Abstract
We previously demonstrated that high levels of IL-6/sIL-6R complexes are present in sera of patients with systemic juvenile idiopathic arthritis (s-JIA) and that the amount of IL-6 estimated in the IL-6/sIL-6R complexes is markedly higher than that measured by the B9 assay. Here, we show that two additional bioassays, employing human myeloma XG-1 cells and human hepatoma Hep3B cells, detected serum IL-6 levels similar to those measured by the B9 assay and approximately 10-fold lower than the IL-6 levels estimated to be present in the IL-6/sIL-6R complex. Using an assay for the measurement of the amount of circulating IL-6 complexed with the sIL-6R and available for binding to gp130 (gp130 binding activity), we show that the IL-6/gp130 binding activity is similar to that detected by the bioassays and again significantly lower than that estimated to be present in the IL-6/sIL-6R complex. Addition of recombinant human IL-6 (rhIL-6) to sera of patients or controls results in a markedly lower increase in the gp130 binding activity in patients than in controls. Moreover, sera from s-JIA patients inhibited in a dose dependent manner the gp130 binding activity assay. These results show that sera from patients with s-JIA contain a factor, or factors, that inhibit(s) the binding of the IL-6/sIL-6R complex to gp130. This inhibitory activity does not appear to be due to soluble gp130, C-reactive protein or autoantibodies to IL-6.
Keywords: interleukin-6, IL-6/, sIL-6R complex, gp130
Introduction
IL-6 is a pleiotropic cytokine with multiple biological activities relevant to the induction and regulation of the immune, inflammatory and haematopoietic responses, and has been implicated in several pathological conditions including inflammatory, autoimmune, and malignant diseases [1, 2, 3, 4, 5, 6, 7, 8]. IL-6 mediates its functions through two membrane proteins: the IL-6R, an 80-kD ligand binding receptor, and gp130, a 130-kD signal transducing element [9,10]. A soluble form of the IL-6R (sIL-6R), lacking the transmembrane and cytoplasmic regions, is present in serum in ng/ml amounts. Upon binding with IL-6 the sIL-6R is able to associate to gp130 and to mediate intracellular signalling, in a manner similar to the membrane IL-6R. Several studies have shown that addition of sIL-6R potentiates responses to IL-6 in vitro and in vivo[11]. While gp130 expression is practically ubiquitous, IL-6R expression appears to be limited to certain cell types. However, the lack of a membrane IL-6R may be completely compensated by the presence of sIL-6R. Examples of this phenomenon, for which the term trans-signalling has been proposed [12], include osteoclast activation [13], synovial fibroblast proliferation [14], CD34 + stem cell proliferation and erythropoiesis [15], endothelial cell chemokine production [16], and vascular smooth muscle cell proliferation [17].
We have previously shown that IL-6/sIL-6R complex can be demonstrated in sera of patients with systemic juvenile idiopathic arthritis (s-JIA) [18], a disease characterized by elevated serum levels of IL-6, that appear to explain several clinical and laboratory features of the disease [19]. When we compared the IL-6 levels measured by the assay employing the murine hybridoma B9, and those estimated in the IL-6/sIL-6R, we found a 10 fold discrepancy with higher amounts of IL-6 measured in the circulating IL-6/sIL-6R complex [18]. Other authors have shown that high serum levels of IL-6, with no or little bioactivity on B9 cells, can be detected by particular immunoassays in high molecular weight complexes ranging from 80 to 600 kD [20–22]. Immunoreactivity and B9 bioactivity of this IL-6 appeared to vary according to the size of the complexes and to the origin of the samples [20–22]. These observations suggest a complex regulation of IL-6 transport and bioavailability in human serum. Experiments by other groups showed that, in addition to the sIL-6R, soluble gp130 (sgp130), C-reactive protein (CRP) and immunoglobulins were among the IL-6-associated proteins in human blood [20–23]. Soluble gp130 has indeed been shown to inhibit IL-6 bioacitivities in vitro[24,25]. Autoantibodies to IL-6 have been found in normal human sera and in patients with rheumatic diseases [26,27]. However, their role on IL-6 bioavailability is yet unclear. On one hand, they have been found to be able to inhibit binding of IL-6 to its cell surface receptors [26]. On the other, they may function as chaperones, being able to release bioactive IL-6 to the surface receptors [22].
