Abstract
Varicella zoster virus (VZV) causes varicella (chickenpox) as the primary infection and zoster (shingles) on reactivation from latency, often many years later. One of the most common and most severe sequela of zoster is postherpetic neuralgia (PHN). Apart from age, factors which predispose towards PHN are unknown. In the present study, the concentration of a variety of Th1 and Th2 cytokines in the serum of 30 zoster patients at the time of the acute disease were correlated with the subsequent development of PHN in nine of these patients, but no association was found. In addition, although some cytokines such as IFN-γ, IL-6 and IL-8 were slightly raised in the zoster group compared with a group of normal healthy subjects of a similar age distribution, these differences only verged on significance. Antibody titres to VZV were raised in the zoster group compared with the controls but these did not differ between the patients who developed PHN and those who did not. Biopsies of zoster lesions were collected from nine patients. There were significantly fewer infiltrating lymphocytes in the lesions of the three patients who subsequently developed PHN compared with the six who did not, although the expression of the neuropeptide, substance P, did not differ between the two groups. It is possible that the poor inflammatory response at the time of the acute zoster may result in less effective containment of the VZV and more damage in the dermatome, thus contributing to the persistence of the neuralgia.
Keywords: cutaneous immune responses, cytokines, lymphocytes, postherpetic neuralgia, zoster
Introduction
Varicella zoster virus (VZV), a human alphaherpesvirus, produces two clinical syndromes, varicella (chickenpox) as the primary infection and zoster (shingles), frequently many years later [reviewed in 1, 2]. The virus is transmitted by inhalation of respiratory secretions or contact with skin lesions. Varicella in northern countries is primarily a disease of childhood. During the primary infection, the virus becomes latent in the dorsal ganglia and zoster is due to reactivation from latency, a process which occurs most often in subjects over the age of 50 years. Generally the lesions of zoster are preceded by intense pain in the involved dermatome. They are unilateral and located at the site affected most severely by varicella, commonly either the facial or the mid-thoracic to upper lumbar dermatomes. The lesions pustulate and crust in 7–10 days but may take several weeks to heal.
On first infection with VZV, CD4 and CD8 T cells are induced, specific for a variety of viral proteins, followed by generation of VZV IgM, IgG and IgA. IFN-α and IFN-γ are found in the serum during the acute phase of infection [3]. On stimulation of T cells with VZV in vitro following vaccination, IFN-γ and IL-10 are produced and this property is maintained, while IL-12 is released as an early but transient event [4]. Memory immunity to VZV is characterized by the persistence of IgG, CD4 T helper cells and both CD4 and CD8 cytotoxic T cells. From cytokine analysis, the memory CD4 T cell response to VZV is predominantly Th1, with production of IL-2 and IFN-γ. The events surrounding reactivation are not well understood, although a decline in VZV-specific cell-mediated immunity, specifically a decreased T cell proliferation in response to the virus, has been reported [5]. Zhang et al. have found that cells with a Th1-like cytokine profile are affected mainly [6]. However, zoster does not occur in all individuals with a decreased T cell immunity and thus this factor is necessary but not sufficient for reactivation. Decreasing antibody titres to VZV do not predispose to reactivation [7].
Postherpetic neuralgia (PHN) is defined as the presence of pain more than 4–6 weeks after the lesions of zoster have resolved. It occurs in 10–15% of all subjects with zoster and in at least 50% of those older than 60 years. PHN can be severe and debilitating: various treatment modalities have been tried without consistent success [1], although prompt antiviral therapy may help. Age has been identified as a consistent predisposing factor in predicting the development of PHN [8]. Other factors include the severity of prodromal pain, the severity of pain on presentation, and ophthalmic distribution [9]. The mechanism by which PHN develops is unknown, although it has been suggested that the persistence of VZV in the blood mononuclear cells may be important [10].
The aim of the present project was to monitor the presence of a variety of Th1 and Th2 cytokines in the serum of patients with zoster during the acute phase of this disease, and to determine whether the concentration of any of these differed from healthy controls or correlated with the subsequent development of PHN. In addition biopsies of the zoster lesions were collected from some subjects and analysed for infiltrating lymphocytes and the expression of the neuropeptide, substance P, to further test for a possible correlation with PHN development. Substance P was selected as it is one of a variety of neurogenic mediators that contribute to cutaneous inflammation and immunomodulation [reviewed in 11].
