Abstract
The addition of a foreign antigen to an inoculum completely inhibits the development of collagen-induced arthritis (CIA). However, the mechanism of this phenomenon, antigen -inhibition, is incompletely understood. Previous studies have demonstrated that the inhibition of arthritis is not mediated through suppression of the antibody response to cartilage antigens. In this paper we investigated cytokine mRNA levels in lymph nodes cells recovered 3, 7 or 16 days from animals immunized with either collagen II in IFA or OVA + collagen II in IFA. At day 7, but not at other time-points, IL-4 mRNA was up-regulated in the lymph nodes of OVA-inhibited non-arthritic animals compared to control animals which all developed arthritis. No significant differences between the two groups could be detected when expression of IFN-γ, IL-2, TNF-α, IL-1β or IL-10 mRNA was analysed. Flow cytometry analysis of draining lymph node cells demonstrated that the T cell marker Ox40 was up-regulated in the OVA-inhibited group. Our results indicate that the complete inhibition of CIA caused by addition of OVA to the collagen II inoculum is due to the presence of a TH2 environment resulting from an increased production of IL-4 mRNA and a parallel increase in Ox40+ T cells.
Keywords: antibodies, autoimmunity, cell populations, cytokines, inhibition, unrelated antigen
Introduction
Collagen-induced arthritis (CIA) is an organ-specific autoimmune disease with many similarities to human rheumatoid arthritis (RA), which is inducible in susceptible rodent and primate animal strains [1,2]. The disease is dependent on both the activation of T cells [3] and on anticollagen antibody production [4,5]. Induction of disease is associated with a strong activation of proinflammatory cytokines in the draining lymph nodes before disease onset [6]. Activation of type 2 cytokines has been associated with amelioration [7,8] and resistance to arthritis [9].
An immune response to unrelated antigens can interfere with the development of CIA in DA rats. The effect is especially strong when the antigen is included in the inoculum of IFA and Collagen II (CII). The inhibition is effective using a number of foreign antigens, whereas self-antigens such as rat serum albumin (RSA) have comparatively little effect on preventing arthritis development [10]. A similar phenomenon of antigen inhibition has been studied in another model of autoimmunity, in which activation of an immune response to keyhole limpet haemocyanin (KLH) inhibited the development of EAE [11].
The mechanism of antigen inhibition is incompletely understood, especially in light of the particularly strong effect this has on the development of arthritis. Previous investigations of the inhibition of CIA demonstrated that the antibody response to CII was not affected in antigen-inhibited animals compared to control CIA rats 28 days after disease induction [10]. This suggested that there are mechanisms other than changes in the humoral response that mediate the inhibitory effect. The fact that the inhibited animals had a T cell-dependent IgG antibody response to CII indicates that they have a functional antigen presentation of collagen that results in generation of T and B cell responses to CII.
We have now investigated further the mechanism of antigen -inhibition of CIA. The anticollagen antibody response was studied initially, and in accordance with previous studies it was little affected. We then investigated the kinetics of the production of cytokine mRNA in OVA-inhibited and control CIA animals. Furthermore, the differences in cellular populations were studied, with particular emphasis on the activation state of T cell populations.
Materials And Methods
Animals
In all experiments female Dark Agouti (DA) rats of age 8–10 weeks were used. All experiments, approved by the Ethical committee of Stockholm North, were performed according to local ethical regulations.
Collagen II preparation
Bovine CII (bCII) dissolved in 0·1 m acetic acid was used in all experiments and was prepared from calf nose cartilage, as described previously [12,13].
Immunizations
Rats were immunized intradermally in the base of the tail with 300 µl of an emulsion of either bCII + IFA + PBS or bCII + IFA + OVA. The inoculate was prepared by first emulsifying bCII (75 µl per rat) and IFA (150 µl per rat) (Difco, Detroit, MI, USA) and subsequently adding and emulsifying the PBS, BSA (Sigma, St Louis, MO, USA) or OVA, respectively (75 µl per rat) (Sigma, St Louis, MO, USA). Each rat thus received 150 µg bCII and 150 µl IFA and in the antigen-inhibited rats, 112 µg OVA (in PBS) or 10 µg BSA (in PBS) was included in the inoculate. In a separate experiment the control group IFA/OVA was added, the rats receiving an emulsion of acetic acid, OVA and IFA mixed as described above.
