Abstract
In this study, we investigated the cytokine profiles of 14 treatment-naive HIV-infected patients on the initiation of highly active antiretroviral therapy (HAART). At baseline, plasma levels of TNF-α and its mRNA in peripheral blood mononuclear cells (PBMC) were highest in the most severely immunocompromised patients (<200 CD4+ cells/mm3). After 12 months of HAART, the virus was undetectable in the plasma of all patients (<200 copies/ml), and median CD4 T cell counts had increased (+164 cells/mm3). We also observed a gradual decrease in the number of proviral DNA copies in PBMC and in immune activation, with lower levels of IFN-γ mRNA in PBMC associated with weaker activation of CD8+ T cells and lower levels of plasma TNF-α. IL-2 mRNA levels in PBMC were found to increase in parallel. The decrease in TNF-α and IFN-γ levels and the increase in IL-2 production appear to be correlated with the efficacy of HAART in naive immunocompromised HIV-infected individuals.
Keywords: cytokines, HAART, HIV, immune restoration, virological response
Introduction
The availability of highly active antiretroviral therapy (HAART) has improved the treatment and outcomes of HIV-1 infection considerably, with a marked reduction in patient mortality and morbidity [1,2]. The effects of HAART on immunological and virological parameters consist of an increase in CD4+ T cell count and a decrease in plasma viral load in the peripheral blood within a few months of treatment initiation [3]. These changes in plasma HIV-1 load and CD4+ T lymphocyte counts, taken together, are valid predictors of disease progression [4,5]. It is not possible to achieve complete viral eradication, as illustrated by the continuing presence of latently infected CD4+ T cells with replication-competent provirus [6,7] and HIV replication, albeit at low levels, even in patients responding well to HAART in whom plasma HIV RNA is persistently undetectable (<50 copies/ml) [8].
In the absence of HAART, the decline in CD4+ T cell count is associated with a dramatic loss of T cell-mediated immune responses and, particularly, of helper T (Th) cell responses. The Th1/Th2 balance, defined by the secretion of interleukin(IL)-2, interferon-γ (IFN-γ) and IL-12 (Th1 response) or IL-4, IL-5 and IL-10 (Th2 response), has been explored intensively in HIV-infected patients. A decrease in the expression and secretion of type 1 cytokines and an increase in the expression and secretion of type 2 cytokines are associated with disease progression [9–12]. In contrast, dominant type 1 cytokine profiles are associated with a lack of disease progression [13,14]. Furthermore, an increase in IL-2 production [15,16] and a decrease in IL-4 production [17] in CD4+ T cells, accompanied by a significant decrease in the number of IFN-γ-producing CD8+ T cells, have been observed in patients undergoing HAART [16,18]. HAART also affects the dysregulated immune system by modifying the inflammatory cytokine profile, as illustrated by the correlations between the decrease in viral load, the increase in the number of CD4+ T cells and the decrease in concentration of components of the TNF system during HAART, and between TNF-α, HIV replication and persistent TNF-α activation in subjects in whom HAART is considered to have failed virologically or immunologically [19,20].
Whereas the majority of the previous works assessing the effects of HAART on the immune system has focused on cross-sectional studies, we therefore investigated changes in cytokine profiles, cell markers and plasma and cellular HIV viral loads longitudinally in patients prior to and after initiating HAART.
Patients And Methods
Patients
Fourteen HAART-naive HIV-1-infected patients (13 men, one woman) were included in this study. The clinical, immunological and virological characteristics of these patients are shown in Table 1. Exclusion criteria were acute HIV-1 infection and accidental HIV contamination. The median age at inclusion was 36 years (range: 22–57). The clinical stages of the patients were as follows: Centers for Disease Control and Prevention (CDC − Atlanta, 1993 definition) stage A (10 patients), B (one patient) and C (three patients). Median CD4+ T cell count was 292 mm3 (5–908; six patients < 200 CD4+ T lymphocytes and eight patients> 200 CD4+ T lymphocytes) and median plasma HIV-1 RNA level was 80 700 copies/ml (3300–12 568 000). For all patients, HAART was initiated on inclusion in the study: 12 patients were treated with a combination of two nucleoside reverse transcriptase inhibitors (NRTI) and one protease inhibitor (PI), one was treated with a combination of one NRTI and one non-nucleoside reverse transcriptase inhibitor (NNRTI), and one patient was treated with two NRTI and one IP combined with hydroxyurea. This study was approved by the local ethics committee, and all patients gave written informed consent before inclusion. Clinical examinations were carried out and blood samples taken for haematological and biochemical parameter determination and for virological and immunological investigations at baseline and 1, 4, 8 and 12 months after HAART initiation.
