Abstract
Allergic asthma, a chronic inflammatory disease of the airways, is characterized by the presence of T helper 2 cells and eosinophils in sputum, bronchoalveolar lavage, and mucosal biopsy specimens. Although the T helper 1-promoting cytokine, interleukin-12, is capable of inhibiting the T helper 2-driven asthma symptoms and bronchial responsiveness, the specific mechanisms underlying these interleukin-12 actions are unclear. The anti-allergic response to interleukin-12 is only partially dependent on interferon-γ, which induces apoptosis by enhancing expression of Fas antigen. We therefore investigated in vivo whether the anti-allergic action of interleukin-12 is mediated through induction of apoptosis. C57BL/6 mice immunized to ovalbumin by intraperitoneal injection were challenged three times with an ovalbumin aerosol every second day for 7 days. Recombinant interleukin-12 was administered intravenously after the final challenge. After the last ovalbumin challenge, mice were examined for effects of interleukin-12 on inflammatory cell infiltration and apoptosis in the lung as detected by terminal deoxynucleotidyl transferase-mediated deoxyribonucleoside triphosphate nick end-labelling.
Administration of interleukin-12 reduced ovalbumin-induced pulmonary eosinophilia (P < 0·01) and CD4+ T cell infiltration (P < 0·01). Moreover, treatment with interleukin-12 shortly after ovalbumin inhalation resulted in both increased interferon-γ production (P < 0·01) and enhanced apoptosis of CD4+ T cells in allergic airway infiltrates (P < 0·05). These results suggest that the beneficial effects of interleukin-12 in asthma may include enhancement of apoptosis of CD4+ T cells in airways.
Keywords: interleukin-12, asthma, CD4 T cell, apoptosis
Introduction
Persistent inflammation of the bronchial mucosa, characterized by eosinophilic infiltration but also involving other cell types including neutrophils, mast cells, basophils, and lymphocytes, is considered important in the pathogenesis of asthma [1,2]. T helper (Th) 2 inflammatory responses have been reported to predominate over Th1 responses in human asthma, where increased interleukin (IL)-4 and IL-5 mRNA expression and protein secretion in bronchial biopsy specimens are related to clinical measures of disease severity [3,4]. Interest is now focused on Th1-type cells, which produce large amounts of interferon (IFN)-γ, as regulators of allergic inflammation that can decrease Th2 immune responses in the airways, protecting against symptoms of allergic asthma [5,6].
The functionally active form of IL-12, a heterodimer (p70) composed of two disulphide-linked chains of 35 (p35) and 40 (p40) kD, is secreted predominantly by antigen-presenting cells (APCs) in response to T cell engagement of the major histocompatibility complex (MHC) class II and CD40 molecules [7,8]. IL-12 has been shown to have potent activity as an immune-activating agent, is effective in the treatment of tumours and infectious diseases, and as an adjuvant in prophylactic and therapeutic vaccines [9,10]. Central to its efficacy in these models is its potent promotion of IFN-γ production by T lymphocytes and natural killer (NK) cells, enhancement of antigen-specific T cell proliferation, and differentiation of naive T cells to a Th1 phenotype. Studies in asthma models concluded that administration of IL-12 before and during the period of allergen challenge prevented allergen-induced airway eosinophilia, airway hyper-responsiveness, production of Th2 cytokines and allergen-specific serum IgE, but the underlying mechanisms remain unclear [11,12].
Accumulation and activation of immune effector cells in the airways of asthmatics may result from increased cell infiltration and/or prolonged cell survival. The latter represents a failure to induce programmed cell death (apoptosis). Recently, interest has increased in eosinophil apoptosis at inflamed sites; such cell death tends to limit inflammatory tissue injury and promote resolution of inflammation. In a previous study, we reported that eosinophil apoptosis could facilitate resolution of airway inflammation in asthma [13]. This finding suggested that inducing apoptosis in immune cells might be an important goal in suppression of allergic responses.
In the present study, we provide evidence that exogenous IL-12 can have an important effect in inhibition of allergic inflammation, occurring at least partially via induction of apoptosis of CD4+ T cells infiltrating the airways.