In this study, by means of a newly developed assay for the measurement of the serum IL-6 that is both complexed with the sIL-6R and available for binding to gp130, we show that the great part of the IL-6/sIL-6R complex present in s-JIA sera is not able to bind gp130. Moreover, the great part of the IL-6/sIL-6R complex is biologically inactive, not only on murine B9 cells, but also in two additional bioassays employing the human myeloma cells XG-1 and by the human hepatoma cells Hep 3B. In addition, s-JIA sera from patients with s-JIA inhibit in a dose-dependent manner the binding of the IL-6/sIL-6R complex to gp130. These results show the presence in s-JIA sera of factor(s) inhibiting IL-6 bioactivities through interference with the binding of the IL-6/sIL-6R complex to gp130. This inhibition does not appear to be secondary to soluble gp130, autoantibodies to IL-6, or CRP.
Materials And Methods
Patients
Serum was obtained from patients with systemic JIA (mean age 8·5 year, range 3–17 year), according to the ILAR diagnostic criteria [28], and from healthy controls (mean age 9·2 year, range 4–17 year), hospitalized for minor surgical procedures or bone marrow donation. Permission to draw extra blood during routine venipuncture was obtained from the parents of all children. The study was approved by the Ethical and Scientific Committee of the IRCCS Policlinico San Matteo where the patients were followed. Samples were obtained from patients with active disease, defined by the presence of synovitis on examination. All patients were receiving nonsteroidal anti-inflammatory drugs; in addition approximately 2/3 of them were receiving low-dose weekly methotrexate (mean dose 12·3 mg/m2/week), and half was receiving oral prednisone (mean dose 0·32 mg/kg/day) on alternate day regimen in the majority of them. Patients receiving high-dose methylprednosolone in i.v. bolus were not included in the study. Blood was allowed to clot for 2 h at room temperature and centrifuged at 1500 × g for 10 min Sera were stored at −70°C until used.
Biological assays for IL-6
Serum IL-6 levels were measured using 3 bioassays [1]. An hybridoma growth factor (HGF) assay employing the murine hybridoma cell line B9, as described [29]. CHO cell-derived recombinant human IL-6 (rhIL-6) (Genzyme Corp., Cambridge, MA, USA) was used as a standard in the assay. The amount of IL-6 required to achieved half-maximal proliferation of B9 cells in each assay was defined as 1 HGF Unit, corresponding to approximately 4·5 pg/ml of rhIL-6. Serial dilutions of serum samples were tested in triplicates and the dilution corresponding to half-maximal proliferation of the B9 cells was extrapolated from the standard curve, and used to calculate the concentration of IL-6 in the sample [2]. The human myeloma cell line XG-1 was used as described by Zhang et al. [30]. XG-1 cells (6000/well) were cultured for 7 days in the presence of increasing concentrations of rhIL-6 or of serial dilutions of heat-inactivated (56°C for 30 min) serum tested in triplicates. 3H-thymidine incorporation, after a 4-h pulse (1 µCi/well), was measured using standard procedures. The concentration of IL-6 was calculated as described above for the B9 assay [3]. A colourimetric assay based on the production of a secreted form of alkaline phosphatase (SEAP) in human hepatoma cells Hep3B, as described [31]. Cells were transfected with a fusion between the promoter of the human C-reactive protein gene and the coding region for a secreted form of alkaline phosphatase (AP). 1 × 105 transfected cells were plated in 24-well plates in DMEM, 10% heat inactivated FCS, 2 mm l-glutamine, 50 mg/ml gentamicine (complete medium), and after adherence, cells were washed with PBS and incubated for 72 h in complete medium supplemented with 500 U/ml of interleukin-1β, in the presence of increasing concentrations of rhIL-6 or serial dilutions of serum in triplicates. The amount of AP in the medium was quantified by recording the A405 of the cell culture supernatants after addition of the AP substrate p-nitrophenylphospate. The amount of IL-6 present in each sample was calculated as described above.
Immunoassay for the detection of the IL-6/sIL-6R complex
The levels of the IL-6/sIL-6R complex were measured, as described [18]. Briefly, each serum, diluted 1:1 in PBS, was added to 96 well plates, previously coated with the monoclonal antibody (mAb) 34·1 to human IL-6 (kindly provided by Interpharm Laboratories, Nes-Ziona, Israel), and incubated for 2 h at 37°C. The AP-conjugated mAb 22·1 to human sIL-6R was added (final concentration 1·3 µg/ml) and the plates were incubated for 1 h at 37°C. After washing the plates, the substrate p-NPP was added, and the A405 recorded. A standard curve was obtained by adding increasing concentrations of rhIL-6 to a reference serum from a healthy adult control, as previously described [18]. This curve was used to extrapolate the amount of IL-6 complexed to the sIL-6R, as previously reported [18].