Table 1.
Clinical, immunological, virological and pharmacological characteristics of 14 HIV-infected patients included in this study
| Cd4 T cell count (cells/mm3) | Plasma HIV-1 RNA (copies/ml) | Proviral HIV DNA (copies/106 CD4 T cells) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age (years) | Antecedents | Year of seroconversion | Clinical stage (CDC, 1993) | Opportunistic infections (before treatment) | M0 | M12 | M0 | M12 | M0 | M12 | Treatment |
| 1 | M | 51 | zona bacterial pulmonary infection | 1997 | C3 | Pneumocystosiscarinii pneumonia | 77 | 187 | 400 120 | <200 | 5 614 | 3 191 | AZT, 3TC, RTV |
| 2 | M | 40 | – | ? | A3 | – | 70 | 465 | 153 800 | <200 | 591 527 | 7 919 | ddI, d4T, IDV |
| 3 | M | 36 | – | 1992 | C3 | disseminated cryptococcosis pulmonary tuberculosis cerebral toxoplasmosis | 5 | 146 | 12 568 000 | <200 | 2 722 713 | 1 181 253 | ddI, d4T, NFV |
| 4 | F | 35 | – | 1998 | A2 | – | 364 | 758 | 82 520 | <200 | 35 247 | 4 727 | ddI, d4T, NFV |
| 5 | M | 53 | labial herpes zona | 1986 | A2 | – | 466 | 446 | 3 300 | <200 | 1 839 | 440 | AZT, 3TC, NFV |
| 6 | M | 36 | chronic active hepatitis B | 1991 | A3 | – | 59 | 166 | 56 120 | <200 | 24 221 | 3 666 | AZT, 3TC, NFV |
| 7 | M | 36 | – | 1998 | C3 | cryptococcal meningitis | 37 | 249 | 574 200 | <200 | 7 077 | 6 872 | AZT, 3TC, NFV |
| 8 | M | 31 | – | 1998 | A1 | – | 835 | 655 | 189 000 | <200 | 25 742 | 5 773 | ddI, d4T, IDV, HU |
| 9 | M | 22 | – | 1998 | A2 | – | 368 | 350 | 78 880 | <200 | 3 503 | 1 239 | AZT, 3TC, IDV |
| 10 | M | 57 | myocardial infarct, zona cured hepatititis B | 1992 | B1 | – | 908 | 1000 | 37 880 | <200 | 24 267 | 4 814 | AZT, 3TC, IDV |
| 11 | M | 34 | hepatitis C cured hepatitis B | 1998 | A3 | – | 105 | 307 | 66 240 | <200 | 25 735 | 575 | d4T, 3TC, NFV |
| 12 | M | 26 | – | 1998 | A2 | – | 321 | 656 | 172 400 | <200 | 3 236 816 | 22 548 | d4T, 3TC, NFV |
| 13 | M | 40 | chronic active hepatitis B syphilis | ? | A2 | – | 262 | 546 | 19 600 | <200 | 711 | 91 | d4T, 3TC, IDV |
| 14 | M | 50 | depression | 1988 | A1 | – | 557 | 597 | 14 760 | <200 | 1 948 | 5 | d4T, 3TC, EFV |
AZT, zidovudine; 3TC, lamivudine; RTV, ritonavir; ddI, didanosine; d4T, stavudine; IDV, indinavir; NFV, nelfinavir; HU, hydroxyurea; EFV, efavirenz.
Extraction of plasma and isolation of PBMC
Blood samples were processed within 3 h of collection. Plasma was collected by centrifugation at 1700 g for 10 min, dispensed into 1 ml–aliquots, frozen and stored at − 80°C. Peripheral blood mononuclear cells (PBMC) were then isolated by Ficoll density gradient centrifugation and counted by trypan blue exclusion assay.