Materials And Methods
Patients
Informed consent was obtained from all the individuals before collecting the samples and the study was approved by the local ethics committee. The subjects with zoster attended the Department of Dermatology, University Hospital of Lodz, Poland, as out-patients who had self-referred or as in-patients. In the original cohort, called group A, there were 22 subjects (nine male, 13 female), aged 14–65, mean 49 years. They were bled on two occasions − on their first visit to hospital, in almost all cases within 10 days of first developing the symptoms of zoster, as reported by the patient, and secondly, one week later. In nine patients from this cohort, 4-mm punch biopies were collected from the area of the zoster lesions at the time of the first visit. These were formalin-fixed and paraffin-embedded. Only lesions occurring on the trunk were biopsied. A control group of healthy volunteers, called A, with a similar age range and distribution as the patients was recruited and consisted of 24 subjects (15 male, nine female), aged 22–66, mean 41 years. They were bled once. A second group of patients with zoster called group B was also used for some cytokine assays − this consisted of 29 subjects, 21 from the original patient group A and eight new subjects (11 males, 18 females) aged 14–65, mean 49 years, who were bled on two occasions with the same timings as described above for group A. The second control group called B consisted of 18 healthy volunteers of whom two were from the original control group A and 16 new subjects (six males, 12 females) aged 22–61, mean 46 years. They were bled once.
In all cases serum was collected from the clotted blood samples and stored at −20°C in aliquots until analysis for cytokine concentrations and antibody titre to VZV.
All the patients with zoster were treated with oral acyclovir in doses from 400 to 800 mg/day five times daily for 5–7 days from the time of their first hospital visit. They were monitored for clinical symptoms, particularly for the development of PHN, on several occasions during the 6 months following the acute zoster outbreak.
VZV antibody titre
The VZV-specific antibody (IgG) titres were measured by ELISA (Launch Diagnostics, Kent, UK) in the serum samples from all the patients and all the controls.
Cytokine analysis
The serum samples from the group A of patients and controls were analysed by ELISA using kits from Endogen (Woburn, MA, USA) for IL-2, IL-4, IL-10, IL-12 and IFN-γ. The serum samples from the group B of patients and controls were analysed by ELISA using kits for sIL-2R (Endogen), IL-6, IL-8, IL-13 (R&D Systems, Minneapolis, USA) and IL-18 (MBL, Nagoya, Japan). High sensitivity kits for the analysis of IL-10 and IL-12 were also used (R&D).
Antibodies and reagents
Monoclonal antibodies for CD3 (mouse antihuman IgG1) and CD56 (mouse antihuman IgG1) were purchased from Dako Ltd (Ely, Cambridgeshire, UK), and for CD4 (mouse antihuman IgG1) and CD8 (mouse antihuman IgG2b) from Novocastra (Vector Laboratories Ltd, Peterborough, UK). The substance P antibody was polyclonal rabbit antihuman (Biogenex, A. Menarini Diagnostics, Wokingham, Berks, UK). A catalysed tyramide signal amplification kit (TSA), ABC horseradish peroxidase reagent kit (ABC-HRP), 3,3′-diaminobenzidine tetrahydrochloride (DAB) substrate and biotinylated F(ab′)2 secondary antibodies (rabbit antimouse and swine antirabbit) were all purchased from Dako. All other reagents were of analytical grade from Sigma-Aldrich (Poole, Dorset, UK).
Immunohistochemistry
The paraffin-embedded sections from the patients were cut into 5-µm sections onto 3-aminotriethoxysilane-coated glass slides. The sections were dewaxed, followed by 0·5% hydrogen peroxide in methanol for 10 min to block endogenous peroxidase. Antigen retrieval was carried out where necessary before the slides were loaded into a Sequenza immunostaining rack (Shandon, UK) in Tris buffered saline or Tris buffered saline containing 0·2% v/v Tween 20. For antigen retrieval, the slides were placed in a plastic rack and immersed in 700 ml antigen retrieval buffer (1 mm EDTA, pH 8) which had been preboiled for 5 min. Sections were microwaved on full power (800 W) for the appropriate number of cycles (4 × 5 min for CD8, 3 × 5 min for CD56 and 3 × 5 for CD4) in a Samsung Model M6148 microwave fitted with a rotary plate. Sections were allowed to cool in the buffer by immersing the microwave container in cold running water for 15 min and then washed extensively in tap water.
