the 24 h study period [Fig. 5].
Fig. 5.

IL-8, MCP-1 and RANTES mRNA expression in human renal epithelial cells, A498 and primary HMC by RT-PCR after stimulation with E. coli CFT-073 for 0, 2, 6 and 24 h. A chemokine/G3PDH band intensity ratio was calculated for each chemokine at each time point (upper panel). Bands are displayed at the different time points for each chemokine (lower panel).
Delayed RANTES secretion in HMC
At the protein level, an IL-8 increase due to E. coli, IL-1β and the combination was observed both in the HREC, A498 and HMC after 6 h (P < 0·001, respectively), with significantly lower levels after E. coli stimulation than after IL-1β stimulation (P < 0·001 and P < 0·02, respectively) [Fig. 6]. For MCP-1, E. coli induced an increase in both cell types after 6 h (P < 0·03 in both cell types). Initially, the MCP-1 levels were higher after IL-1β compared with E. coli stimulation (P < 0·01 at 6 h in both cell types). Interestingly, the combination yielded lower levels than IL-1β stimulation alone in the HREC, A498 cell line (P < 0·01 at 24 h) and HMC (P < 0·004 at 6 h, respectively). No detectable RANTES levels were observed after E. coli stimulation at 2 or 6 h in the two tested cell types. After 24 h, a prominent increase versus unstimulated cells was seen in HREC, A498 and HMC after stimulation with either E. coli (P < 0·02 and P < 0·001, respectively), IL-1β (P < 0·01 and P < 0·01, respectively) or the combination (P < 0·001, respectively).
Fig. 6.
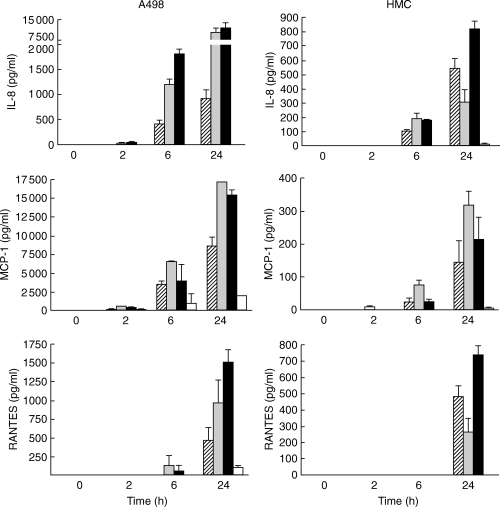
IL-8, MCP-1 and RANTES secretion following stimulation of human renal epithelial cells, A498 and primary HMC by E. coli CFT-073 (striped bars), IL-1β (grey bars) or in combination (black bars) expressed as means with s.d.. Non-stimulated controls (open bars) were usually under detection limit (n = 3 in each group).
Discussion
In this study we demonstrate a rapid increase of the chemokines MIP-2, MCP-1 and RANTES in response to E. coli strain CFT-073 in a mouse model of acute pyelonephritis. Contrary to our hypotheses, we found a significant increase of IL-8 in the IL-1β-deficient mice as compared with their wild-type littermates. We also show the same chemokines in human renal epithelial cells and in primary human mesangial cells as well as the effect of IL-1β on the chemokine response.
IL-8 is not only a potent chemoattractant for neutrophils but it has also been shown to trigger the release of cytotoxic products produced by the neutrophils. These products, which include lysozyme, elastase and myeloperoxidase are important for the development of renal damage [24]. Furthermore, recent studies have focused on the important role of IL-8 receptors in the pathogeneses of renal scarring. Both IL-8 receptor knockout mice [25] and wild-type mice treated with anti-MIP-2 antibody [5] have been shown to undergo neutrophil accumulation under the pelvic epithelium leading to renal scarring. It was therefore suggested that chemokine receptors are of pivotal importance for neutrophil clearance of kidney tissue by allowing for the neutrophils to migrate across the epithelial barrier [5]. However, Olszyna et al. showed that by blocking the IL-8 receptor in mice, the kidneys presented large necrotic areas, but with almost no influx of neutrophils [26]. Moreover, it has also been shown that neutrophils of children with recurrent pyelonephritis have a lower number of the IL-8 receptor CXCR-1 expression than the neutrophils of healthy controls, thus indicating their clinical importance [27]. In the present study we observed early MIP-2 mRNA and protein increase, with levels rather levelling off after 48 h.