Materials And Methods
Animals
Male C57BL/6 mice, free of specific pathogens, were purchased at 6–8 weeks of age from Charles River Laboratories (Atsugi, Japan). Mice were kept in standard animal housing facilities and given free access to tap water and ovalbumin (OVA)-free rodent chow.
Immunization and challenge exposure
Immunization and subsequent challenge exposures in mice were carried out according to the method of Foster et al. [14]. C57BL/6 mice were immunized to OVA (Sigma Chemical, St. Louis, MO, USA) by intraperitoneal injection of 50 µg of OVA adsorbed to 1 mg of alum on days 0 and 12. On day 24, mice were exposed three times to an aerosol of OVA (10 mg/ml) in 0·9% saline for 30 min using an ultrasonic nebulizer, and then every second day for 7 days. Control mice were injected intraperitoneally with 0·5 ml of sterile saline and then exposed to aerosolized sterile saline using similar equipment and schedules. OVA-sensitized and challenged mice were divided into two groups. One hour after the last challenge with OVA, some animals were treated with a single injection via the tail vein with murine recombinant IL-12 (rIL-12; purity> 97%, Genzyme, Cambridge, MA), 500 ng in 0·25 ml of saline, based on the experiments by Hofstra et al. [15]. Control animals were treated with 0·25 ml of saline vehicle. On day 31, 24 h after the last OVA or saline inhalation, airways inflammation was compared between IL-12-treated and saline-treated mice, sensitized and challenged with OVA. Each experimental group consisted of at least eight mice.
Lung histology
After the lungs were excised and immediately frozen using dry ice, 4-µm-thick sections were cut on a cryostat and thaw-mounted onto gelatinized glass slides. Lung sections were fixed in 4% paraformaldehyde in 0·1 m phosphate buffer (pH 7·4), and then subjected to Giemsa staining. For immunohistochemical analyses of CD4+ T cells that had migrated into the lung, sections were fixed with acetone at room temperature for 5 min Sections were treated with 0·3% H2O2 in methanol overnight at room temperature to quench the endogenous peroxidase. Immunostaining was performed overnight at 4°C with rat anti-mouse CD4 antibody (L3T4; Pharmingen, San Diego, CA, USA), diluted 1 : 200 with 1% normal goat serum (NGS) in phosphate-buffered saline (PBS). After incubation with biotinylated secondary antibody (goat anti-rat IgG; Tago, Camarillo, CA, USA) diluted 1 : 500 with 1% NGS in PBS, immunoreactivity was detected by an avidin-biotin peroxidase complex technique (Vectastain ABC kit; Vector, Burlingame, CA, USA) or using the alkaline phosphatase/anti-alkaline phosphatase (APAAP) method. Specificity of each antibody was confirmed by replacement of primary antibodies with non-immunized rat IgG2a,k (clone G155-178). Eosinophils and CD4+ T cells were counted using a light microscope in all medium-sized airways present in each section, and image analysis was performed (Interaktive Build-Analyse System; Kontron-Bildanalyse, Oberkochen, Germany). These counts reflected the number of cells per unit length of bronchial basement membrane in medium-sized airways.
Quantification of cytokines in lung tissue extracts
After sampling of blood, lungs were removed, snap-frozen in liquid nitrogen, and stored at − 80°C. Later, the lungs were homogenized with hypotonic lysis buffer as described previously [16]. Each lung was removed and washed with PBS. Disruption of the organ in lysis buffer was accomplished with 10 strokes in a Wheaton Dounce homogenizer. Homogenates then were centrifuged at 15 000 g for 10 min. Supernatants were stored at − 80°C until testing. Protein concentrations of the extracts were adjusted to 1 mg/ml after measurement by Bradford's method (Protein Assay Kit; Bio-Rad Laboratories, Richmond, VA, USA). ELISAs for IFN-γ, IL-12 (p70), and IL-18 were performed using matching antibody pairs (IFN-γ and IL-12, Amersham, Buckinghamshire, UK; IL-18, Medical and Biological Laboratories, Nagoya, Japan). The secondary antibodies were conjugated to horseradish peroxidase. Subtractive readings at 550 nm and 450 nm were converted to pg/ml using values obtained with recombinant IFN-γ, IL-12, and IL-18 standards. Limits of detection in these assays were 5 pg/ml, 15 pg/ml, and 25 pg/ml, respectively. Amounts of cytokines in tissue extracts were expressed relative to total protein content.