Endogenous immunoglobulin binding sites were blocked by incubation for 30 min in 20% v/v normal rabbit or swine serum diluted in Tris buffered saline, according to the species of the secondary antibody. The incubation with the primary antibodies was overnight at 4°C and the optimal dilutions of each were determined empirically – they were 1 : 100 for CD3, 1 : 10 for CD4, 1 : 40 for CD8, 1 : 30 for CD56, neat for substance P. The ABC-HRP method was used as described previously [12] to detect CD3, CD4, CD56 and substance P staining. The TSA method [13] was used to detect CD8, according to the manufacturer's instructions. Negative controls consisted of the addition of species-matched non-immune antisera or non-immune IgG subclass-specific antibody for the primary antibodies and the omission of the primary antibody as a negative control for the secondary antibody. Following staining, the slides were washed extensively in running tap water before counterstaining for 1 min in Harris's haematoxylin and blueing for 1 min in Scott's tap water substitute. The sections were dehydrated through graded ethanol, and cleared in xylene before being permanently mounted in DePeX (BDH, Poole, Dorset, UK).
Sections were examined in a blinded manner and 10 fields were viewed per sample at × 400 magnification. The CD staining was scored on a five-point scale with 0 = no positive cells, + = < 25% inflammatory cells positive, + + = 25–50% inflammatory cells positive, + + + = 50–75% inflammatory cells positive, + + + + = > 75% inflammatory cells positive. The staining for substance P could not be scored on an individual cell basis but was distinguished into three groups with + for marginal positivity, + + for intermediate positivity and +++ for intense positivity.
Statistical analysis
For the VZV antibody and cytokine results, the Mann–Whitney test was used. For relating the day of collecting the first clinical samples from the patients and subsequent PHN development, rank Spearman's test was used. For ensuring that the age and sex of the patient and control groups were not significantly different, the χ2 and Mann–Whitney tests were used. For testing the significance of the infiltration of lymphocytes in PHN cases and the differences in ages of all the PHN+ and PHN− subjects and those with PHN who were biopsied, the Mann–Whitney test was used.
Results
Clinical symptoms
The average time until resolution of the lesions was 10–14 days and PHN was defined as the persistence of pain for more than 4 weeks following the resolution of the lesions. Of a total of 30 subjects with zoster, nine developed PHN (30%), of whom three had zoster on the face and six on the trunk. The mean age of the zoster patients with PHN was 55·4 (s.d. ± 7·6) and of the non-PHN zoster patients was 46·1 (s.d. ± 17·2), a difference which was statistically significant, P = 0·025. The age and sex of the control groups A and B were not significantly different from the zoster groups A and B.
VZV antibodies
The mean VZV antibody titre of the patients with zoster on their first visit to the hospital was 91·2 EU/ml (sd ± 47·2, range 13·5–161·2) and this rose to a mean of 123·9 EU/ml (s.d. ± 33·0, range 41·1–163·1) when tested in the second serum sample a week later. The mean antibody titre in the control subjects was 50·9 EU/ml (s.d. ± 21·0, range 20·1–87·5). The titres in both sets of samples from the patients were significantly higher than in the controls: first sample versus control P < 0·001, second sample versus control P < 0·0001. Thus, as might be expected, the reactivation of VZV and the emergence of the zoster lesions boosted the antibody titre to the virus.
The mean VZV antibody titre in samples 1 and 2 of the patients who subsequently developed PHN was 101·1 (s.d. ± 38·5) and 133·5 (s.d. ± 40·6), respectively, while in samples 1 and 2 of those who did not develop PHN, it was 87·7 (s.d. ± 50·3) and 120·5 (s.d. ± 30·4), respectively. There was no significant difference between the mean titres of the PHN patients and the non-PHN patients at either time point.