Immunological parameters
Phenotypic analysis of lymphocyte subsets
Cell-surface markers expressed on lymphocytes were analysed by flow cytometry, using three-colour direct immunofluorescence and a FACScan flow cytometer (Becton Dickinson–Pharmingen, Mountain View, CA, USA). Anti-CD3-FITC, anti-CD4-PerCP, anti-CD8-PerCP and anti-CD14-FITC MoAbs were obtained from Becton Dickinson–Pharmingen and the MoAbs anti-CD28-PE and anti-HLA-DR-PE were obtained from Immunotech (Marseille, France).
Levels of plasma cytokines
Cytokines were determined in plasma, using specific ELISA kits (TNF-α and sTNF-RII: Biosource, Nivelles, Belgium; IL-1β and IL-6: Cayman Chemical, Ann Arbor, USA; IL-2, IL-4, IL-10, IL-12p40: Quantikine™ ELISA, R&D Systems, Minneapolis, USA), according to the manufacturer's recommendations.
Levels of cytokine mRNA in PBMC
The expression levels of mRNA for the endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and for TNF-α, IL-1β, IL-6, IL-2, IFN-γ, IL-12p40, IL-4 and IL-10, were quantified by real-time quantitative RT-PCR (TaqMan, PE Applied Biosystems, Courtaboeuf, France), as described previously for the quantification of cytokine mRNA [21,22]. Briefly, mRNA were extracted by a method derived from that of Chomczynski [23], and reverse-transcribed as described previously [24,25]. For each sample, amplified cytokine signals were normalized with amplified GAPDH signals. Calculations were carried out according to the recommendations of PE Applied Biosystems and results are expressed as arbitrary units. The reproducibility of the method was checked and each sample was tested in duplicate.
Virological parameters
Plasma viral load
Plasma HIV RNA loads were determined with the RT Assay kit (Roche Products), with a detection threshold of 200 copies/ml.
Cellular viral load in PBMC
Total DNA was extracted from 3 million PBMC with the MasterPure DNA Purification kit (Epicentre Technologies, Madison, WI, USA). Proviral DNA loads were quantified in PBMC by PCR, using primers from the HIV gag gene (SK38: ATAATCCACCTATCCCAGTAGGAG AAAT − SK39: TTTGGTCCTTGTCTTATGTCCAGAATGC; 115 base pair sequence) [26] and from the β-globin gene (sense primer: CAACTTCATCCACGTACTCC − antisense primer: GAAGAGCCAAGGACAGGTAC; 380 base pair sequence), a housekeeping gene. In parallel, we plotted standard curves systematically, using chronically infected 8E5 cells.
The PCR mixture contained 10 µm of each dNTP, 100 ng of each specific primer, 15 mm or 25 mm MgCl2, respectively, for the β-globin and gag genes, and 1·25 U of Taq polymerase (Appligene, Pleasanton, USA) in a final volume of 50 µl. PCR amplification was performed in a Crocodile II thermocycler (Appligene), with 30 cycles of denaturation at 94°C for 30 s, primer annealing at 58°C for 30 s and extension at 72°C for 30 s for the β-globin gene and with 40 cycles of denaturation at 94°C for 45 s, primer annealing at 57°C for 1 min and extension at 72°C for 1 min for the gag gene. After amplification, 8 µl of the PCR products were resolved in agarose gels, stained with ethidium bromide, visualized under ultraviolet light and analysed by densitometric scanning (NIH Image 1·52, Bethesda, MD, USA). For each sample, amplified gag gene signals were normalized using amplified β-globin gene signals, and the results were expressed in cellular viral HIV DNA copies per million CD4+ T cells.
Statistical analysis
If significant results were obtained in a non-parametric Friedman test, further statistical analysis was carried out, using the non-parametric Wilcoxon test. Patients were classified into two groups, corresponding to those with <200 CD4+ T cells/mm3 (n = 6) and those with>200 CD4+ T cells/mm3 (n = 8). The results (P) of multiple tests were corrected by Bonferroni test as follows: P × 2n (n corresponding to the number of tests). Differences were considered as significant if P < 0·05. Results are given as medians and 10th−90th percentiles (unless stated otherwise), open circles representing values above and below the 10th and 90th percentiles. Mean baseline values and correlations were assessed by using the nonparametric Mann–Whitney and Kendall tests, respectively. Statistical analysis was performed with StatView 4·51·1 software (Abacus Concepts Incorporation, Berkeley, CA, USA).