Detection of apoptotic cells
Terminal deoxynucleotidyl transferase (TdT)-mediated d-UTP-biotin nick end labelling (TUNEL) was performed as described previously [13]. After the lungs were excised and quickly frozen in dry ice, 4-µm-thick sections were cut on a cryostat and thaw-mounted onto gelatinized glass slides. Sections were fixed with 4% paraformaldehyde in 0·1 m phosphate buffer (pH 7·4) for 30 min and then incubated in 0·3% H2O2 in absolute methanol overnight before TUNEL staining. Briefly, TdT was used to incorporate biotinylated deoxyuridine at sites of DNA cleavage, with avidin-peroxidase used to detect labelling. Positive controls (deoxyribonuclease-treated cell preparations and also thymocytes) stained positively, indicating DNA fragmentation.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed by using anova followed by multiple comparison corrected t-test. A P-value of <0·05 was considered statistically significant.
Results
Inhibition by exogenous IL-12 of established allergic airway responses
We examined histological findings in the lungs and analysed cells infiltrating into the lung tissue in the same specimens. The OVA-challenged, vehicle-treated group showed a marked increase in eosinophils and CD4+ T cells in the infiltrates. A single administration of IL-12 caused a significant decrease in antigen-induced eosinophilia (P < 0·01). Numbers of CD4+ T lymphocytes were also significantly reduced following IL-12 administration (P < 0·01; Fig. 1). In OVA-challenged mice, a single administration of IL-12 caused significant inhibition of the antigen-induced airway hyperresponsiveness (data not shown).
Fig. 1.
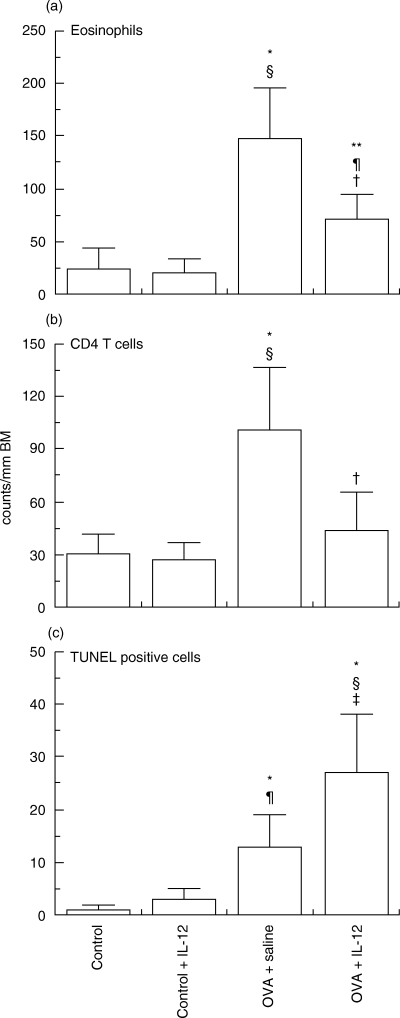
Effects of interleukin (IL)-12 on numbers of lung eosinophils (a), CD4+ T cells (b) and apoptotic (TUNEL-positive) cells (c) in C57BL/6 mice sensitized systemically and then challenged by aerosolized ovalbumin. Data represent means ± SD for indicated groups of mice (*P < 0·01 and **P < 0·05 compared with control mice after saline challenge; §P < 0·01 and ¶P < 0·05 compared with rIL-12 treated-mice after saline challenge; †P < 0·01 and ‡P < 0·05 compared with placebo treated-mice and rIL-12-treated mice after OVA challenge). BM, basement membrane.