Cytokine analysis
Serum samples from the patients with zoster were collected on two occasions, the first on entry into hospital as close to the beginning of the acute phase of the lesions as was possible and the second 1 week later. The control group consisted of subjects in good health who were similar in age distribution and sex as the patients and they were bled on one occasion. The sera were analysed for the concentrations of IFN-γ, IL-2, sIL-2R, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13 and IL-18, and the results are shown in Table 1. The high-sensitivity kits for IL-10 and IL-12 did not change the results for these two cytokines significantly.
Table 1.
Cytokine concentrations (mean ± s.d.) in serum samples from two groups of patients with zoster taken on their first visit to hospital (S1) and 1 week later (S2), and from two groups of healthy controls
| Group A patients | Group A controls | Group B patients | Group B controls | |||
|---|---|---|---|---|---|---|
| n= 22 | n = 24 | n = 29 | n = 18 | |||
| S1 | S2 | S1 | S1 | S2 | S1 | |
| IFN-γ pg/ml | 1·8 ± 2·9 | 0·7 ± 1·3 | 0·3 ± 1·7 | |||
| IL-2 pg/ml | ND | ND | ND | |||
| sIL-2R U/ml | 501 ± 337 | 509 ± 236 | 437 ± 410 | |||
| IL-4 pg/ml | ND | ND | ND | |||
| IL-6 pg/ml | 2·0 ± 3·1 | 2·9 ± 4·3 | 0·7 ± 0·6 | |||
| IL-8 pg/ml | 28·5 ± 22·2 | 33·5 ± 94·0 | 18·1 ± 19·0 | |||
| IL-10 pg/ml | 5·9 ± 5·0 | 4·7 ± 3·3 | 5·7 ± 4·5 | |||
| IL-12 pg/ml | 128 ± 65 | 128 ± 70 | 112 ± 99 | |||
| IL-13 pg/ml | ND | ND | ND | |||
| IL-18 pg/ml | 289 ± 269 | 275 ± 177 | 296 ± 159 | |||
ND = below detectable limit.
Large variations between individuals were found as is reflected in the high standard deviations of the groups. Although for some cytokines, namely IFN-γ, IL-6 and IL-8, increased levels were detected in the patient samples compared with the controls, the only significant difference between the cytokine concentrations in the sera of the zoster patients at either time-point compared with the controls was for IL-6. Here the sample 1 of the patients was higher than the controls, P = 0·03. No cytokine changed concentration significantly between the two samples taken from each individual patient. In addition, for all the mediators tested, there were no significant differences between the cytokine levels in those zoster patients who subsequently developed PHN compared with those who did not. Finally, a comparison between these two groups regarding the time at which the first sample was collected did not reveal any significant difference.
Local immune response in zoster lesions
Biopsies of zoster lesions from nine patients were collected at the time of the first visit to hospital: three of these patients subsequently developed PHN. The mean age of those biopsied who developed PHN was 59·3 (s.d. ± 5·8) and those who did not was 43·3 (s.d. ± 5·8) which was just significantly different (P = 0·046). Specimens were taken only from lesions on the trunk to avoid scarring the face and the number examined was limited, as many patients were reluctant to participate in this part of the study. Immunohistochemistry was performed on sections of the biopsies to monitor lymphocyte infiltration and the expression of substance P. Representative sections are shown in Fig. 1 and the results in Table 2. The substance P staining was not cell-associated, indicating secretion, and was scored on an arbitrary basis into three categories. Positive staining was seen in all the biopsies and there was no significant difference between the amount in those subjects who later developed PHN and those who did not. In contrast, the lymphocytic infiltration did differ between these two groups. There were significantly fewer CD3, CD8 and CD56-positive cells in the lesions of the zoster patients who later developed PHN (P = 0·004, 0·013 and 0·027 for CD3, CD8 and CD56, respectively). In addition, fewer CD4-positive cells were seen in the PHN patients compared with the non-PHN, although this difference was not significant (P = 0·078).
Fig. 1.
