Abstract
The immunological mechanisms by which respiratory syncytial virus (RSV) contributes to the development of asthma are poorly understood. γδ T cells are important in mucosal defence, and may contribute to the establishment of primary immune responses by producing cytokines early during respiratory infections. Thus, we used flow cytometry and intracellular cytokine staining to investigate the expression of interferon (IFN)-γ and interleukin (IL)-4 by mitogen-stimulated γδ T cells from the peripheral blood of 15 hospitalized infants with RSV bronchiolitis, seven rotavirus-infected infants and eight normal controls. γδ T cells from RSV-infected infants had a lower proportion of IFN-γ-producing cells (median, 4.00%; range, 0.58–6.60%) and a slightly but significantly higher proportion of IL-4-producing cells (median, 0.40%; range, 0.13–2.76%) than rotavirus-infected infants (median, 32.10%; range, 14.43–61.21%; P < 0·01, median, 0.00%; range, 0.00–0.00%; P < 0·05) in the acute phase. By contrast, differences in cytokine production by total CD3+ T cells did not differ significantly between patient groups. Thus, reduced IFN-γ-production by γδ T cells in the peripheral blood of RSV-infected infants is accompanied by increased Th2 cytokine production during the acute phase of disease. At follow-up, eight children had recurrent episodes of wheezing. The frequencies of IFN-γ-producing γδ T cells were significantly lower in patients who developed recurrent wheezing (median, 0.65%; range, 0.02–1.75%) than in patients without recurrent wheezing (median, 6.90%; range, 5.25–10.98%; P < 0·005). Cytokine production by γδ T cells may therefore be important in the pathogenesis of acute RSV disease, and play a part in the development of recurrent childhood wheezing after bronchilolitis.
Keywords: bronchilolitis, IFN-γ, IL-4, γδ T cells, respiratory syncytial virus
Introduction
Respiratory syncytial virus (RSV) is the most important cause of lower respiratory tract disease in infants [1]. In addition, a history of RSV bronchiolitis is related to the development of recurrent episodes of bronchial obstruction, specific IgE antibodies and established asthma [2–6] in some infants or children with a family history of atopy. Although the precise mechanisms for the development of asthma and allergy by RSV infection are not clear, a relative predominance of Th2 over Th1 cytokine has been demonstrated to be important [7,8]. The observation that RSV can induce virus-specific IgE antibodies that correlate with subsequent wheezing episodes [9] suggests this possibility. A shift to relative Th2 cytokine balance could be due either to enhanced interleukin (IL)-4 production or to diminished interferon (IFN)-γ production. Defective IFN-γ production by peripheral blood mononuclear cells (PBMC) has been reported to be correlated with the severity of RSV bronchiolitis and the subsequent development of asthma [10–12], suggesting that suppressed IFN-γ may be responsible for the relative dominance of Th2 cytokine. IFN-γ is produced by subsets of immune cells such as NK cells, αβ T cells, and γδ T cells [13–16]. It is very important to clarify the source of IFN-γ in RSV bronchiolitis, but there have been few studies of the cell source of IFN-γ in the acute infectious phase in humans [17,18].
Although the biological role and repertoire of γδ T cells have not been fully defined, recent studies suggest that γδ T cells play an important role in the immune response not only to bacterial infections such as tuberculosis [19, >20] but also to viral infections such as cytomegalovirus or Epstein–Barr virus infection [21,22]. In mice, early during the course of infection, γδ T cells may contribute to development of αβ T helper subset responses [23,24], and be essential for Th2-mediated airway inflammation [25]. In contrast, little is known of the immune responses of γδ T cells during acute lower respiratory infection caused by RSV in humans. Thus, we compared the frequencies of IFN-γ or IL-4 producing γδ T cells in mitogen-stimulated peripheral blood from RSV-infected infants and rotavirus-infected infants by flow cytometry combined with intracellular cytokine staining. In addition, we studied whether the cytokine responses of γδ T cells in the acute phase of RSV bronchiolitis are associated with the subsequent development of recurrent wheezing during a 2-year follow-up period.