Enhancement of apoptosis by exogenous IL-12
To study the effect of exogenous IL-12 on apoptosis of infiltrating cells in lung tissue sections, apoptotic cells were examined in antigen-challenged mice with established allergic responses after treatment with IL-12 or saline. We previously studied the dynamics of appearance of TUNEL-positive cells in the airways of allergen-challenged mice [13]. In agreement with our previous results, almost no TUNEL-positive cells were detected in the airways of mice given nebulized saline alone. One day after nebulized antigen challenge followed by treatment with saline, a few TUNEL-positive cells were detected. Administration with IL-12 in actively immunized C57BL/6 mice decreased the number of eosinophils and CD4+ T cells (Fig. 1), while significantly increasing the number of TUNEL-positive cells infiltrating lung tissue (Figs 1 and 2).
Fig. 2.
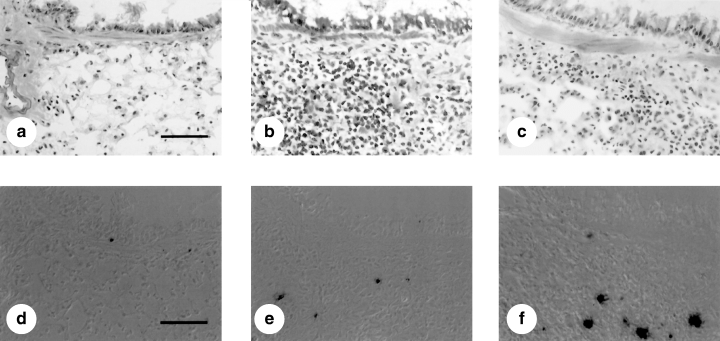
Histological preparations showing inflammatory cells and apoptotic cells in lung sections from C57BL/6 mice sensitized systemically and then challenged via the airways with ovalbumin. Light micrographs of formalin-fixed tissue from sensitized C57BL/6 mice that were saline-challenged (a,d) or ovalbumin challenged (b,c,e,f) and treated with recombinant murine IL-12 (c,f) or vehicle (b,e) after the last aerosol exposure are shown. Lung sections were stained by Giemsa (a–c) or terminal deoxynucleotidyl transferase-mediated deoxyribonucleoside triphosphate nick end-labelling (TUNEL) methods (d–f). Dark brown-stained apoptotic cells were present beneath the bronchial epithelium after aerosol antigen challenge (bar = 50 µm).
To identify the nature of the apoptotic cells, adjacent sections from lungs of mice treated with murine recombinant IL-12 were examined. This revealed that a significant amount TUNEL-positivity correlated with CD4+ immunoreactive T cells (Fig. 3a,b). Using a combination of immunostaining and TUNEL, we found that treatment with IL-12 after nebulized antigen challenge decreased the number of non-apoptotic CD4+ T cells, while significantly increasing in the numbers of both apoptotic CD4+ T cells and CD4− cells infiltrating lung tissue (Fig. 4). These findings suggest that treatment with IL-12 induced the apoptosis of infiltrating CD4+ T cells and CD4− cells in this model of allergic airway inflammation.
Fig. 3.
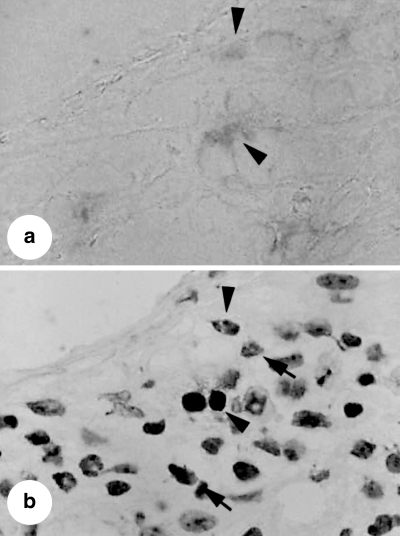
Photomicrographs of mouse lung sections obtained from mice sensitized systemically and then challenged via the airways with ovalbumin and treated with recombinant murine IL-12. Lung sections were stained for CD4 (a) or terminal deoxynucleotidyl transferase-mediated deoxyribonucleoside triphosphate nick end-labelling (TUNEL) methods (b) Some TUNEL-positivity corresponded to CD4+ T cells in adjacent sections (Arrow heads). The arrows indicate TUNEL+, chromatin-condensed inflammatory cells not associated with CD4+ T cells.