Evaluation of arthritis
Rats were scored blindly by two independent investigators according to a previously described procedure [14]. In brief, 1 point signifies swelling of one group of joints [for example metatarsophalangeal (MTP), metacarpophalangeal (MCP) joints or proximal interphalangeal (PIP) joints], 2 points signifies two groups of swollen joints, 3 points signifies three groups of swollen joints (for example PIP, MCP and wrist or ankle joints), and 4 points signifies swelling of the entire paw. The maximum score for each rat was 16.
Quantification of anti-bCII antibodies
Individual sera were collected days 21 and 30 post-immunization (p.i.) from bCII/IFA immunized rats with either PBS or OVA in the inoculate. The sera were stored at − 20°C until the total IgG anti-CII levels were analysed according to a previously described ELISA procedure [15]. A pool of sera from bCII immunized rats was used as standard. The 1 : 50 dilution of this standard serum was defined arbitrarily to contain 2 units of anti-bCII antibody.
Determination of anti-bCII IgG isotypes IgG1, IgG2a and IgG2b was performed by ELISA as described previously [16].
Cytokine quantification
The inguinal lymph nodes were dissected from the rats at days 3, 7 or 16 p.i. Lymph nodes were frozen after dissection and kept at − 70°C until RNA preparation. Extraction of RNA and synthesis of cDNA was performed as previously described [7].
Quantitative PCR analysis of cDNA was performed using the Taqman™ methodology (Perkin Elmer, Norwalk, CT, USA), according to the manufacturer's instructions. Cytokine values for each animal were normalized against the housekeeping enzyme GAPDH. Primers and probes were designed, using the software Primerexpress™, to span over introns in order to avoid amplification of genomic DNA. The primers and probes used are described elsewhere [17] except for GAPDH (sense: TCAACTAC ATGGTCTACATGTTCCAG; antisense: TCCCATTCTCAGC CTTGACTG; probe: FAM-TGACTCTACCCACGGCAAGT TCAACG-TAMRA).
Flow cytometric analysis
For three-colour flow cytometric analysis, OVA-treated and normal CIA rats were sacrificed on days 7 or 30 p.i. The draining inguinal lymph nodes were dissected, passed through a steel mesh, washed twice in PBS and resuspended in PBS supplemented with 2% FCS; 5 × 105 cells/sample were incubated with monoclonal antibodies at saturating concentrations for 30 min at 4°C. The antibodies used were: biotinylated Ox40 (Pharmingen, San Diego, CA, USA), antirat TCR αβ-FITC (clone R73, Serotec, Oxford, UK), FITC antirat CD8 (clone OX8, Pharmingen), antirat CD25-PE (clone Ox-39, Pharmingen), antirat CD45RC-FITC (clone Ox-22, Serotec), antirat CD62L-FITC (clone HRL-1, Pharmingen), antirat CD4-Cy chrome (clone Ox-35, Pharmingen) and antirat NK-RP1-FITC (clone 10/78, Serotec). For stainings in which one of the primary antibodies was biotinylated Ox40, cells were washed once with PBS/2% FCS, and incubated thereafter with streptavidin-PE (Becton Dickinson, Mountain View, CA, USA) for 30 min at 4°C. After a final wash, cells were fixed in PBS/1% paraformaldehyde. Simultest™ Control γ1/γ2a antibodies (Becton Dickinson) were used as negative control. Cells were also incubated with streptavidin-PE alone to exclude non-specific staining from this reagent.
Finally, 10–50 000 events per sample were acquired on a FACSsort flow cytometer (Becton Dickinson, San Jose, CA, USA) and analysed using CellQuest software (Becton Dickinson). A wide lymphocyte gate was set to also include NK cells and monocytes.
Statistics
The arthritis score values were analysed using the nonparametric Mann–Whitney U-test for two-group analysis. The antibody titres were analysed using Student's t-test.
Cytokine mRNA production and cell populations were analysed using non-parametric analysis as above, i.e. Mann–Whitney U-test.