Results
Clinical events and adverse effects
No major clinical event was observed except for one case of CMV retinitis (patient 3 at month 12). Nevertheless, 11 mild adverse events were observed in eight patients, consisting mainly of PI-related hypertriglyceridaemia or hypercholesterolaemia.
T cell immunophenotype
Significant median increases in CD4+ T cell count and percentages were observed 12 months after initiating HAART [+ 164 cells/mm3 (range − 180 –+ 395) and + 8% (-10 –+ 20), respectively; P < 0·05]. Subgroup analysis according to CD4+ T cell count showed that this increase remained significant only in patients with less than 200 CD4+ T cells/mm3 (P < 0·05). No significant decrease in CD8+ T cell counts was observed (P = 0·592). Nevertheless, the median CD4/CD8 T cell ratio had increased significantly after 12 months of HAART: 0·30 at baseline versus 0·56 at month 12 (P < 0·01). The percentages of CD3+ CD8+ and of CD3+ CD8+ HLA-DR+ cells declined significantly after 12 months of HAART (P < 0·05 and P < 0·01, respectively), and the percentage of CD3+ CD8+ CD28+ cells increased, but this trend was not statistically significant (P = 0·06).
Plasma viral load
During HAART, the number of patients with undetectable levels of HIV RNA copies gradually increased. Seven of the 14 patients after 1 month of HAART, 12 after 4 months, and all patients after 12 months of HAART had achieved and maintained the plasma viraemia nadir (<200 copies/ml; Table 1). The decrease in median plasma viral load was statistically significant (P < 0·01) as early as the first month after HAART initiation. Moreover, as expected, a negative correlation was found at baseline between plasma viral load and CD4+ T cell count (P < 0·05).
Cellular viral load in PBMC
After 12 months of HAART, the number of proviral DNA copies had significantly decreased in all patients (P < 0·01). The median number of copies of proviral DNA was 24 244 copies/million CD4+ T cells at baseline (range 711–3 236 816), and 4727 copies/million CD4+ T cells at month 12 (range 5–1 181 253; Table 1). The median decrease in proviral DNA load was 0·71 log10 copies/million CD4+ T cells (range 0·01–2·62), with a median half-life of 2·3 ± 1·1 years. The decrease in the median copy number of proviral DNA was detected at all times after HAART initiation. However, this decrease became significant only after 8 or 12 months of HAART (P < 0·05 and P < 0·01, respectively). Subgroup analysis showed that this decrease remained significant at month 12 only in those patients who had less than 200 CD4+ T cells/mm3 (P < 0·05; data not shown). No correlation was found either between cellular and plasma viral loads or between CD4+ T cell count and cellular viral load.
Levels of plasma cytokines
In the absence of blood stimulation, IL-2, IL-4, IL-10 and IL-12p40 were not detected in any of the plasma samples obtained from the 14 patients. IFN-γ was detected in the plasma of only five of the 14 patients at baseline, and in two others at month 12 (data not shown). In contrast, proinflammatory cytokines (TNF-α, IL-1β and IL-6) and the soluble TNF-α type II receptor (sTNF-RII) were detected consistently in the plasma of all patients (Fig. 1). At baseline, plasma levels of these proinflammatory factors were highest in the most severely immunocompromised patients (<200 CD4+ T cells/mm3; Fig. 1). The decrease in median plasma TNF-α levels was statistically significant at months 8 and 12 (P < 0·05 and P < 0·01, respectively, Fig. 2). In subgroup analysis, this decrease in plasma TNF-α levels remained statistically significant only in patients with less than 200 CD4+ T cells/mm3 (month 12 versus baseline, P < 0·05; data not shown). Moreover, plasma levels of TNF-α were inversely correlated with CD4+ T cell counts 12 months after HAART initiation (P < 0·01). Although a correlation was found between TNF-α and sTNF-RII levels at baseline (P < 0·05), we found no evidence for a decrease in plasma sTNF-RII levels in response to HAART (Fig. 2). Similarly, no significant change in plasma IL-1β and IL-6 levels was observed during the 12 months of HAART (Fig. 2).
Fig. 1.