Methods
Subjects
Fifteen infants (five males, 10 females) were hospitalized RSV-positive bronchiolitis patients aged 6–28 months (13·5 ± 7·0 months, mean ± s.d.). The diagnosis of bronchiolitis was based on the clinical criteria of tachypnoea, coryzal symptoms and respiratory distress. None of the patients was in need of mechanical ventilation. As controls with viral infection, seven rotavirus-positive enterocolitis patients (four males, three females) aged 8–35 months (17·3 ± 8·1 months) were selected during the same winter season. All were born mature and healthy until the time of infection. Ten RSV-infected patients (67%) and four rotavirus-infected patients (57%) had a positive family history of atopy. RSV antigens in nasal swabs and rotavirus antigens in stools were detected by EIA (Enzyme Immunoassay) (Abbott Laboratories, Abbott Park, IL, USA) according to the manufacturer's protocol. Eight control infants (four males, four females) aged 6–31 months (17·0 ± 9·8 months) without evidence of infection were selected for this study. Included were infants prior to minor surgery, healthy infants screened for congenital disorders and infants with mild liver dysfunction. After informed consent, a maximum 4 ml of blood was drawn into heparinized tube within 24 h of admission for bronhiolitis or enterocolitis after it was established that there was no underlying anaemia. Subjects received conventional therapy that did not include either Ribavirin or corticosteroids while they were hospitalized. Three to 4 weeks later, in the convalescent phase, heparinized blood was taken again from five of the RSV patients. All families consented to regular follow-up for 2 years. Follow-up was performed using diaries that were developed for this study. The parents were taught how to use the diary and the differences between wheezing and other noisy breathing while the infants were hospitalized. At the end of the follow-up period, patients were classified as ‘recurrent wheezing’ if more than two episodes of wheezing were noted after discharge. The study was approved by the ethics committee of National Shimoshizu Hospital.
Reagents
The mouse monoclonal antibodies (MoAbs) used for flow cytometry analysis were purchased from the following sources. Phycoerythrin (PE)-conjugated anti-IFN-γ, anti-IL-4, mouse-IgG1 control, mouse IgG2a control, FITC-conjugated anti-pan γδ T cell receptor, mouse IgG1 control, PerCP-conjugated anti-CD3 and mouse IgG1 control were from Becton Dickinson Immunocytometry Systems (BDIS) (Mountain View, CA, USA). These MoAbs were used at the concentrations recommended by the manufacturer. Brefeldin A (BFA), phorbol 12-myristate 13-acetate (PMA), and ionomycin were obtained from Sigma Chemical Co. (St Louis, MO, USA). RPMI-1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 2 mm l-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin and 2 × 10−5 m mercaptoethanol was used.
Cell culture and staining
A total of 500 µl heparinized venous blood was added to 500 µl of RPMI-1640 in 15 ml conical polypropylene tubes (Becton Dickinson Labware, Franklin Lakes, NJ, USA), and incubated with the secretion inhibitor BFA (1 µg/ml) in the presence of PMA (25 ng/ml) and ionomycin (1 µm) at 37°C, 7% CO2 for 4 h. Surface staining was then carried out at room temperature (RT) for 15 min in the dark. Blood samples were then lysed and fixed in FACS™ Lysing Solution (BDIS) for 10 min at RT. The cells were subsequently resuspended in FACS™ Permeabilizing Solution for 10 min at RT. After permeabilization, the cells were washed in cold wash buffer (PBS, 0·5% BSA, 0·1% NaN3) and stained for 30 min at RT in the dark with PE-conjugated cytokine-specific MoAbs. Isotype-matched control antibodies were included to detect non-specific binding. After staining, the samples were washed and fixed in 1% paraformaldehide. Cytokine production in a sample obtained from the same healthy adult was included in every experiment to assess whether the stimulation used throughout the study was optimal.
Flow cytometric analysis
Three-colour flow cytometric analysis was performed on a FACSCalibur™ flow cytometer (BDIS). T cells were gated using FL3 (CD3 PerCP) and FSC/SSC parameters, and 1 × 104−2 × 104 cells were analysed using CELLQuest™ software (BDIS). The results are expressed as the percentage of cytokine-producing cells in each CD3+T cell or γδ T cell sample.
Statistical analysis
The percentages of cytokine producing cells were analysed by the Mann–Whitney U-test or the Kruskall–Wallis H-test. If the Kruskall–Wallis H-test showed significant differences, the Bonferroni correction was performed in order to analyse which groups have significantly different values and to calculate P-values. Values of P < 0·05 were considered significant.