Results
Antigen inhibition of bovine CIA using irrelevant antigen
In a number of experiments we could inhibit completely the bCII-induced CIA with an unrelated antigen (Table 1) using either OVA or BSA. The inhibition was complete, i.e. no arthritis was observed, whereas the incidence was nearly 100% in all simultaneously performed control experiments when bCII + IFA was injected without the presence of foreign antigen. Inhibition was effective with as little as 10 µg BSA/rat.
Table 1.
Unrelated antigens can inhibit bovine CIA: effects on antibody levelsa
| Exp. | Inhibitor | n | Incidence (%) | Maximum score | α-CII IgG day 21 | α-CII IgG day 30 |
|---|---|---|---|---|---|---|
| 1 | OVA | 5 | 0 | 0 | n.d. | 0·23 ± 0·20 |
| none | 5 | 100 | 2·6 | n.d. | 0·37 ± 0·08 | |
| 2 | OVA | 5 | 0 | 0 | 1·4 ± 1·2 | n.d. |
| none | 5 | 100 | 6·6 | 0·76 ± 0·79 | n.d. | |
| 3 | BSA | 7 | 0 | 0 | n.d. | n.d. |
| none | 7 | 85 | 8·4 | n.d. | n.d. |
The number of animals in each treated group (n) is indicated in one column. The values for maximum score are mean values. The total α-CII IgG value was determined relative to a standard serum that was set to the arbitrary value of 2. The values indicated are mean ± s.d. None of the values for α-CII IgG are statistically significant.
Anti-CII levels in antigen-inhibited rats
Serum levels of anti-bCII antibodies were determined in sera recovered at day 21 or 30, respectively (Table 1). Total levels of anti-bCII IgG were not significantly different between groups at either time-point. The individual antibody isotypes were also determined. As previously reported [18] the IgG2a anti-bCII response was dominant, with IgG1 and IgG2b accounting for only a fraction of the total IgG anti-bCII. There was no consistent difference between the two groups for any of the three isotypes IgG1, IgG2a and IgG2b (data not included).
Cytokine analysis of lymph node cells before and after the onset of arthritis
Cytokine mRNA expression was determined in the draining lymph nodes at different time-points before and after the onset of disease, at days 3, 7 or 16 after CII immunization. The relative value for each cytokine mRNA is expressed as the quantity of the respective cytokine mRNA in the OVA-treated animals compared to the control CIA immunized animals (as percentage) (Table 2). IL-4 mRNA increased in treated as compared to control immunized animals at day 7 p.i. This result was confirmed in a second set of experiments. No significant differences in IL-4 mRNA between treated and control groups was recorded at other time-points and no statistically significant differences could be detected in IFN-γ, TNF-α, IL-1β and IL-10 levels at any of the time-points analysed.
Table 2.
Analysis of the cytokine production in inguinal lymph nodes at days 3, 7 and 16a
| CIA | OVA | |||
|---|---|---|---|---|
| Mean | s.e.m. | Mean | s.e.m. | |
| (a) Day 3 | ||||
| IL-4/GADPH | 100 | 34 | 113 | 24 |
| IFN-γ/GADPH | 100 | 25 | 81 | 22 |
| IL-10/GADPH | 100 | 10 | 78 | 13 |
| (b) Day 7 | ||||
| IL-4/GADPH | 100 | 17 | 228* | 22 |
| IFN-γ/GADPH | 100 | 18 | 90 | 15 |
| IL-10/GADPH | 100 | 17 | 83 | 6 |
| IL-1β/GAPDH | 100 | 18 | 59 | 8 |
| TNF-α/GAPDH | 100 | 10 | 77 | 5 |
| (c) Day 16 | ||||
| IL-4/GADPH | 100 | 56 | 65 | 6 |
| IFN-γ/GADPH | 100 | 17 | 78 | 9 |
| IL-10/GADPH | 100 | 13 | 88 | 9 |
The individual values for each cytokine were determined from a standard curve of either a plasmid construct or control cDNA prepared from ConA stimulated cells. The cytokine value for each animal was normalized to the housekeeping enzyme GAPDH. The resulting mean value for the control group for each cytokine was set to 100. Since RNA preparation, cDNA synthesis and PCR experiments were performed in different experiments for each day and cytokine, only OVA-treated and CIA animals can be compared.
P < 0·05 using Mann–Whitney U-test.