Fig. 4.
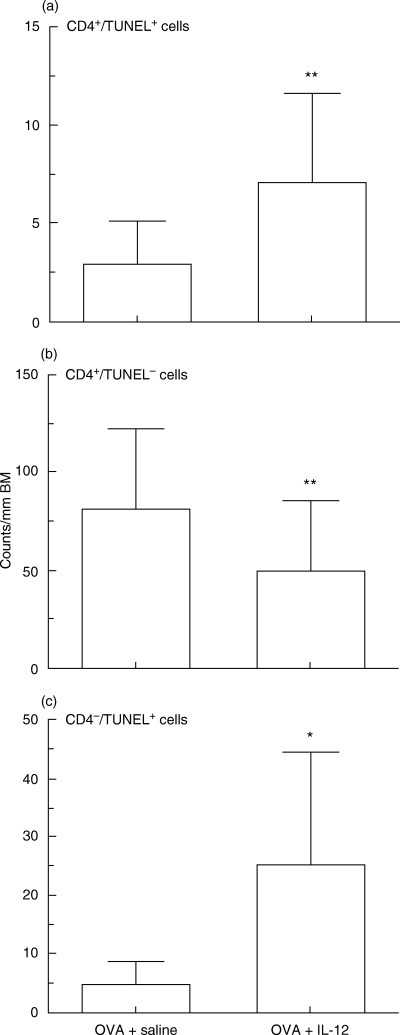
Correlations between individual numbers of apoptotic cells, non-apoptotic cells and intensity of CD4 expression in C57BL/6 mice sensitized systemically and then challenged by aerosolized ovalbumin following treatment of IL-12 and saline. Data represent means ± SD for indicated groups of mice (*P < 0·01 and **P < 0·05 compared with saline treated-mice and rIL-12-treated mice after OVA challenge). BM, basement membrane.
Enhancement of IFN-γ production following treatment with IL-12
Amounts of IFN-γ, IL-12, and IL-18 were not altered in mice after exposure to OVA (IFN-γ; 197 ± 11 and 186 ± 15, IL-12; 193 ± 22 and 185 ± 42, IL-18; 15·5 ± 1·5 and 14·2 ± 1·6 pg/mg protein, respectively), suggesting selective induction of a Th2-type cytokine profile in the lungs of OVA-challenged mice (Fig. 5). Intravenous administration of IL-12 increased the concentration of IL-12 in the lung even after 1 day. Administration of IL-12 to saline-challenged mice caused a slight but significant increase in IFN-γ in the lung (187 ± 21 and 302 ± 75 pg/mg protein, respectively). In antigen-challenged mice, a single dose of IL-12 significantly increased the concentration of IFN-γ in antigen-challenged mice (Fig. 5), but did not affect IL-18 (IFN-γ; 186 ± 15 and 416 ± 180, IL-18; 14·2 ± 1·6 and 16·2 ± 3·5 pg/mg protein, respectively).
Fig. 5.
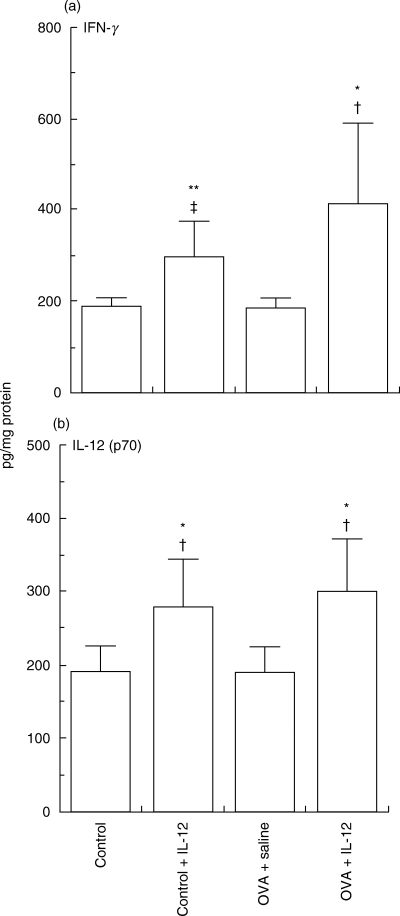
Effects of interleukin (IL-12) on (a) interferon (IFN)-γ and (b) IL-12 (p70) in lung tissue extracts from mice sensitized systemically and then challenged via the airways with ovalbumin. Data represent means ± SD for indicated groups of mice (*P < 0·01 **P < 0·05 compared with control mice after saline challenge; †P < 0·01 and ‡P < 0·05 compared with placebo treated-mice after OVA challenge).