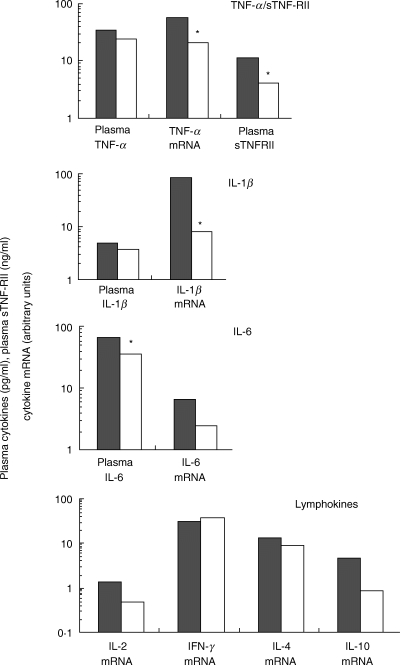
Median levels of cytokine mRNA and cytokines at baseline in HIV-infected patients with < 200 CD4+ T lymphocytes and> 200 CD4+ T lymphocytes. Results are expressed in arbitrary units and pg/ml, respectively (*P < 0·05).  , < 200 LT CD4+/mm3 (n = 6); □,> 200 LT/CD4+/mm3 (n = 8).
, < 200 LT CD4+/mm3 (n = 6); □,> 200 LT/CD4+/mm3 (n = 8).
Fig. 2.
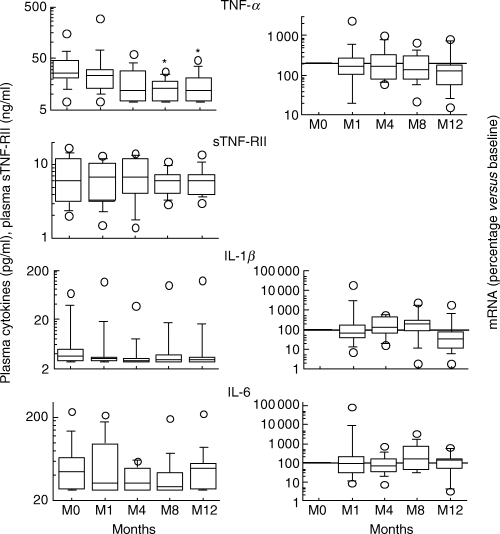
Expression of proinflammatory cytokines (mRNA in PBMC and protein in plasma) during the first 12 months following HAART initiation. Results are expressed as a percentage of baseline values and pg/ml, respectively (*P < 0·05).
Cytokine mRNA levels in PBMC
Cytokine and endogenous control (GAPDH) mRNAs were detected in all samples, except for IL-12p40. At baseline, mRNA levels were significantly higher for TNF-α and IL-1β than for the other cytokines (P < 0·05). As expected, a correlation was observed at baseline between the levels of mRNA for TNF-α and IL-1β (P < 0·05), TNF-α and IL-6 (P < 0·05), and IL-1β and IL-6 (P < 0·01). Furthermore, a correlation was also found at baseline between IFN-γ mRNA levels and plasma viral load (P < 0·05).
During HAART, levels of TNF-α and IL-1β mRNA decreased, but this decrease was not significant (Fig. 2). In parallel, IL-2 mRNA levels increased significantly during HAART (Fig. 3;P < 0·05 at months 8 and 12) whereas IFN-γ mRNA levels decreased significantly (Fig. 3;P < 0·05 at month 8).
Fig. 3.
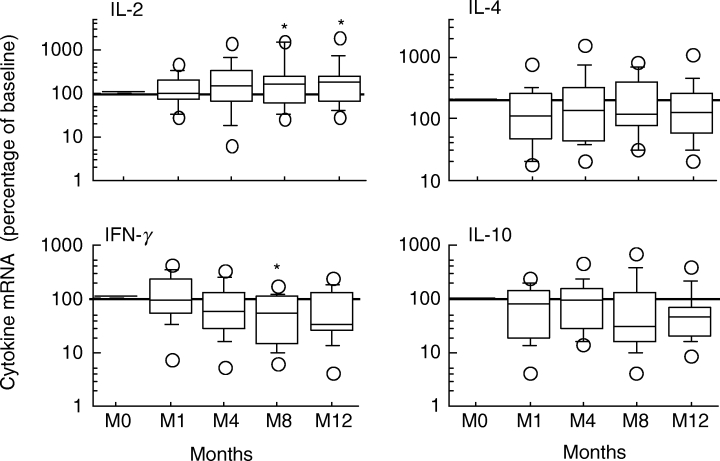
Expression of Th1 and Th2 cytokines during the first 12 months following HAART initiation. Results are expressed as a percentage of baseline values (*P < 0·05).