In a separate experiment the up-regulation of IL-4 mRNA in antigen-inhibited animals was compared to a second control group immunized with OVA/IFA alone. Compared to CIA in animals receiving OVA/FIA the IL-4 mRNA expression was up-regulated (198% ± 60 (mean ± s.e.m.) relative to the group CIA) but not to the same extent as in CIA/OVA animals (563% ± 215 relative to the group CIA that was set to 100% ± 21).
FACS analysis of changes in cell populations after antigen inhibition
On day 7 p.i., the fraction of αβTCR+CD3+ T cells of total lymphocytes in the inguinal lymph nodes was lower in the OVA-treated group than in the control CIA group (Fig. 1a). This decrease was reflected in both the CD8+ and the CD4+ compartments, as both these populations were decreased in the OVA-inhibited animals (Fig. 1b,c).
Figure 1.
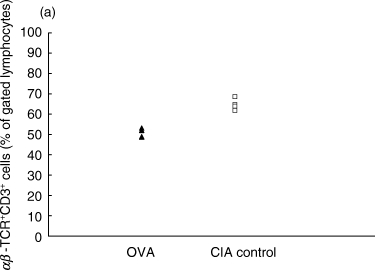
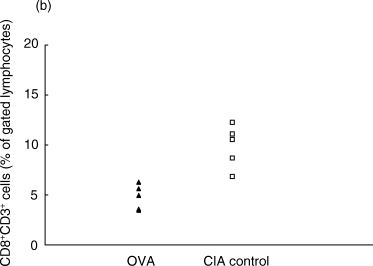
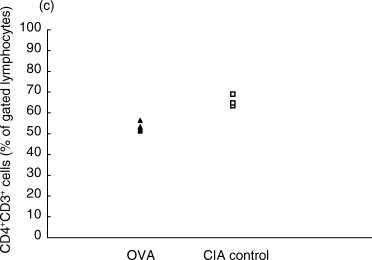
Proportions of αβ T cell populations in OVA-inhibited (n = 5) and control CIA (n = 5) animals at day 7 after immunization, as determined by flow cytometry. (a) The total T cell population defined by CD3+αβ-TCR+ cells was lower in the OVA-inhibited group (P < 0·01) using Student's t-test. This decrease was reflected in both the (b) CD8+ CD3+ population (P < 0·01) and in the (c) CD4+ CD3+ population (P < 0·01). This experiment was repeated once with similar results. ▴, OVA; □, CIA control.
These cellular populations were also analysed in animals at a later time-point in the disease process (day 30) but no differences between the treated and untreated groups were observed (data not included). The total number of cells in the inguinal lymph nodes was also estimated and no significant differences could be detected between treatment groups at any time-point analysed (data not included).
The frequency of NKT cells or CD3+αβTCR− cells, which include the γδ cell population, was not significantly different on day 7 or day 30 p.i. (data not included).
FACS analysis of changes in T cell phenotypes
Since some T cell populations may regulate autoimmunity, different phenotypes of the CD4+ T cell population were investigated with respect to activation markers. On day 7 a marker often used to assess T cell activation, CD25, had similar expression levels in the two treatment groups (Fig. 2a). Later in the disease (day 30 p.i.) expression of this receptor was higher in the arthritic CIA control animals (Fig. 2a).
Figure 2.
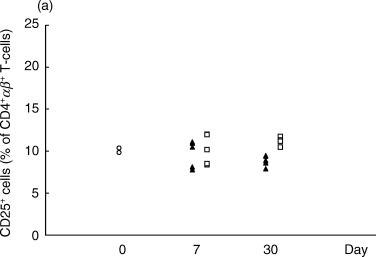
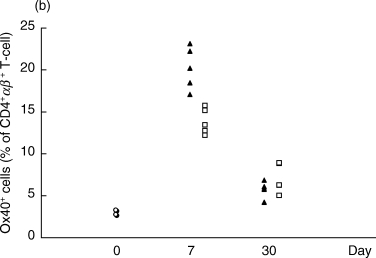
Proportions of CD4+αβ+ T cells expressing the activation markers CD25 (a) or Ox40 (b) in the OVA-inhibited (n = 5) and control CIA animals (n = 5) at days 7 and 30 after immunization, as determined by flow cytometry. The proportion of CD4+αβ+ T cells with CD25 was higher in the CIA control group (P < 0·01) at day 30 p.i. (a). The proportion of CD4+αβ+ T cells expressing Ox40 was higher in the OVA-inhibited group (P < 0·01) at day 7 p.i. (b). This experiment was repeated once with similar results. ▴, OVA; □, CIA control; ^, normal.