IL-1β is rapidly induced by E. coli and obstruction demonstrated earlier by us [19,28], and IL-1β is important for both the induction of inflammatory and anti-inflammatory responses. It is also well known that IL-1β induces chemokines and may be involved in the scarring process partly by inducing TGF-β and matrix metalloproteinases [28,29]. Unexpectedly, when we induced pyelonephritis in IL-1β-deficient mice, a higher MIP-2 response was noted, which suggests that IL-1β gives rise, not only to pro-inflammatory mediators, but may also induce anti-inflammatory cytokines like IL-1 receptor antagonist, IL-10 and IL-4 [30]. We demonstrate here, that the IL-1β-deficient mice are more severely affected by inflammatory changes than the wild-type littermates. At 48 h, the bacterial count does not differ (data not shown) so it can be argued that the bacterial number alone is not the sole reason for scarring. Here we show that one of the possible reasons for increased renal scarring in the IL-1β-deficient mice is a higher MIP-2 response, resulting in increased and prolonged influx of neutrophils, which cause renal damage by releasing cytotoxic products. A total blockade of IL-1β production or of signalling via the IL-1 type 1 receptor would thus not be desirable chronically in the epithelial cells as it worsens outcome of bacterial infections.
Furthermore, it has been shown that children with pyelonephritis under 1 year of age have higher urine IL-8 levels than those aged over 1 year [6]. This correlates with the fact that the very young children with pyelonephritis are more prone to develop renal scarring. A correlation between urinary IL-8 levels due to E. coli urinary tract infection and haemolysin-producing organisms has also been demonstrated [31]. It is therefore noteworthy that the strain used in our experiments is producing haemolysin.
Monocytes and macrophages are important mediators of renal inflammatory reactions [32]. During experimental urethral obstruction, an increase in the number of macrophages correlating to the MCP-1 expression by tubular epithelial cells has been demonstrated [33]. Furthermore, macrophage accumulation adjacent to tubular epithelial cells correlates with tubular damage indicating that molecules responsible for cell death are released [34]. MCP-1 has also been shown to harbour fibrinogenic properties by inducing fibroblast collagen expression via specific receptors and endogenous up-regulation of TGF-β expression [35]. Accordingly, blocking of MCP-1 has led to a decrease in TGF-β and collagen formation in rat glomerulonephritis [36] and reduced glomerular crescent formation and collagen I deposition in mouse crescentic nephritis [37]. In contrast, MCP-1, but not RANTES was down-regulated by TGF-β in proximal tubular cells [38], indicating that during inflammation a relationship might exist to regulate the influx of inflammatory cells and the degree of inflammation.
The chemokine RANTES does not only have a chemoattractant activity but is also a powerful leucocyte activator, a feature potentially relevant in a range of inflammatory disorders [39]. Glomeruli of rats with endotoxemia have previously been reported to express and secrete RANTES and when isolated from these glomeruli, RANTES was shown to be chemotactic for monocytes [40]. To our knowledge, we show here for the first time RANTES gene expression and protein secretion located in the tubular epithelial cells in a model of acute pyelonephritis. In the present study, RANTES is induced at a slower rate and is detectable for a prolonged time compared with IL-8 and MCP-1. Interestingly, in studies with fibroblasts, which are closely related to mesangial cells, IL-1 seemed not to be responsible for RANTES production [41]. We show here that IL-1β induces RANTES in mesangial cells although not as potently as in epithelial cells.
It has previously been shown that permanent obstruction alone can induce a cytokine or chemokine response [42]. The sustained elevation of MCP-1 suggested the assumption of a particular involvement in the pathogenesis of renal interstitial fibrosis. In our model, both the MCP-1 and RANTES mRNA and protein levels were increased during the first 48 h. We have previously, in the same experimental model, also noted prolonged increase of TGF-β mRNA as well as pro-inflammatory cytokines after short time obstruction only [19], indicating that even the short-time obstruction may be hazardous to the patient.