Discussion
We analysed prospectively the virological and immune status of treatment-naive HIV-infected individuals initiating HAART. In this study, the plasma concentrations of cytokines and their mRNA levels in PBMC were determined in parallel in unstimulated ex vivo conditions. This experimental procedure has been validated previously in SIV-infected macaques [24]. This study shows that informative data can be obtained from the plasma for proinflammatory cytokines (TNF-α, sTNF-RII, IL-6 or IL-1β) but not for Th cytokines (IL-2, IL-4, IL-10, IL-12p40 and IFN-γ), as Th cytokines remained undetectable. In contrast, it was possible to quantify mRNA levels in PBMC for all the cytokines studied by real-time PCR.
The prognostic value of plasma viral load, associated with CD4+ T cell count, at any time in HIV infection has been demonstrated conclusively [5,27]. As expected, HAART interfered with HIV replication, inducing a strong decrease in HIV plasma viraemia and an increase in the number of CD4 cells in the 14 patients studied. This increase depended on CD4 T cell counts at the time at which therapy was initiated. Although HAART decreased HIV replication in the bloodstream, together with AIDS-related mortality and morbidity, this treatment alone is unlikely to eradicate latent reservoirs completely. Some authors have demonstrated the presence of latently infected CD4 cells in HIV-infected patients in whom the virus was undetectable in the plasma and, due to the long half-life of these cells, the estimated time required to achieve eradication with HAART alone is 12–60 years [28,29]. We observed a gradual decrease in the number of copies of proviral DNA in all patients, in response to HAART. This decrease was statistically significant 8 months after HAART initiation. This slow decay rate for cell populations constituting latent reservoirs was confirmed by the absence (i) of a significant decrease in the number of copies of proviral DNA in the least immunocompromised patients (>200 CD4+ T cells/mm3), and (ii) of any correlation between plasma HIV RNA and proviral DNA levels and between CD4+ T cell count and proviral DNA levels.
The initiation of HAART increased CD4+ T cell count and decreased the percentage of circulating CD8+ T cells and their level of activation, consistent with previous observations [3,16]. The decrease in viral loads eliminated the antigenic stimuli responsible for inducing the CD8+ T cell response [30,31]. These data are consistent with the cellular dynamics underlying CD4+ and CD8+ T cell counts after several months of HAART (i.e. the combination of a redistribution of CD4+ and CD8+ T cells from lymphoid tissues and a homeostatic response of the host to HIV-related CD4 cell death) [32–34].
Before HAART, only the levels of mRNA for TNF-α and IL-1β and plasma sTNF-RII and IL-6 concentrations were significantly higher in the most severely immunocompromised patients (<200 CD4+ T cells/mm3) than in the other patients. No significant difference in types 1 or 2 cytokine profiles was observed between the two groups of patients (< and> 200 CD4+ T cells/mm3). After HAART initiation plasma TNF-α levels decreased, whereas no significant change in plasma sTNF-RII levels was observed. TNF-α and sTNF-RII levels were correlated before HAART initiation. These data are consistent with those previously published by Aukrust et al. [20]. These authors reported that the decline in TNF-α levels may be involved with adequate virological and immunological responses in HAART-treated patients even if plasma TNF-α concentrations are not normalized − indicating that full immunological normalization is not achieved. Moreover, levels of sTNF-RII decreased transiently and were not significant at the end of a long-term HAART treatment. Thus, TNF-α could be considered to be the most appropriate marker in TNF components. A significant increase in mRNA levels for IL-2, a type 1 cytokine, was observed 8 months after HAART initiation. As demonstrated previously, the improvement of CD4+ T cell functions after a few months of HAART is associated with a restoration of the capacity of these cells to produce IL-2 following, or in the absence of, mitogenic stimulation [15,16]. In parallel, IFN-γ mRNA levels decrease significantly in patients receiving HAART. The results of a number of studies are consistent with those reported here, showing high levels of IFN-γ mRNA in PBMC before HAART and a decrease in these levels during HAART [16,35–37]. The decrease in IFN-γ mRNA levels may reflect the efficacy of HAART, which reduces stimulation by HIV antigens and subsequent CD8+ T cell activation, as shown by the decrease in the number of CD3+/CD8+/HLA-DR+ cells, and evidence presented elsewhere [30,31].