Discussion
IL-12 is an important regulator of the balance between Th1 and Th2 cells. Besides inducing Th1 responses, IL-12 was reported to suppress Th2 responses. IL-12 production has been proposed to be an inducer of anti-allergic immune responses by virtue of these inhibitory effects on Th2 cytokine expression. Striking decreases in eosinophil and CD4+ T cell numbers after administration of IL-12 indicate the possible usefulness of this approach in the treatment of allergic rhinitis and atopic dermatitis.
In our previous study we demonstrated that exogenous IL-12 reduced established airway responses such an eosinophilic infiltration and airway hyperresponsiveness in a mouse model [17]. Furthermore, in cytokine analysis, single intravenous administration of IL-12 after the final antigen challenge inhibited antigen-induced expression of Th2 cytokines such as IL-4 and IL-13 [17]. While these results suggested that IL-12 could suppress airway allergic inflammation by inhibiting production of Th2 cytokines, the present results suggest that the mechanism by which exogenous IL-12 inhibits allergen-induced eosinophilia and CD4+ T cell infiltration in vivo involves induction of apoptosis.
Using immunostaining and detection of TUNEL-positivity, we found that a significant number of CD4+ T cells in lung tissue undergo apoptosis following treatment with IL-12. Many studies reported that interaction of Fas antigen with its ligand (FasL) results in eosinophil and T cell apoptosis. Similarly, we observed that Fas antigen deficiency was associated with prolonged inflammation caused by an extended survival of inflammatory cells due to reduced apoptosis [18]. IL-12 itself also induces Fas antigen, an apoptosis–triggering cell surface molecule. A previous study suggested that pulmonary T lymphocytes from asthmatic patients do not express Fas antigen, but display normal amounts of FasL [19]. These data suggest that in asthma exogenous IL-12 may enhance expression of Fas antigen by CD4+ T cells, which may lead apoptosis in the airways.
In this study, we observed that non-CD4+ cells in lung tissue also undergo apoptosis following treatment with IL-12. Recent animal studies suggest that treatment with exogenous IL-12 in murine models of asthma abolished pulmonary eosinophilia and airway hyperresponsiveness, while causing an increase in IFN-γ and a decrease in IL-4 and IL-5 expression. Recently, Nutku et al.[20] reported that surface expression of IL-12 receptor β1 and β2 subsets in blood eosinophils were expressed. Interestingly, they observed that IL-12 increased eosinophil apoptosis in vitro. Accordingly, it is possible that some of the apoptotic non-CD4+ T cells were eosinophils, although we could not exclude the possibility that other inflammatory cells were TUNEL-positive.
The present study demonstrates that only a few TUNEL-positive cells could be stained as CD4+ cells, so that most CD4+ cells, following treatment with IL-12 are not apoptotic. However, the increase in numbers of apoptotic CD4+ cells following treatment with IL-12 is rather increased significantly. In a previous in vitro study of apoptosis induced by anti-Fas antibody, the total dying process proceeded for over 3·5 h [21]. The data suggest that apoptotic cells are likely removed quickly from the tissue following ingestion by macrophages, one of the important mechanisms in the resolution of airway inflammation.