Results
Cytokine production by CD3+T cells
To determine the cytokine patterns of CD3+ T cells from RSV-infected or rotavirus-infected infants in the acute phase, we measured the capacity of T cells stimulated by PMA and ionomycin to produce IFN-γ and IL-4. After surface staining for CD3, cells were permeabilized and stained with MoAb against cytokine. Intracellular cytokine expression was analysed by flow cytometry of gated lymphocytes using forward and side scatter. Figure 1 shows the proportion of IFN-γ and IL-4 producing CD3+ T cells from RSV-infected or rotavirus-infected infants and from healthy controls. No significant differences were seen among the three groups.
Fig. 1.
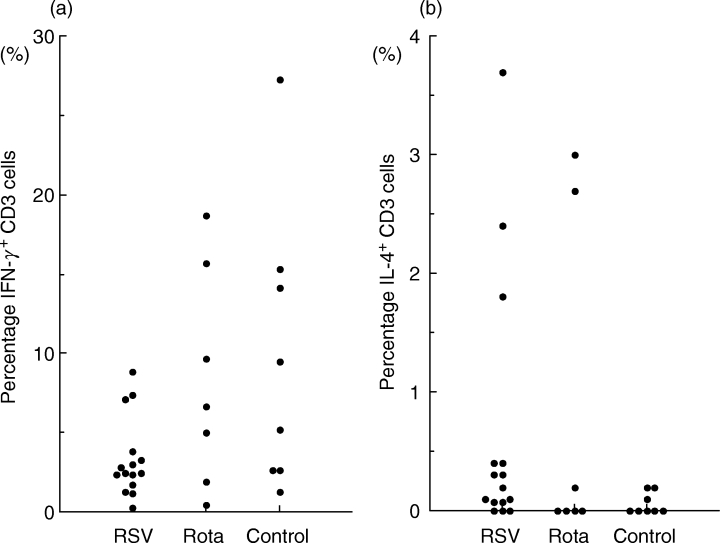
The percentage of CD3+ T cells from RSV- or rotavirus-infected infants and from controls that produce IFN-γ (a) and IL-4 (b) upon stimulation with PMA and ionomycin for 4 h. Each symbol represents one individual tested. No significant differences were seen among the three groups by the Kruskall–Wallis H-test.
Cytokine production by γδ T cells
Figure 2 shows representatives of intracellular cytokine expression gated using FL3 (CD3 PerCP) from RSV-positive bronchiolitis or rotavirus-positive enterocolitis patients. Both γδ TCR+ and γδ TCR− CD3+ T cells produced IFN-γ, but the percentage of IFN-γ-producing cells among the γδ TCR+CD3+ T cells was higher than among the γδ TCR− CD3+ T cells. Figure 3 shows the proportion of IFN-γ and IL-4-producing γδ T cells from RSV- or rotavirus-infected infants in the acute phase and from healthy controls. γδ T cells from RSV-infected infants had a significantly lower proportion of IFN-γ-producing cells (median 4·0%; range 0·58–6·6%) than γδ T cells from rotavirus-infected infants (median 32·10%; range 14·43–61·21%; P < 0·01 by Bonferroni) or controls (median 22·20%; range 11·35–37·40%; P < 0·01 by Bonferroni). γδ T cells from RSV-infected infants had a significantly higher proportion of IL-4 producing cells (median 0·4%; range 0·13–2·76%) than those from rotavirus-infected infants (median 0·00%; range 0·00–0·00%; P < 0·05 by Bonferroni) in the acute phase or controls (median 0·50%; range 0·00–0·50%; P < 0·05 by Bonferroni). In all five RSV patients tested again, the numbers of IFN-γ producing γδ T cells returned to normal in the convalescent phase (Fig. 4).
Fig. 2.