In conclusion, quantification of mRNA levels and plasma concentrations of proinflammatory cytokines, and of types 1 and 2 cytokine mRNA levels, may be useful in clinical trials for assessment of the response of the immune system to therapeutic intervention in HIV-infected patients. In this study, decreases in TNF-α and IFN-γ levels and an increase in IL-2 production were found to be associated with the efficacy of HAART in immunocompromised HIV-infected individuals in the first year after HAART initiation.
Acknowledgments
We would like to thank Marie-Christine Burland for excellent technical assistance and Sandra Roloff for monitoring the collection of patient blood samples. This work was supported by the Agence Nationale de Recherches sur le SIDA (ANRS, Paris, France), the Commissariat à l’Energie Atomique (CEA, Paris, France), the Association des Professeurs de Pathologies Infectieuses et Tropicales (APPIT, Paris, France).
References
- 1.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. HIV Outpatient Study Investigators. [DOI] [PubMed] [Google Scholar]
- 2.Sepkowitz KA. Effect of HAART on natural history of AIDS-related opportunistic disorders. Lancet. 1998;351:228–30. doi: 10.1016/S0140-6736(05)78279-9. [DOI] [PubMed] [Google Scholar]
- 3.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 4.Mellors JW, Kingsley LA, Rinaldo C, et al. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–9. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien WA, Hartigan PM, Martin D, et al. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. N Engl J Med. 1996;334:426–31. doi: 10.1056/NEJM199602153340703. Veterans Affairs Cooperative Study Group on AIDS. [DOI] [PubMed] [Google Scholar]
- 6.Chun TW, Carruth L, Finzi D, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4 (+) T cells during primary HIV-1 infection. Proc Natl Acad Sci USA. 1998;95:8869–73. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340:1605–13. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Hakim FT, Venzon DJ, et al. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:759–65. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Than S, Hu R, Oyaizu N, et al. Cytokine pattern in relation to disease progression in human immunodeficiency virus-infected children. J Infect Dis. 1997;175:47–56. doi: 10.1093/infdis/175.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, Michelet C, Peguillet I, et al. Persistent alterations in T-cell repertoire, cytokine and chemokine receptor gene expression after 1 year of highly active antiretroviral therapy. AIDS. 1999;13:185–94. doi: 10.1097/00002030-199902040-00006. [DOI] [PubMed] [Google Scholar]
- 12.Altfeld M, Addo MM, Kreuzer KA, et al. T(H)1 to T(H)2 shift of cytokines in peripheral blood of HIV-infected patients is detectable by reverse transcriptase polymerase chain reaction but not by enzyme-linked immunosorbent assay under nonstimulated conditions. J Acquir Immune Defic Syndr. 2000;23:287–94. doi: 10.1097/00126334-200004010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Barker E, Mackewicz CE, Levy JA. Effects of TH1 and TH2 cytokines on CD8+ cell response against human immunodeficiency virus: implications for long-term survival. Proc Natl Acad Sci USA. 1995;92:11135–9. doi: 10.1073/pnas.92.24.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clerici M, Balotta C, Salvaggio A, et al. Human immunodeficiency virus (HIV) phenotype and interleukin-2/interleukin-10 ratio are associated markers of protection and progression in HIV infection. Blood. 1996;88:574–9. [PubMed] [Google Scholar]
- 15.Weiss L, Ancuta P, Girard PM, et al. Restoration of normal interleukin-2 production by CD4+ T cells of human immunodeficiency virus-infected patients after 9 months of highly active antiretroviral therapy. J Infect Dis. 1999;180:1057–63. doi: 10.1086/315025. [DOI] [PubMed] [Google Scholar]