On day 7, the T cell activation marker Ox40 was up-regulated in the OVA-inhibited group, 20·2 ± 1·1% (mean ± SEM) versus 13·9 ± 0·7% of CD4+αβ-TCR+ lymphocytes (Fig. 2b). However, the levels of Ox40+ cells in the CD4+αβ-TCR+ T cell population were not significantly changed between the two groups at day 30 (Fig. 2b).
In a separate experiment the increased frequency of Ox40+ T cells in antigen-inhibited animals was compared to a second control group immunized with OVA/IFA alone. The Ox40 expression on CD4+αβ-TCR+ T cells was slightly higher in animals receiving OVA/IFA (14·9% ± 1·5, mean ± s.e.m.) than in animals receiving CII/IFA (13·2% ± 0·9) but not up-regulated to the to the same extent as in CIA/OVA/IFA animals (17·3% ± 0·8).
FACS-analysis of Ox40+ T cells
In order to investigate further the phenotype of the Ox40+ population, two other markers previously associated with regulation of autoimmunity, CD62L (l-selectin) and CD45RC [19,20], were investigated at day 7 p.i.
In rats, CD4 is expressed on T cells (CD4+TCR+) or macrophages/monocytes (CD4+TCR−). However, 7 days after immunization with bCII/IFA/PBS or bCII/IFA/OVA, very few (1·0%) CD4+ CD3− cells were detected in LN cell preparations. In addition, in the experiment depicted in Fig. 2b only 2·0% of the CD4+ Ox40+ cells were αβ−TCR−. Thus, in this experimental set-up it was sufficient to use only CD4+ as a marker for CD4+ T cells.
When analysing the CD4+ Ox40+ cell population on day 7, we observed a difference between OVA-inhibited and control CIA animals in the CD62L− but not in the CD62L+ population (Fig. 3a). Similarly, in the CD4+ Ox40+ cell population, there was a difference between the treated and untreated groups in the CD45RC− but not in the CD45RC+ population (Fig. 3b).
Figure 3.
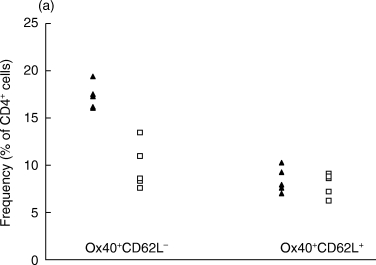
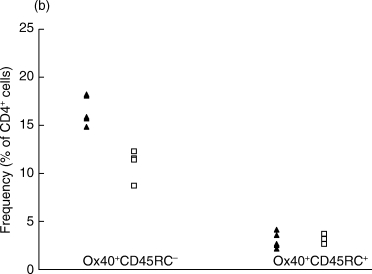
The analysis of the CD4+ Ox40+ population co-expressing (a) CD62L or (b) CD45RC phenotypes by flow cytometry at day 7 p.i. (a) The proportions of the CD4+Ox40+CD62L− and the CD4+Ox40+CD62L+ populations were compared. The difference between OVA-inhibited (n = 5) and control CIA (n = 5) is significant in the CD4+Ox40+CD62L− population (P < 0·01). (b) The proportions of the CD4+Ox40+CD45RC− and the CD4+Ox40+CD45RC+ populations were compared. The difference between OVA-inhibited (n = 5) and control CIA (n = 5) is significant in the CD4+Ox40+CD45RC− population (P < 0·01). ▴, OVA; □, CIA control.
Discussion
In this study, we confirmed that an immune response to unrelated antigens completely inhibited CIA induced with CII [10]. Our most significant findings were that antigen inhibition was associated with an up-regulation of IL-4 mRNA and an increased frequency of Ox40+ CD4+ T cells in the draining lymph nodes 7 days after immunization.
Co-inoculation of the unrelated antigen OVA with CII did not cause any significant change in either the total serum levels of IgG anti-CII or of the respective isotype compared to animals inoculated with CII alone. Thus the dramatic effect of antigen-inhibition on disease is not reflected by any change in the humoral immune response to CII.