In the present study, we show the chemokine release from HREC and HMC upon E. coli stimulation in the presence and absence of IL-1β. Our data indicate what responses these cells can mount. They do, however, not fully reflect how human epithelial and mesangial cells respond in vivo where E. coli and IL-1β both induce a multitude of other signal substances for which these cells have receptors. Thus, the reported chemokine response is only a portion of the response these cell are capable of.
It has been suggested that chemokines are not essential for most normal tissue functioning [43]. However, during inflammation, chemokines are induced and their sustained release by injured cells resulting in a concentration gradient that is maximal within the damaged tissue and is responsible for the unidirectional movement of infiltrating cells is observed [44]. Therefore, blockade of induction of chemokines is an attractive target for possible therapeutic intervention. A better knowledge of chemokine action and regulation during pyelonephritis and infection-induced renal scarring may therefore help to explore new therapeutic strategies in the future.
Acknowledgments
This study was supported by Funds from the Karolinska Institute, Magn. Bergvalls Foundation, Stiftelsen Clas Groschinskys Minnesfond and Stiftelsen Frimurare Barnhuset. Milan Chromek is supported by a scholarship from the Swedish Institute. We thank Dr Ingeborg van der Ploug for G3PDH primers.
References
- 1.Pohl HG, Rushton HG, Park JS, Chandra R, Majd M. Adjunctive oral corticosteroids reduce renal scarring: the piglet model of reflux and acute experimental pyelonephritis. J Urol. 1999;162:815–20. doi: 10.1097/00005392-199909010-00067. [DOI] [PubMed] [Google Scholar]
- 2.Huang A, Palmer LS, Hom D, Anderson AE, Kushner L, Franco I. Ibuprofen combined with antibiotics suppresses renal scarring due to ascending pyelonephritis in rats. J Urol. 1999;162:1396–8. [PubMed] [Google Scholar]
- 3.Janeway C, Travers P, editors. Immunobiology. 5. New York: Garland Publishing, Churchill Livingstone; 2001. [Google Scholar]
- 4.Schlondorff D, Nelson PJ, Luckow B, Banas B. Chemokines and renal disease. Kidney Int. 1997;51:610–21. doi: 10.1038/ki.1997.90. [DOI] [PubMed] [Google Scholar]
- 5.Hang L, Haraoka M, Agace WW, Leffler H, Burdick M, Strieter R, Svanborg C. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J Immunol. 1999;162:3037–44. [PubMed] [Google Scholar]
- 6.Tullus K, Fituri O, Burman LG, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in the urine of children with acute pyelonephritis. Pediatr Nephrol. 1994;8:280–4. doi: 10.1007/BF00866334. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson SH, Hylander B, Wretlind B, Brauner A. Interleukin-6 and interleukin-8 in serum and urine in patients with acute pyelonephritis in relation to bacterial-virulence-associated traits and renal function. Nephron. 1994;67:172–9. doi: 10.1159/000187923. [DOI] [PubMed] [Google Scholar]
- 8.Ko YC, Mukaida N, Ishiyama S, Tokue A, Kawai T, Matsushima K, Kasahara T. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993;61:1307–14. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson M, Jodal U, Agace W, Hellstrom M, Marild S, Rosberg S, Sjostrom M, Wettergren B, Jonsson S, Svanborg C. Interleukin (IL)-6 and IL-8 in children with febrile urinary tract infection and asymptomatic bacteriuria. J Infect Dis. 1996;174:1080–4. doi: 10.1093/infdis/174.5.1080. [DOI] [PubMed] [Google Scholar]