IL-12 induces release of IFN-γ, which is known to have anti-proliferative effects on many cell types; at least some of these effects may be connected to induction of apoptotic cell death [22]. The protein product of Bcl-2, an antiapoptotic gene cloned from the breakpoint of a t [15,8], translocation present in human B-cell lymphomas, is crucial to survival of haematogenous and lymphoid cells [23]. A specific profile of Bcl-2-related apoptotic molecules is expressed in eosinophils [24]. The effect of IFN-γ is paralleled by up-regulation of Bax, which opposes Bcl-2, as well as by down-regulation of Bcl-2 [25]. These findings suggest that production of IFN-γ in response to IL-12 may reduce allergic responses by decreasing Bcl-2 expression in airway inflammatory cells.
IL-18, which is widespread and produced by many cell types, including macrophages [26], strongly augments IFN-γ production by T cells in combination with IL-12. A recent study suggested that IL-18 has an apoptotic effect on tumour cells via a Fas-dependent pathway [27]. Previously, we observed that exogenous IL-12 in the presently used asthma model increased IL-18 receptor α (IL-18Rα) expression on the surfaces of lymphocytes in airway tissues, resulting in enhanced expression of FasL [17]. The finding suggests that in the presence of exogenous IL-12, endogenous IL-18 can better target cells expressing more IL-18Rα, indicating increased apoptosis in immune cells because of enhancement of FasL expression. Thus, IL-12, IL-18, and IFN-γ regulate the allergic immune response via apoptotic signals such as Fas/FasL interactions [16]. Although our preliminary experiments have failed so far to implicate Fas/FasL-mediated apoptosis in the protection observed in this model, this mechanism was not excluded. These questions will be the subject of a separate study.
In summary, our present findings indicate that administration of the Th1-inducing cytokine, IL-12, is protective in a Th2-dependent model of allergic airway inflammation, at least partly via enhanced apoptosis of CD4 T cells. Specific promotion of apoptosis of lung inflammatory cells may provide a new therapeutic approach in asthma. Further studies are necessary to determine whether interactions of IL-12 with apoptotic pathways can provide a novel therapeutic option.
Acknowledgments
We thank Ms. Kyoko Obata and Ms. Diana Nabighian for their technical assistance with the experiments and Dr Hideki Fukuda, Dr Daizo Ihaku, Dr Shigeru Miyata Dr Nobuaki Miyahara for helpful discussion. The work was supported in part by Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan and by grants HL-36577 and HL-61005 from the National Institutes of Health and the Environmental Protection Agency (R825702).
References
- 1.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Ann Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- 3.Robinson D, Hamid Q, Ying S, Bentley A, Assoufi B, Durham S, Kay AB. Prednisolone treatment in asthma is associated with modulation of bronchoalveolar lavage cell interleukin4, interleukin-5, and interferon-γ cytokine gene expression. Am Rev Respir Dis. 1993;148:401–6. doi: 10.1164/ajrccm/148.2.401. [DOI] [PubMed] [Google Scholar]
- 4.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for Interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–6. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon γ regulates antigen-induced eosinophil recruitment into the mouse airway by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573–6. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lack G, Bradley KL, Hamelmann E, Renz H, Loader J, Leung DYM, Larsen G, Gelfand EW. Nebulized IFN-γ inhibits the development of secondary allergic responses in mice. J Immunol. 1996;157:1432–9. [PubMed] [Google Scholar]
- 7.Shu U, Kiniwa M, Wu CY, Maliszewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40–CD40 ligand interaction. Eur J Immunol. 1995;25:1125–8. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 8.Scheicher C, Mehlig M, Dienes HP, Reske K. Uptake of microparticle-adsorbed protein antigen by bone marrow-derived dendritic cells results in up-regulation of interleukin-1α and interleukin-12 p40/p35 and triggers prolonged, efficient antigen presentation. Eur J Immunol. 1995;25:1566–72. doi: 10.1002/eji.1830250615. [DOI] [PubMed] [Google Scholar]