Cytokine patterns have been used as important determinants of whether or not an autoimmune immune response becomes pathogenic [9], which prompted us to investigate which cytokines were most affected by OVA inhibition.
There was generally little difference in cytokine mRNA expression between OVA-inhibited and CIA animals. The reason for this may be that in this animal model it is only possible to measure the total expression of cytokines and not that of CII-specific T cells. The fact that we analysed lymph node cells and not the target organ may also make it more difficult to distinguish differences in a specific pathogenic T cell population.
IL-1β has been implicated strongly as one of the determinants of CIA development [21,22], especially when present in the joint. The fact that the average value of this cytokine mRNA is reduced (although not statistically significant) by OVA treatment may reflect the importance of this cytokine in arthritis development.
Nevertheless, in this study the cytokine that was most affected by OVA-inhibition was IL-4 at day 7 after immunization. Previous studies have demonstrated that the production of many cytokines is increased at this time-point [6]. Indeed, up-regulation of IL-4 has been associated previously with amelioration [7,23] or resistance [9] of arthritis. In addition, administration of IL-4 has an ameliorating effect on CIA [24,25]. Thus, the beneficial effect of an unrelated antigen in CIA may be mediated via an increase of IL-4 production. This conclusion is compatible with observations of other autoimmune models. In the study by Falcone et al., in which an immune response to KLH inhibited MBP-induced EAE, there was an increased IL-4 production in MBP-specific T cells from KLH-inhibited animals compared to uninhibited control EAE animals [11]. The authors suggested that the mechanism for protection was immune deviation towards a type 2 immune response acting in the regional lymph node and not in the peripheral tissue.
The total IL-4 mRNA production was slightly higher in animals receiving OVA/IFA than in those receivingCII/IFA, indicating that OVA is a stronger TH2-inducing antigen than is CII. However, because IL-4 mRNA production was even higher in OVA/CII/IFA immunized animals there was probably also a contribution from CII-specific T cells or due to the fact that these animals had a higher total antigenic load.
There are many descriptions in the literature of lymphocyte populations with autoimmune regulatory capacity. It was therefore our aim to study if antigen inhibition activated a T cell population that had been described previously as regulatory. Mason and coworkers have described autoimmune regulating populations in the peripheral T cell pool with the phenotype CD4+CD25+CD45RC− and CD62L+[19,26]. In our study there was no expansion of the CD25+CD4+ subset in association with antigen inhibition, nor did we find any increased frequencies of γδ T cells or NKT cells in antigen-inhibited animals, populations which may also have autoimmune regulatory function [27,28]. The T cell populations that are elevated by inhibiting antigen in our study (TCRαβ+CD4+Ox40+CD45RC− and TCRαβ+CD4+Ox40+CD62L−) have not been described previously as autoimmune regulatory cells.
On day 7 after immunization the CD4+αβ-TCR+ T cells from OVA-inhibited animals had increased expression of the activation marker Ox40, which is up-regulated transiently on activated CD4+ T cells [29]. When the antigen dependence of this Ox40 up-regulation was investigated in animals receiving OVA, CII or OVA + CII, respectively, the group receiving OVA alone had an intermediate expression level of Ox40. This suggests that both CII and OVA-specific T cells contribute to increased Ox40 expression. In the literature, there are suggestions that Ox40+ T cells may be involved in mounting a type 2 immune response in vitro[30,31] and in vivo[32]. However, it has also been demonstrated recently that Ox40+ T cells can also mount a Th1 response [33] in an experimental model of colitis.
Our data suggest that the Ox40+ population that differs between treated and untreated animals has the phenotype CD45RC− and CD62L−. T cells that are recent thymic emigrants are CD45RC+ and upon activation they convert to CD45RC−[34,35]. Expression of CD62L (l-selectin) on T cells is important in mediating homing to lymph nodes [36,37]. Loss of CD62L may also be a sign of activation [36]. This indicates that the Ox40+ CD4+ T cells associated with antigen-inhibition of CIA have phenotypical signs of active non-naive T cells.