- 10.Brauner A, Soderhall M, Jacobson SH, Lundahl J, Andersson U, Andersson J. Escherichia coli-induced expression of IL-1 alpha, IL-1 beta, IL-6 and IL-8 in normal human renal tubular epithelial cells. Clin Exp Immunol. 2001;124:423–8. doi: 10.1046/j.1365-2249.2001.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olszyna DP, Prins JM, Dekkers PE, De Jonge E, Speelman P, Van Deventer SJ, Van Der Poll T. Sequential measurements of chemokines in urosepsis and experimental endotoxemia. J Clin Immunol. 1999;19:399–405. doi: 10.1023/a:1020554817047. [DOI] [PubMed] [Google Scholar]
- 12.Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Grone HJ, Schlondorff D. Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–87. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- 13.Heptinstall RH. The development of renal pathology. Am J Kidney Dis. 1990;16:568–73. doi: 10.1016/s0272-6386(12)81042-0. [DOI] [PubMed] [Google Scholar]
- 14.Marcussen N, Olsen TS. Atubular glomeruli in patients with chronic pyelonephritis. Laboratory Invest. 1990;62:467–73. [PubMed] [Google Scholar]
- 15.Schwarz M, Radeke HH, Resch K, Uciechowski P. Lymphocyte-derived cytokines induce sequential expression of monocyte- and T cell-specific chemokines in human mesangial cells. Kidney Int. 1997;52:1521–31. doi: 10.1038/ki.1997.482. [DOI] [PubMed] [Google Scholar]
- 16.Read RC, Camp NJ, di Giovine FS, Borrow R, Kaczmarski EB, Chaudhary AG, Fox AJ, Duff GW. An interleukin-1 genotype is associated with fatal outcome of meningococcal disease. J Infect Dis. 2000;182:1557–60. doi: 10.1086/315889. [DOI] [PubMed] [Google Scholar]
- 17.Chabaud M, Page G, Miossec P. Enhancing effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory protein-3alpha production in rheumatoid arthritis: regulation by soluble receptors and Th2 cytokines. J Immunol. 2001;167:6015–20. doi: 10.4049/jimmunol.167.10.6015. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H, Fletcher D, Kozak W, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 19.Khalil A, Brauner A, Bakhiet M, Burman LG, Jaremko G, Wretlind B, Tullus K. Cytokine gene expression during experimental Escherichia coli pyelonephritis in mice. J Urol. 1997;158:1576–80. [PubMed] [Google Scholar]
- 20.Hedges SR, Bjarnadottir M, Agace W, Hang L, Svanborg C. Immunoregulatory cytokines modify Escherichia coli induced uroepithelial cell IL-6 and IL-8 responses. Cytokine. 1996;8:686–97. doi: 10.1006/cyto.1996.0091. [DOI] [PubMed] [Google Scholar]
- 21.Olsson T, Bakhiet M, Hojeberg B, et al. CD8 is critically involved in lymphocyte activation by a T. brucei brucei-released molecule. Cell. 1993;72:715–27. doi: 10.1016/0092-8674(93)90400-k. [DOI] [PubMed] [Google Scholar]
- 22.Lim CS, Zheng S, Kim YS, Ahn C, Han JS, Kim S, Lee JS, Chae D-W, Koo JR, Chun RW, Noh JW. Th1/Th2 predominance and proinflammatory cytokines determine the clinicopathological severity of IgA nephropathy. Nephrol Dial Transplant. 2001;16:269–75. doi: 10.1093/ndt/16.2.269. [DOI] [PubMed] [Google Scholar]
- 23.Aguilar-Santelises M, Rottenberg ME, Lewin N, Mellstedt H, Jondal M. Bcl-2, Bax and p53 expression in B-CLL in relation to in vitro survival and clinical progression. Int J Cancer. 1996;69:114–9. doi: 10.1002/(SICI)1097-0215(19960422)69:2<114::AID-IJC8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–99. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 25.Hang L, Frendeus B, Godaly G, Svanborg C. Interleukin-8 receptor knockout mice have subepithelial neutrophil entrapment and renal scarring following acute pyelonephritis. J Infect Dis. 2000;182:1738–48. doi: 10.1086/317599. [DOI] [PubMed] [Google Scholar]
- 26.Olszyna DP, Florquin S, Sewnath M, Branger J, Speelman P, van Deventer SJ, Strieter RM, van der Poll T. CXC chemokine receptor 2 contributes to host defense in murine urinary tract infection. J Infect Dis. 2001;184:301–7. doi: 10.1086/322030. [DOI] [PubMed] [Google Scholar]