- 9.Rao JB, Chamberlain RS, Bronte V, Carroll MW, Irvine KR, Moss B, Rosenberg SA, Restifo NP. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7–1 expression. J Immunol. 1996;156:3357–65. [PMC free article] [PubMed] [Google Scholar]
- 10.Lu H, Zhong G. Interleukin-12 production is required for chlamydial antigen-pulsed dendritic cells to induce protection against live chlamydia trachomatis infection. Infect Immunol. 1999;67:1763–9. doi: 10.1128/iai.67.4.1763-1769.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokin expression in mice. J Exp Med. 1995;182:1527–36. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sur S, Lam J, Bouchard P, Sigounas A, Holbert D, Metzger WJ. Immunomodulatory effects of IL-12 on allergic lung inflammation depend on timing of doses. J Immunol. 1996;157:4173–80. [PubMed] [Google Scholar]
- 13.Kodama T, Matsuyama T, Miyata S, Nishimura H, Nishioka Y, Kitada O, Sugita M. Kinetics of apoptosis in the lung of mice with allergic airway inflammation. Clin Exp Allergy. 1998;28:1435–43. doi: 10.1046/j.1365-2222.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 14.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin-5 deficiency abolishes eosinophilia, airway hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofstra CL, Van Ark I, Hofman G, Kool M, Nijkamp FP, Van Oosterhout AJ. Prevention of Th2-like cell responses by co-administration of IL-12 and IL-18 is associated with inhibition of antigen-induced airway hyperresponsiveness, eosinophilia, and serum IgE levels. J Immunol. 1998;161:5054–60. [PubMed] [Google Scholar]
- 16.Kodama T, Matsuyama T, Kuribayashi K, Nishioka Y, Sugita M, Akira S, Nakanishi K, Okamura H. IL-18 deficiency selectively enhances allergen-induced eosinophilia in mice. J Allergy Clin Immunol. 2000;105:45–53. doi: 10.1016/s0091-6749(00)90176-3. [DOI] [PubMed] [Google Scholar]
- 17.Kuribayashi K, Kodama T, Okamura H, Sugita M, Matsuyama T. Effects of postinhalation treatment with interleukin (IL)-12 on airway hyperreactivity, eosinophilia, and IL-18 receptor expression in mouse model of asthma. Clin Exp Allergy. 2002;32:641–9. doi: 10.1046/j.0954-7894.2002.01346.x. [DOI] [PubMed] [Google Scholar]
- 18.Duez C, Tomkinson A, Shultz LD, Bratton DL, Gelfand EW. Fas deficiency delays the resolution of airway hyperresponsiveness after allergen sensitization and challenge. J Allergy Clin Immunol. 2001;108:547–56. doi: 10.1067/mai.2001.118288. [DOI] [PubMed] [Google Scholar]
- 19.Spinozzi F, Fizzotti M, Agea E, et al. Defective expression of Fas messenger RNA and Fas receptor on pulmonary T cells from patients with asthma. Ann Intern Med. 1998;128:353–9. doi: 10.7326/0003-4819-128-5-199803010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Nutku E, Zhuang Q, Soussi-Gounni A, Aris F, Mazr B, Hami Q. Functional expression of IL-12 receptor by human eosinophils: IL-12 promotes eosinophil apoptosis. J Immunol. 2001;167:1039–46. doi: 10.4049/jimmunol.167.2.1039. [DOI] [PubMed] [Google Scholar]
- 21.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 22.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Cross-linking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing Interferon-γ and Tumor necrosis factor-α secretion. Blood. 1994;84:2622–31. [PubMed] [Google Scholar]
- 23.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t (14; 18) chromosome translocation. Science. 1984;226:1097–9. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 24.Dewson G, Walsh GM, Wardlaw AJ. Expression of Bcl-2 and its homologues in human eosinophils modulation by interleukin-5. Am J Respir Cell Mol Biol. 1999;20:720–8. doi: 10.1165/ajrcmb.20.4.3453. [DOI] [PubMed] [Google Scholar]
- 25.Koshiji M, Adachi Y, Soga S, et al. Apoptosis of colorectal adenocartinoma (COLO 201) by tumor necrosis factor-alpha (TNF-α) and/or interferon-gamma (IFN-γ), resulting from down-modulation of Bcl-2 expression. Clin Exp Immunol. 1998;111:211–8. doi: 10.1046/j.1365-2249.1998.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura H, Tsutsui H, Kashiwamura SI, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto W, Osaki T, Okamura H, et al. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999;163:583–9. [PubMed] [Google Scholar]