- 16.Giovannetti A, Pierdominici M, Mazzetta F, et al. T cell responses to highly active antiretroviral therapy defined by chemokine receptors expression, cytokine production, T cell receptor repertoire and anti-HIV T-lymphocyte activity. Clin Exp Immunol. 2001;124:21–31. doi: 10.1046/j.1365-2249.2001.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew E, Gallagher L, Kuehnert M, et al. Intracellular cytokines in the acute response to highly active antiretroviral therapy. Aids. 2001;15:1665–70. doi: 10.1097/00002030-200109070-00009. [DOI] [PubMed] [Google Scholar]
- 18.Levacher M, Bouscarat F, Landman R, et al. Frequency of cytokine-producing T cells in HIV-infected patients treated with stavudine, didanosine, and ritonavir. AIDS Res Hum Retroviruses. 2000;16:1869–75. doi: 10.1089/08892220050195829. [DOI] [PubMed] [Google Scholar]
- 19.Hittinger G, Poggi C, Delbeke E, et al. Correlation between plasma levels of cytokines and HIV-1 RNA copy number in HIV-infected patients. Infection. 1998;26:100–3. doi: 10.1007/BF02767768. [DOI] [PubMed] [Google Scholar]
- 20.Aukrust P, Muller F, Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179:74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 21.Hartel C, Bein G, Kirchner H, et al. A human whole-blood assay for analysis of T-cell function by quantification of cytokine mRNA. Scand J Immunol. 1999;49:649–54. doi: 10.1046/j.1365-3083.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Wang X. Application of real-time polymerase chain reaction for the quantitation of interleukin-1beta mRNA upregulation in brain ischemic tolerance. Brain Res Brain Res Protoc. 2000;5:211–7. doi: 10.1016/s1385-299x(00)00015-5. [DOI] [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Benveniste O, Vaslin B, Le Grand R, et al. Comparative IL-2/IFNgamma and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SIVmac251. Proc Natl Acad Sci USA. 1996;93:3658–63. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benveniste O, Vaslin B, Le Grand R, et al. Interleukin 1 beta, interleukin 6, tumor necrosis factor alpha, and interleukin 10 responses in peripheral blood mononuclear cells of cynomolgus macaques during acute infection with SIVmac251. AIDS Res Hum Retroviruses. 1996;12:241–50. doi: 10.1089/aid.1996.12.241. [DOI] [PubMed] [Google Scholar]
- 26.Ou CY, Kwok S, Mitchell SW, et al. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988;239:295–7. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- 27.Hubert JB, Burgard M, Dussaix E, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. Aids. 2000;14:123–31. doi: 10.1097/00002030-200001280-00007. The SEROCO Study Group. [DOI] [PubMed] [Google Scholar]
- 28.Finzi D, Siliciano RF. Viral dynamics in HIV-1 infection. Cell. 1998;93:665–71. doi: 10.1016/s0092-8674(00)81427-0. [DOI] [PubMed] [Google Scholar]
- 29.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 30.Schooley RT. Longer-term immunologic effects and side effects of successful antiretroviral therapy. Clin Infect Dis. 1999;29:12–8. doi: 10.1086/520139. [DOI] [PubMed] [Google Scholar]
- 31.Ogg GS, Jin X, Bonhoeffer S, et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 33.Cohen Stuart JW, Hazebergh MD, Hamann D, et al. The dominant source of CD4+ and CD8+ T-cell activation in HIV infection is antigenic stimulation. J Acquir Immune Defic Syndr. 2000;25:203–11. doi: 10.1097/00126334-200011010-00001. [DOI] [PubMed] [Google Scholar]
- 34.Hazenberg MD, Stuart JW, Otto SA, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART) Blood. 2000;95:249–55. [PubMed] [Google Scholar]
- 35.Fan J, Bass HZ, Fahey JL. Elevated IFN-gamma and decreased IL-2 gene expression are associated with HIV infection. J Immunol. 1993;151:5031–40. [PubMed] [Google Scholar]
- 36.Breen EC, Salazar-Gonzalez JF, Shen LP, et al. Circulating CD8 T cells show increased interferon-gamma mRNA expression in HIV infection. Cell Immunol. 1997;178:91–8. doi: 10.1006/cimm.1997.1115. [DOI] [PubMed] [Google Scholar]
- 37.Westby M, Marriott JB, Guckian M, et al. Abnormal intracellular IL-2 and interferon-gamma (IFN-gamma) production as HIV-1-associated markers of immune dysfunction. Clin Exp Immunol. 1998;111:257–63. doi: 10.1046/j.1365-2249.1998.00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