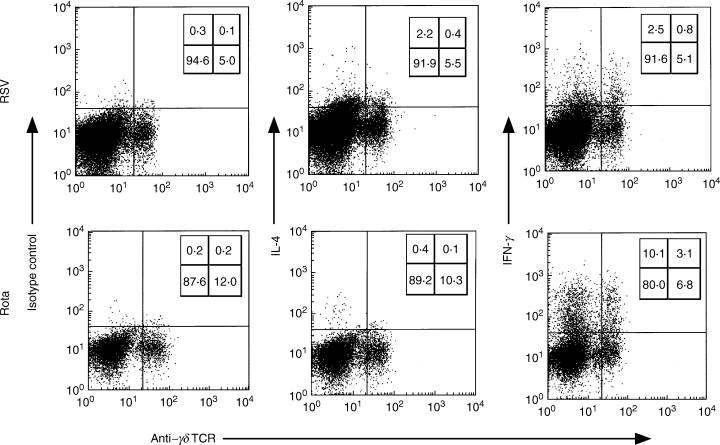
Representative plots of IL-4 and IFN-γ production among the CD3+ T cells from RSV- or rotavirus-infected infants upon stimulation with PMA and ionomycin for 4 h. Numbers indicate the percentage of CD3+ T cells stained in each of four quadrants.
Fig. 3.
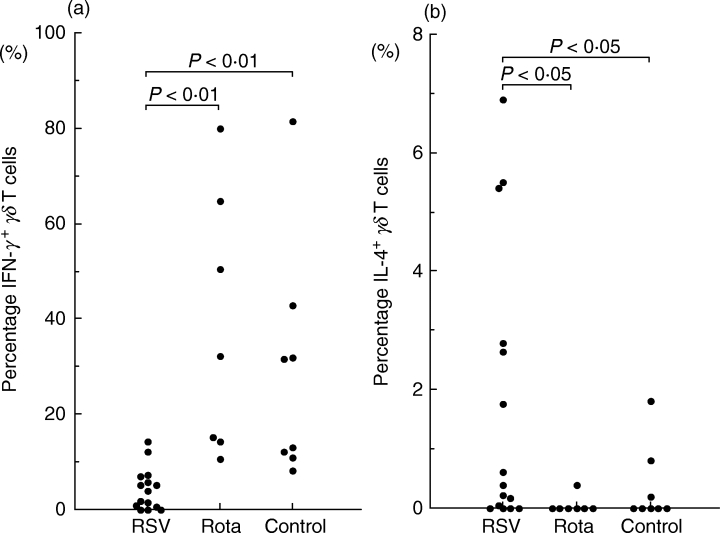
The percentage of γδ T cells from RSV- or rotavirus-infected infants and from controls that produce IFN-γ (a) and IL-4 (b) upon stimulation with PMA and ionomycin for 4 h. Each symbol represents one individual tested. γδ T cells from RSV-infected infants had a significantly lower proportion of IFN-γ-producing cells (P < 0·01 by Bonferroni) and a slightly but significantly higher proportion of IL-4-producing cells (P < 0·05 by the Bonferroni) than rotavirus-infected infants in the acute phase.
Fig. 4.
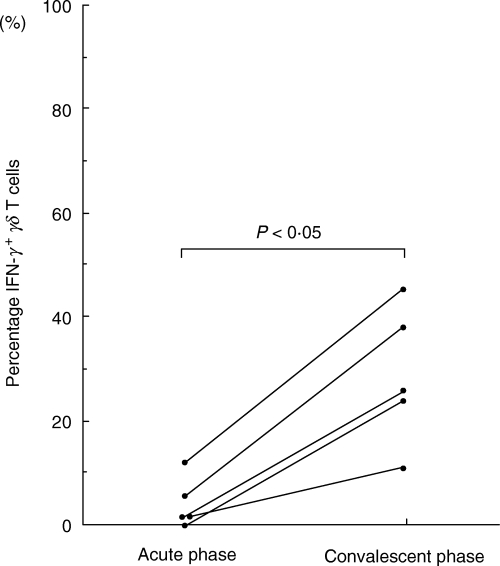
IFN-γ production by γδ T cells during the acute and convalescent phase in five RSV-infected infants. Data represent individual values. The numbers of IFN-γ-producing γδ T cells returned to normal in the convalescent phase.
The association between IFN-γ response and recurrent wheezing
Eight children had two or more episodes of wheezing during the follow-up period. IFN-γ responses of γδ T cells in the acute phase were significantly lower in infants with recurrent wheezing than in those without recurrent wheezing (P < 0·005 by Mann–Whitney U-test) (Fig. 5). IL-4 responses of γδ T cells were not associated with recurrent wheezing during follow-up.
Fig. 5.