- 27.Frendeus B, Godaly G, Hang L, Karpman D, Lundstedt AC, Svanborg C. Interleukin 8 receptor deficiency confers susceptibility to acute experimental pyelonephritis and may have a human counterpart. J Exp Med. 2000;192:881–90. doi: 10.1084/jem.192.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalil A, Tullus K, Bartfai T, Bakhiet M, Jaremko G, Brauner A. Renal cytokine responses in acute Escherichia coli pyelonephritis in IL-6-deficient mice. Clin Exp Immunol. 2000;122:200–6. doi: 10.1046/j.1365-2249.2000.01377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, McCluskey K, Fujii K, Wahl LM. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J Immunol. 1998;161:3071–6. [PubMed] [Google Scholar]
- 30.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–4. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 31.Jantausch BA, O'Donnell R, Wiedermann BL. Urinary interleukin-6 and interleukin-8 in children with urinary tract infection. Pediatr Nephrol. 2000;15:236–40. doi: 10.1007/s004670000456. [DOI] [PubMed] [Google Scholar]
- 32.Paterson D, Lan H, Atkins R. Macrophages in immune renal injury. In: Neilson E, Courser WG, editors. Immunological renal disease. New York: Raven Press; 1997. pp. 575–92. [Google Scholar]
- 33.Diamond JR, Kees-Folts D, Ding G, Frye JE, Restrepo NC. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol. 1994;266:F926–33. doi: 10.1152/ajprenal.1994.266.6.F926. [DOI] [PubMed] [Google Scholar]
- 34.Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor beta1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–84. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- 36.Schneider A, Panzer U, Zahner G, Wenzel U, Wolf G, Thaiss F, Helmchen U, Stahl RA. Monocyte chemoattractant protein-1 mediates collagen deposition in experimental glomerulonephritis by transforming growth factor-beta. Kidney Int. 1999;56:135–44. doi: 10.1046/j.1523-1755.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 37.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–80. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerritsma JS, van Kooten C, Gerritsen AF, van Es LA, Daha MR. Transforming growth factor-beta 1 regulates chemokine and complement production by human proximal tubular epithelial cells. Kidney Int. 1998;53:609–16. doi: 10.1046/j.1523-1755.1998.00799.x. [DOI] [PubMed] [Google Scholar]
- 39.Appay V, Rowland-Jones SL. RANTES. a versatile and controversial chemokine. Trends Immunol. 2001;22:83–7. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 40.Haberstroh U, Stilo K, Pocock J, Wolf G, Helmchen U, Wenzel U, Zahner G, Stahl RA, Thaiss F. 1-arginine suppresses lipopolysaccharide-induced expression of RANTES in glomeruli. J Am Soc Nephrol. 1998;9:203–10. doi: 10.1681/ASN.V92203. [DOI] [PubMed] [Google Scholar]
- 41.Michelson S, Dal Monte P, Zipeto D, Bodaghi B, Laurent L, Oberlin E, Arenzana-Seisdedos F, Virelizier JL, Landini MP. Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J Virol. 1997;71:6495–500. doi: 10.1128/jvi.71.9.6495-6500.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommer M, Eismann U, Deuther-Conrad W, Wendt T, Mohorn T, Funfstuck R, Stein G. Time course of cytokine mRNA expression in kidneys of rats with unilateral ureteral obstruction. Nephron. 2000;84:49–57. doi: 10.1159/000045538. [DOI] [PubMed] [Google Scholar]
- 43.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–8. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rovin BH, Phan LT. Chemotactic factors and renal inflammation. Am J Kidney Dis. 1998;31:1065–84. doi: 10.1053/ajkd.1998.v31.pm9631856. [DOI] [PubMed] [Google Scholar]