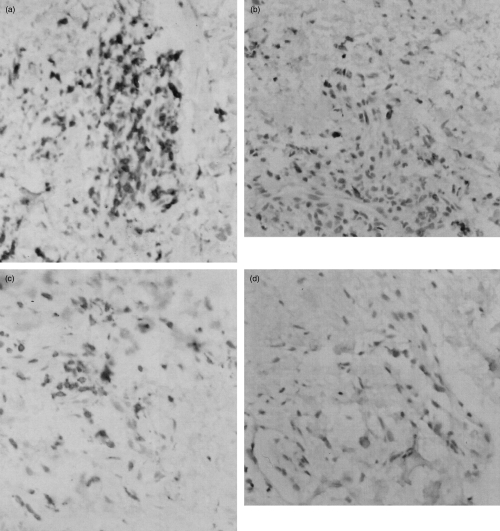
Immunostaining showing the influx of CD3-positive and CD56-positive lymphocytes into the zoster skin lesions of patient 1 (a and c, respectively) who did not develop HPN subsequently, and patient 9 (b and d, respectively) who developed PHN subsequently. Original magnification ×100.
Table 2.
Infiltration of lymphocytes and expression of substance P (SP) in skin lesions of patients with zoster, some of whom subsequently developed postherpetic neuralgia (PHN)
| Number | Sex | Age* | Day after first symptoms | Development of PHN | SP | CD3† | CD4 | CD8‡ | CD56§ |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 48 | 8 | – | + + | + + + + | + | + + + | + |
| 2 | F | 29 | 4 | – | + | + + + + | + + | + + + + | + |
| 3 | F | 58 | 3 | – | + + + | + + + + | + + | + + + + | + + |
| 4 | M | 22 | 3 | – | + + + | + + + | + | + + + + | + |
| 5 | M | 47 | 6 | – | + | + + + + | + + + | + + + + | + + |
| 6 | F | 56 | 12 | – | + | + + + + | + + + | + + + + | + |
| 7 | F | 63 | 30 | + | + | + + | + | + + | + |
| 8 | F | 67 | 3 | + | + | + + | 0 | + + + | 0 |
| 9 | F | 48 | 5 | + | + + | + + | + + | + + + | 0 |
SP staining: + marginal, + + intermediate, + + + intense positivity. CD staining: 0 = no positive cells, + = < 25%, + + = 25–50%, + + + = 50–75%, + + + + = > 75% inflammatory cells positive.
PHN+ significantly older than PHN− (P = 0·046)
PHN+ significantly lower than PHN− (P = 0·004)
PHN+ significantly lower than PHN− (P = 0·013)
PHN+ significantly lower than PHN− (P = 0·027).
Discussion
It is known that age is a significant predictor of severity and duration of pain following zoster [8]: for example, the prevalence of PHN is around 10–15% in all subjects with zoster and rises to at least 50% when older than 60 [14,15]. Other risk factors for PHN have been proposed, including the severity of the initial pain, the extent of the rash and psychosocial stress but, in contrast to age, none of these show strong associations. McQuay has suggested that, if the symptoms of zoster are treated effectively, then the risk of developing PHN should be reduced [16]. However, acyclovir, which until recently was the antiviral agent of choice in the treatment of zoster, reducing both acute pain and time to healing, has no benefit in decreasing the duration of PHN [17]. In the present study, the systemic production of a range of cytokines and the local inflammatory infiltrate at the time of the acute zoster were evaluated in subjects, some of whom later developed PHN, to find out if these responses could influence the subsequent prolongation of pain.
In the zoster patient cohort, age was a predisposing factor for PHN, both within the whole group and in the subgroup from whom biopsies were taken. The first sample of blood and the biopsy were collected as soon as the subject presented at the hospital and, although the period between first developing the clinical symptoms of zoster and this time-point varied, in most instances it was within 10 days. Although it might have been preferable to obtain the clinical samples earlier in the course of the acute phase of zoster and at a standard time, this was not possible in practice. However, the fact that the VZV antibody titre was considerably higher in the second blood sample collected one week after the first, indicates that immunity to the virus was still actively increasing.