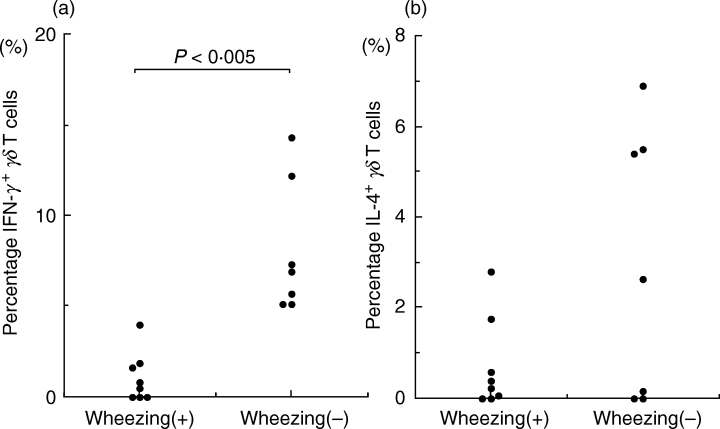
Relationship between IFN-γ (a) or IL-4 (b) production by γδ T cells in PMA and ionomycin-stimulated whole-blood cultures and the development of recurrent wheezing after RSV bronchiolitis. IFN-γ responses of γδ T cells in the acute phase were significantly lower in infants with recurrent wheezing than in those without recurrent wheezing (P < 0·005 by the Mann–Whitney U-test).
Discussion
This study shows that the proportion of γδ T cells that produce IFN-γ after mitogen stimulation is significantly reduced in patients with RSV bronchiolitis. The proportion of IL-4-producing γδ T cells is higher but this is not prominent compared to the reduction in IFN-γ producing cells.
The reduction in the number of IFN-γ-producing γδ T cells in RSV bronchiolitis was not due to a general immune defect in the patients, such as immaturity of the immune response, because the number of IFN-γ producing γδ T cells returned to normal level in the convalescent phase. Furthermore, the number of IFN-γ producing γδ T cells was not affected in age-matched patients with acute rotavirus enteritis. In separate experiments, we examined the cytokine responses of γδ T cells in schoolchildren with mycoplasma or influenza infection and found that the number of IFN-γ-producing γδ T cells was not suppressed in comparison with healthy controls (Aoyagi et al. manuscript in preparation). Taken together, these results suggest strongly that RSV is unique in terms of its strong effect on IFN-γ production by γδ T cells, and provokes a relatively Th2-skewed immune response in comparison with other pathogens in the acute phase. This hypothesis is compatible with the observation by Hall et al. that interferon production in nasal washes in acute RSV infection is significantly lower in influenza and parainfluenza virus infection [26].
γδ T cells comprise a minor population in the circulation. However, these cells play an important role in the first line of immune responses to various infections. Certain microbial infections have been associated with the maturation of the immune system from low Th1 to high Th1 in the postnatal period. In this process, γδ T cells play an important role by inducing Th1 immune responses and thus protect against primary allergic sensitization [27].
McMenamin et al. showed that γδ T cells from ovalbumin-tolerant mice selectively suppress immunoglobulin E antibody production by inducing high levels of IFN-γ[28]. Since γδ T cells contribute to the modulation of inflammatory processes and the development of an αβ T helper subset in a murine model [23,24], these primary immune responses of γδ T cells in RSV infection may influence the cytokine milieu and be an important factor in the increase in allergen-specific IgE antibodies and the development of asthma after bronchiolitis in some infants. The role of γδ T cells is especially important in young infants because γδ T cells are enriched in young rather than adult animals [27] and γδ T cell-deficient animals show defects in immunoprotection only during the early phases of infection in very young mice [29].
IFN-γ is produced by several T cell subsets, and the identification of cell populations showing defective IFN-γ production is of special interest in RSV bronchiolitis. Bendelja et al. showed that the number of IFN-γ producing cells in both CD4+ and CD8+ T cells is decreased in the acute phase of RSV bronchiolitis compared to healthy controls [17]. Although we studied relative small number of patients, we found no statistical difference in the number of IFN-γ-producing CD3+ T cells between RSV bronchiolitis and rotavirus enteritis patients. The discordance of our results from those of Bendelja may be explained by the age of the subjects studied. Their study included much younger RSV bronchiolitis patients than our study. As already demonstrated previously, T cells from young infants produce significantly less IFN-γ than those from older infants [30]. The other possibility is that main source of IFN-γ in the acute phase of RSV bronchiolitis is really αβ T cell receptor-negative T cells such as γδ T cells or NK cells. Because we examined cell numbers of cytokine-producing cells, but not cytokines themselves, this cannot be determined from the present study. Thus, the main cell populations producing IFN-γ remain to be elucidated in the future.