The introduction of a competing immune response (in this case against OVA) abolishes the arthritogenic immune response. Despite the fact that the anti-CII antibody response is unchanged, the anti-OVA immune response is dominant, at least in determining the Th1/Th2 type of the immune response. This strategy, trying to induce a dominant, unrelated immune response that interferes with the pathological immune response, has also been demonstrated using live foreign microorganisms [38].
This is the first study investigating changes in lymphocyte populations and cytokine production as a result of an unrelated antigen inhibition of collagen-induced arthritis. We suggest that the modulation of the CIA by antigen inhibition is due to an increased production of IL-4 in the draining LN. This is also the first description of an association between protection of autoimmunity and expansion of the T cell phenotype TCRαβ+CD4+Ox40+. This phenotype is thus interesting to investigate in further studies.
Acknowledgments
Financial support was obtained from the Swedish Medical Research Council, King Gustav V 80-years fund and Nanna Svartz foundation. The authors wish to thanks Assoc. Prof. R. A. Harris and Dr V. Malmström for critical reading of the manuscript.
References
- 1.Larsson P, Kleinau S, Holmdahl R, Klareskog L. Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis Rheum. 1990;33:693–701. doi: 10.1002/art.1780330512. [DOI] [PubMed] [Google Scholar]
- 2.Trentham DE. Collagen arthritis as a relevant model for rheumatoid arthritis. Arthritis Rheum. 1982;25:911–16. doi: 10.1002/art.1780250801. [DOI] [PubMed] [Google Scholar]
- 3.Goldschmidt TJ, Holmdahl R. Therapeutic effects of monoclonal antibodies to alpha beta TCR but not to CD4 on collagen-induced arthritis in the rat. Cell Immunol. 1994;154:240–8. doi: 10.1006/cimm.1994.1072. [DOI] [PubMed] [Google Scholar]
- 4.Holmdahl R, Vingsbo C, Mo JA, et al. Chronicity of tissue-specific experimental autoimmune disease: a role for B cells. Immunol Rev. 1995;144:109–35. doi: 10.1111/j.1600-065x.1995.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 5.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–6. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mussener Å, Klareskog L, Lorentzen JC, Kleinau S. TNF-a dominates cytokine mRNA expression in lymphoid tissues of rats developing collagen- and oil-induced arthritis. Scand J Immunol. 1995;42:128–34. doi: 10.1111/j.1365-3083.1995.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 7.Mattsson L, Lorentzen JC, Svelander L, et al. Immunization with alum-collagen II complex suppresses the development of collagen-induced arthritis in rats by deviating the immune response. Scand J Immunol. 1997;46:619–24. doi: 10.1046/j.1365-3083.1997.d01-163.x. [DOI] [PubMed] [Google Scholar]
- 8.Persson S, Mikulowska A, Narula S, Ogarra A, Holmdahl R. Interleukin-10 suppresses the development of collagen type II-induced arthritis and ameliorates sustained arthritis in rats. Scand J Immunol. 1996;44:607–14. doi: 10.1046/j.1365-3083.1996.d01-355.x. [DOI] [PubMed] [Google Scholar]
- 9.Mussener Å, Lorentzen JC, Kleinau S, Klareskog L. Altered Th1/Th2 balance associated with non-major histocompatibility complex genes in collagen-induced arthritis in resistant and non-resistant rat strains. Eur J Immunol. 1997;27:695–9. doi: 10.1002/eji.1830270318. [DOI] [PubMed] [Google Scholar]
- 10.Lorentzen JC, Erlandsson H, Mussener Å, et al. Specific and long-lasting protection from collagen-induced arthritis and oil-induced arthritis in DA rats by administration of immunogens. Scand J Immunol. 1995;42:82–9. doi: 10.1111/j.1365-3083.1995.tb03629.x. [DOI] [PubMed] [Google Scholar]
- 11.Falcone M, Bloom BR. A T helper cell 2 (Th2) immune response against non-self antigens modifies the cytokine profile of autoimmune T cells and protects against experimental allergic encephalomyelitis. J Exp Med. 1997;185:901–7. doi: 10.1084/jem.185.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BD, Martin GR, Miller EJ, Dorfman A, Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975;166:181–6. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- 13.Andersson M, Holmdahl R. Analysis of type II collagen-reactive T cells in the mouse. I. Different regulation of autoreactive vs. non-autoreactive anti-type II collagen T cells in the DBA/1 mouse. Eur J Immunol. 1990;20:1061–6. doi: 10.1002/eji.1830200517. [DOI] [PubMed] [Google Scholar]