Antibody titres to VZV in both the first blood samples and the second did not distinguish between the patients who later developed PHN and those who did not. This result was not surprising, as antibodies appear to play little part in the control of VZV, indicated, for example, by the lack of correlation between decreasing titres and reactivation of the virus from latency [7].
The concentration of nine cytokines, plus sIL-2R, were measured in the sera of the zoster patients and, although some showed slight increases in concentration over the levels in the controls, the differences were slight, did not increase in sample 2 and were considered unlikely to have functional significance. Furthermore, no correlation could be established between cytokine concentration and the severity of zoster or the development of PHN. It has been reported that IFN-γ in particular is important in the inhibition of VZV replication [18] and is produced in the serum following the viraemic phase and within the first 3 days of rash development in children with varicella [3]. In addition, if lymphocytes from immune adults are stimulated in vitro with VZV, Th1 cytokines are released predominantly but also some Th2 cytokines [19]. Furthermore, immunization of adults with varicella vaccine induces viraemia with a prolonged increase in IFN-γ and a more transient increase in IL-10 concentration on stimulation of lymphocytes in vitro with the virus [20]. On the other hand, when sera from adult cases of varicella, collected within 24 h of rash development, were assessed for TNF-α, IL-2 and IFN-γ, IL-2 and IFN-γ were detectable at low levels in a minority of samples only [4]. TNF-α was elevated frequently, although its concentration did not correlate with the severity of the varicella. It has also been reported that the frequency of VZV-specific CD4-positive T cells in the blood decreases with age, a factor which correlates with the increasing incidence and severity of zoster [21]. The lowered frequency is accompanied by a lowered in vitro T cell proliferation in response to VZV and a lowered production of IFN-γ. Finally, it should be noted that, in zoster, there is localized reactivation of VZV within the ganglion and limited spread to the local dermatome, with viraemia occurring rarely. Under these circumstances, detectable changes in serum cytokine concentrations may be unlikely and stimulation of lymphocytes in vitro with VZV antigens might be required to demonstrate modulations in cytokine production as a result of zoster.
In contrast to the cytokine and VZV antibody results, the immunohistochemistry of the zoster lesions revealed differences between those patients who later developed PHN and those who did not. Although more biopsies would have strengthened these results, the patients rarely agreed to participate in this part of the study, mainly due to the considerable local pain they were suffering at the time of our request. Even with the small numbers available, it was clear that there were fewer inflammatory cells in the lesions of those who suffered subsequently from PHN. The mechanism of this is unknown. It is possible that the limited inflammatory response at the time of the acute zoster may result in less effective containment of the virus and more damage in the dermatome, thus contributing to the persistence of the neuralgia. VZV DNA has been reported to persist in the mononuclear cells of PHN patients compared with zoster patients without PHN [10], which might reflect the lack of clearance of the virus at the time of the dermatomal rash. In addition VZV has a variety of immune evasion mechanisms that limit presentation of viral peptides by MHC Class I and II pathways, thus allowing local replication of VZV in the skin [reviewed in 22]. These mechanisms could be more effective in individuals with particular HLA haplotypes or genetic polymorphisms: such analyses of host genetic factors have not been undertaken as yet for VZV, as far as we are aware.
Finally the expression of substance P was also monitored by immunohistochemistry in the zoster lesions. Although it was detected, its expression did not differ significantly between patients who later developed PHN and those who did not. Thus, the quantity of substance P in the acute phase of zoster is unlikely to be a prognostic factor for PHN.
In conclusion, patients with zoster who subsequently developed PHN were older than the PHN-negative patients and also had lowered infiltration by CD3, CD8 and CD56-positive cells into their skin lesions at the time of the acute zoster.
Acknowledgments
M.Z-P. and M.N. wish to thank the British Council for funding a British-Polish Joint Collaborative Programme. The work was also supported by the Medical University of Lodz, Poland Internal Grant 502-11-782 (25) (M.Z-P.) and the Foundation for Skin Research (R.C.M.).