Our results demonstrate that the reduced IFN-γ responses of γδ T cells during the acute phase of RSV bronchiolitis are associated with the development of subsequent recurrent episodes of wheezing. In contrast, no relationship was found between increased IL-4 responses of γδ T cells and recurrent episodes of wheezing. These results support the importance of defective IFN-γ production during acute viral infection in the development of postbronchiolitis wheezing, which was clearly demonstrated by Sorkness et al. in an animal model of postbronchiolitis airway hyperreactivity [31]. Recently, they demonstrated further that defective IFN-γ production by natural killer cells from brown Norway rats is responsible for this postviral chronic airway dysfunction [32]. These results indicate that IFN-γ produced by innate immunity plays a major role in preventing the acquisition of bronchial hyperresponsiveness after respiratory viral infections. In humans, Renzi and colleagues showed that lower IFN-γ production by PBMC in response to IL-2 at the time of bronchiolitis is linked to later abnormal pulmonary function and to the development of asthma [33]. These data, in combination with the animal study, suggest an important role of defective IFN-γ production from innate immunity such as γδ T cells in the development of asthma in selected subjects after RSV bronchiolitis.
In summary, we have demonstrated that IFN-γ production by γδ T cells is suppressed in patients with acute RSV-positive bronchiolitis, while that of the CD3+ T cell population is largely unaffected. This suppressed IFN-γ pattern is related to the subsequent development of recurrent wheezing. The results suggest a novel and important role for γδ T cells in RSV bronchiolitis in terms of the development of respiratory allergy. Future work needs to focus on the regulatory effects of γδ T cells in younger infants. Elucidating these mechanisms will provide useful information to be used for the prevention of asthma and the development of practical vaccines.
Acknowledgments
We thank Dr M. D. Ohto for reviewing the manuscripts. This work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture.
References
- 1.Everard ML, Milner AD. The respiratory syncitial virus and its role in acute bronchiolitis. Eur J Pediatr. 1992;151:638–51. doi: 10.1007/BF01957564. [DOI] [PubMed] [Google Scholar]
- 2.Mok JYK, Simpson H. Symptoms, atopy and bronchial reactivity after lower respiratory infection in children. Arch Dis Child. 1984;59:299–305. doi: 10.1136/adc.59.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss ST, Tager IB, Muoz A, Speizer FE. The relationship of respiratory infection in early childhood to the occurrence of increased levels of bronchial responsiveness and atopy. Am Rev Respir Dis. 1985;131:573–8. doi: 10.1164/arrd.1985.131.4.573. [DOI] [PubMed] [Google Scholar]
- 4.Sly PD, Hibbert ME. Childhood asthma following hospitalization with acute viral bronchiolitis in infancy. Pediatr Pulmonol. 1989;7:153–8. doi: 10.1002/ppul.1950070307. [DOI] [PubMed] [Google Scholar]
- 5.Welliver RC, Sun M, Rinaldo D, Ogra PL. Predictive value of respiratory syncytial virus-specific IgE responses for recurrent wheezing following bronchiolitis. J Pediatr. 1986;109:776–80. doi: 10.1016/s0022-3476(86)80692-8. [DOI] [PubMed] [Google Scholar]
- 6.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–7. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 7.Frick OL. Effect of respiratory and other virus infection on IgE immunoregulation. J Allergy Clin Immunol. 1986;78:1013–81. doi: 10.1016/0091-6749(86)90295-2. [DOI] [PubMed] [Google Scholar]
- 8.Welliver RC, Wong DT, Sun M, Middleton E, Vaughan RS, Ogra PL. The development of RSV-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841–6. doi: 10.1056/NEJM198110083051501. [DOI] [PubMed] [Google Scholar]
- 9.Welliver RC, Duffy L. The relationship of RSV-specific immunoglobulin E antibody responses in infancy, recurrent wheezing and pulmonary function at age 7–8 years. Pediatr Pulmonol. 1993;15:19–27. doi: 10.1002/ppul.1950150104. [DOI] [PubMed] [Google Scholar]