- 14.Åkerlund K, Erlandsson Harris H, Tracey KJ, et al. Anti-inflammatory effects of a new tumour necrosis factor-alpha (TNF-alpha) inhibitor (CNI-1493) in collagen-induced arthritis (CIA) in rats. Clin Exp Immunol. 1999;115:32–41. doi: 10.1046/j.1365-2249.1999.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorentzen JC, Klareskog L. Susceptibility of DA rats to arthritis induced with adjuvant oil or rat collagen is determined by genes both within and outside the major histocompatibility complex. Scand J Immunol. 1996;44:592–8. doi: 10.1046/j.1365-3083.1996.d01-354.x. [DOI] [PubMed] [Google Scholar]
- 16.Larsson P, Mattsson L, Klareskog L, Johnsson C. A vitamin D analogue (MC 1288) has immunomodulatory properties and suppresses collagen-induced arthritis (CIA) without causing hypercalcaemia. Clin Exp Immunol. 1998;114:277–83. doi: 10.1046/j.1365-2249.1998.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svelander L, Holm BC, Bucht A, Lorentzen JC. Responses of rat immune system to arthritogenic adjuvant oil. Scand J Immunol. 2001. pp. 599–605. [DOI] [PubMed]
- 18.Firth SA, Morgan K, Evans HB, Holt PJ. IgG subclasses in collagen-induced arthritis in the rat. Immunol Lett. 1984;7:243–7. doi: 10.1016/0165-2478(84)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Seddon B, Saoudi A, Nicholson M, Mason D. CD4+CD8- thymocytes that express L-selectin protect rats from diabetes upon adoptive transfer. Eur J Immunol. 1996;26:2702–8. doi: 10.1002/eji.1830261123. [DOI] [PubMed] [Google Scholar]
- 20.Fowell D, Powrie F, Saoudi A, Seddon B, Heath V, Mason D. The role of subsets of CD4+ T cells in autoimmunity. Ciba Found Symp. 1995;195:173–82. doi: 10.1002/9780470514849.ch12. [DOI] [PubMed] [Google Scholar]
- 21.Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF a, anti-IL-1 a/b, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 22.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–55. [PubMed] [Google Scholar]
- 23.Gumanovskaya ML, Myers LK, Rosloniec EF, Stuart JM, Kang AH. Intravenous tolerization with type II collagen induces interleukin-4- and interleukin-10-producing CD4+ T cells. Immunology. 1999;97:466–73. doi: 10.1046/j.1365-2567.1999.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsfall AC, Butler DM, Marinova L, et al. Suppression of collagen-induced arthritis by continuous administration of IL-4. J Immunol. 1997;159:5687–96. [PubMed] [Google Scholar]
- 25.Lubberts E, Joosten LA, Chabaud M, et al. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest. 2000;105:1697–710. doi: 10.1172/JCI7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fowell D, Mason D. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–36. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. a/b-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujihashi K, Dohi T, Kweon MN, et al. γδ T cells regulate mucosally induced tolerance in a dose-dependent fashion. Int Immunol. 1999;11:1907–16. doi: 10.1093/intimm/11.12.1907. [DOI] [PubMed] [Google Scholar]
- 29.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–17. [PubMed] [Google Scholar]
- 30.Ohshima Y, Yang LP, Uchiyama T, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 31.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiba H, Miyahira Y, Atsuta M, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J Exp Med. 2000;191:375–80. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malmström V, Shipton D, Singh B, et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:6972–81. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 34.Mackay CR. Immunological memory. Adv Immunol. 1993;53:217–65. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- 35.Vitetta ES, Berton MT, Burger C, Kepron M, Lee WT, Yin XM. Memory B and T cells. Ann Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- 36.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–7. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 37.Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 38.Mattsson L, Larsson P, Erlandsson Harris H, Klareskog L, Harris RA. Parasite-mediated down-regulation of collagen-induced arthritis (CIA) in DA rats. Clin Exp Immunol. 2000;122:477–83. doi: 10.1046/j.1365-2249.2000.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