References
- 1.McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1–14. doi: 10.1016/s0190-9622(99)70398-1. [DOI] [PubMed] [Google Scholar]
- 2.LaGuardia JJ, Gilden DH. Varicella-zoster virus: a re-emerging infection. J Invest Dermatol Symp Proc. 2001;6:183–7. doi: 10.1046/j.0022-202x.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- 3.Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–9. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins DE, Redman RL, Meng Lam E, Liu C, Lin I, Arvin AM. Interleukin (IL)-10, IL-12, and interferon-γ production in primary and memory immune responses to varicella-zoster virus. J Infect Dis. 1998;178:940–8. doi: 10.1086/515702. [DOI] [PubMed] [Google Scholar]
- 5.Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis. 1992;165:119–26. doi: 10.1093/infdis/165.1.119. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Cosyns M, Levin MJ, Hayward AR. Cytokine production in varicella zoster virus-stimulated limiting dilution lymphocyte cultures. Clin Exp Immunol. 1994;98:128–33. doi: 10.1111/j.1365-2249.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster A, Grint P, Brenner MK, Prentice HG, Griffiths PD. Titration of IgG antibodies against varicella zoster virus before bone marrow transplantation is not predictive of future zoster. J Med Virol. 1989;27:117–9. doi: 10.1002/jmv.1890270209. [DOI] [PubMed] [Google Scholar]
- 8.Helgason S, Petursson G, Gudmundsson S, Sigurdsson JA. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ. 2000;321:794–6. doi: 10.1136/bmj.321.7264.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decroix J, Partsch H, Gonzalez R, et al. Factors influencing pain outcome in herpes zoster: an observational study with valaciclovir. J Eur Acad Dermatol Venereol. 2000;14:23–33. doi: 10.1046/j.1468-3083.2000.00020.x. [DOI] [PubMed] [Google Scholar]
- 10.Mahalingam R, Wellish M, Brucklier J, Gilden DH. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–3. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- 11.Lambert RW, Granstein RD. Neuropeptides and Langerhans cells. Exp Dermatol. 1998;7:73–80. doi: 10.1111/j.1600-0625.1998.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 12.Szepietowski JC, Walker C, McKenna DB, Hunter JAA, McKenzie RC. Leukaemia inhibitory factor (LIF) and interleukin (IL)-8 expression in non-melanoma skin cancers. Clin Exp Dermatol. 2001;26:72–8. doi: 10.1046/j.1365-2230.2001.00765.x. [DOI] [PubMed] [Google Scholar]
- 13.Adams JC. Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem. 1992;40:1457–63. doi: 10.1177/40.10.1527370. [DOI] [PubMed] [Google Scholar]
- 14.Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 15.McKendrick MW, McGill JI, Wood MJ. Lack of effect of acyclovir on post-herpetic neuralgia. Br Med J. 1989;298:431. doi: 10.1136/bmj.298.6671.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McQuay HJ. Antidepressants and chronic pain. Effective analgesia in neuropathic pain and other syndromes. Br Med J. 1997;314:763–4. doi: 10.1136/bmj.314.7083.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bean B, Braun C, Balfour HH. Acyclovir therapy for acute herpe zoster. Lancet. 1982;2:118–21. doi: 10.1016/s0140-6736(82)91090-x. [DOI] [PubMed] [Google Scholar]
- 18.Rabalais GP, Berkowitz FE, Hayward AR, Levin MJ. Inhibition of varicella-zoster virus in vitro by human peripheral blood mononuclear cells. Clin Exp Immunol. 1989;75:381–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Hayward AR, Cosyns M, Jones M, et al. Cytokine production in varicella-zoster virus-stimulated cultures of human blood lymphocytes. J Infect Dis. 1998;178(Suppl. 1):s95–8. doi: 10.1086/514266. [DOI] [PubMed] [Google Scholar]
- 20.Wallace MR, Woelfl I, Bowler WA, et al. Tumor necrosis factor, interleukin-2 and interferon-gamma in adult varicella. J Med Virol. 1994;43:69–71. doi: 10.1002/jmv.1890430113. [DOI] [PubMed] [Google Scholar]
- 21.Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, and cytomegalovirus by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–66. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 22.Abendroth A, Arvin AM. Immune evasion as a pathogenic mechanism of varicella zoster virus. Sem Immunol. 2001;13:27–39. doi: 10.1006/smim.2001.0293. [DOI] [PubMed] [Google Scholar]