- 10.Román M, Calhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like response. Am J Respir Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 11.Aberle JH, Aberle SW, Dworzak MN, et al. Reduced interferon-γ expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160:1263–8. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 12.Bont L, Heijnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–9. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 13.Dunn P, North RJ. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharton TM, Scott P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–77. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon γ, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–5. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christmas SE, Meager A. Production of interferon-gamma and tumour necrosis factor-alpha by human T-cell clones expressing different forms of the γδ receptor. Immunology. 1990;71:486–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Bendelja K, Gagro A, Bace A, et al. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol. 2000;121:332–8. doi: 10.1046/j.1365-2249.2000.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussell T, Openshaw PJ. Intracellular IFN-γ expression in natural killer cells precedes lung CD8 T cell recruitment during respiratory syncytial virus infection. J Gen Virol. 1998;79:2593–601. doi: 10.1099/0022-1317-79-11-2593. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Kojiro N, Ikeda T, Ito T, Funada J, Kokubu T. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberuculosis. Chest. 1992;102:195–8. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 20.Saunders BM, Frank AA, Cooper AM, Orme IM. Role of γδ T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect Immun. 1998;66:5508–14. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Déchanet J, Merville P, Lim A, et al. Implication of γδ T cells in the human immune response to cytomegalovirus. J Clin Invest. 1999;103:1437–49. doi: 10.1172/JCI5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Paoli P, Gennari D, Martelli P, Cavarzerani V, Comoretto R, Santini G. γδ T cell receptor-bearing lymphocytes during Epstein–Barr virus infection. J Infect Dis. 1990;161:1013–6. doi: 10.1093/infdis/161.5.1013. [DOI] [PubMed] [Google Scholar]
- 23.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373:255–7. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh B, Schrenzel MD, Mulvania T, Lepper H, DiMolfetto-Landon L, Ferrick DA. In vivo cytokine production in murine listeriosis. Evidence for immunoregulation by γδ T cells. J Immunol. 1996;156:232–7. [PubMed] [Google Scholar]
- 25.Zuany-Amorim C, Ruffié C, Hailé S, Vargaftig BB, Pereira P, Pretolani M. Requirement for γδ T cells in allergic airway inflammation. Science. 1998;280:1265–7. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 26.Hall CB, Douglas RG, Jr, Gelman RL. Interferon production in children with respiratory syncytial, influenza, and parainfluenza virus infections. J Pediatr. 1978;93:28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- 27.Hayday AC, Roberts S, Ramsburg E. γδ cells and regulation of mucosal immune responses. Am J Respir Crit Care Med. 2000;162:S161–3. doi: 10.1164/ajrccm.162.supplement_3.15tac4. [DOI] [PubMed] [Google Scholar]
- 28.McMenamin C, McKersey M, Khnlein P, Hnig T, Holt PG. γδ T cells down-regulate IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390–4. [PubMed] [Google Scholar]
- 29.Waters WR, Harp JA. Cryptosporidium parvum infection in T-cell receptor (TCR)-alpha- and TCR-delta-deficient mice. Infect Immun. 1996;64:1854–7. doi: 10.1128/iai.64.5.1854-1857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell DE, Fryga AS, Bol, Kemp A. Intracellular interferon-gamma production in normal children and children with atopic dermatitis. Clin Exp Immunol. 1999;115:377–82. doi: 10.1046/j.1365-2249.1999.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorkness RL, Castleman WL, Kumar A, Kaplan MR, Lemanske RF., Jr Prevention of chronic postbronchioitis airway sequelae with IFN-gamma treatmt in rats. Am J Respir Crit Care Med. 1999;160:705–10. doi: 10.1164/ajrccm.160.2.9810002. [DOI] [PubMed] [Google Scholar]
- 32.Mikus LD, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Reduced interferon-gamma secretion by natural killer cells from rats susceptible to postviral chronic airway dysfunction. Am J Respir Cell Mol Biol. 2001;24(1):74–82. doi: 10.1165/ajrcmb.24.1.4125. [DOI] [PubMed] [Google Scholar]
- 33.Renzi PM, Turgeon JP, Marcotte JE, et al. Reduced interferon-γ production in infants with bronchiolitis and asthma. Am J Respir Crit Care Med. 1999;159:1417–22. doi: 10.1164/ajrccm.159.5.9805080. [DOI] [PubMed] [Google Scholar]