Assay for the IL-6/gp130 binding activity
In order to evaluate the amount of IL-6/sIL-6R complex available for the binding to gp130, we developed an immunoassay based on the use of mAb 22·1 to IL-6R for capture and a recombinant human soluble gp130 tagged at the C-terminus with the FLAG epitope (gp130FLAG) for detection. gp130FLAG was obtained as previously described [32]. The mAb 22·1 was chosen because it has been previously shown not to inhibit the binding of rIL-6 to human natural sIL-6R [33] and because it did not affect proliferative responses of XG-1 cells to human IL-6 (data not shown). 96-wells polystyrene plates (EIA/RIA Costar, NY, USA) were coated overnight with mAb 22·1 to the sIL-6R (10 µg/ml in carbonate buffer, pH 9·6). The nonspecific binding sites were blocked with a 2-h incubation at 37°C with PBS, 2% BSA. Mixtures of rhsIL-6R (100 ng/ml), obtained as described[34], and increasing concentrations of rhIL-6 or serum samples were pre-incubated 60 min at 37°C with 250 ng/ml of gp130FLAG, diluted 1:1 (v/v) in PBS, pH 7·4, added to the plates and incubated for 2 h at 37°C. Following this incubation, plates were not washed. We chose not to wash the plates because preliminary experiments with rIL-6 and rsIL-6R, as well as with human serum, showed that washing the plates resulted in marked decrease in the optical density. This was conceivably due to the prompt dissociation of the IL-6/IL-6R/gp130FLAG complex during the subsequent incubations at 37°C. Therefore, following the 2 h incubation, 20 µl/well of a preformed mixture of the biotinylated mAb M2 to the FLAG epitope (Eastman Kodak Company, New Haven, CT, USA) (final concentration 2 µg/ml) and AP–conjugated streptavidin (Boheringer-Mannheim) (final dilution 1:1000) were directly added and plates incubated for 1 h at 37°C. In order to remove unbound AP–conjugated streptavidin, plates were then washed 3 times with PBS, pH 7·4. In order to minimize dissociations of the complex, and subsequent loss of O.D., washes were performed with ice-cold PBS, that was immediately removed from the plates. The AP substrate p-NPP was added and the A405 recorded. The results are expressed as specific optical density obtained by subtracting the optical density obtained in wells coated with PBS alone to the total optical density observed in wells coated with the anti-IL-6R monoclonal antibody. The concentration of IL-6, present in the IL-6/sIL-6R complex and available for binding to gp130, was extrapolated from a standard curve obtained by adding increasing concentrations of rhIL-6 to a reference serum of a healthy adult control. This serum had 100 ng/ml of sIL-6R and contained neither detectable IL-6 in the B9 assay, nor detectable IL-6/sIL-6R complex (specific OD 0·000). In order to obtain acceptable resolution over a wide range of concentrations of IL-6, the A405 was recorded, without stopping the reaction, after different times of incubation with the substrate (see Results and Fig. 1).
Fig. 1.
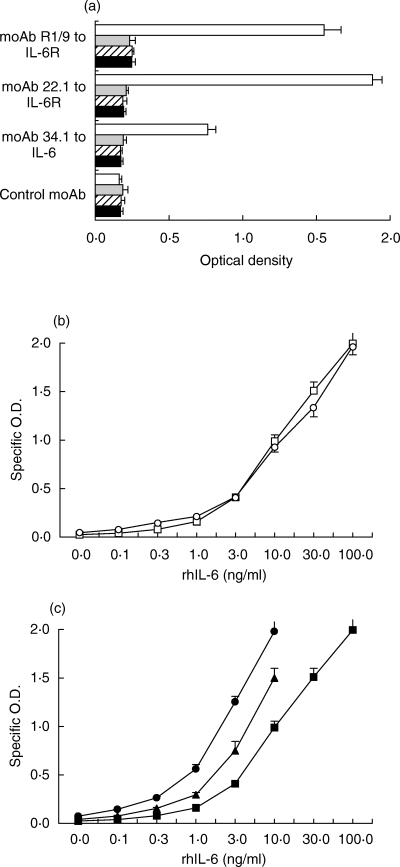
Assay for the IL-6/gp130 binding activity. (a) Efficacy of two different mAbs to the sIL-6R (22·1 and R1/9) for capture in the evaluation of the IL-6/gp130 binding activity. Wells were incubated at 37°C for 1 hour with buffer alone (j) or with soluble gp130FLAG (250 ng/ml), was incubated at 37°C for 1 h in the absence ( ) or in the presence of rhsIL-6R alone (100 ng/ml,
) or in the presence of rhsIL-6R alone (100 ng/ml,  ;), or of rhsIL-6R (100 ng/ml) and rhIL-6 (3 ng/m)(h) and then added to wells coated with the indicated mAb. j None. The IL-6/sIL-6R complex bound to gp130FLAG was revealed with a mAb to the FLAG epitope. Results are shown as total O.D., as means + SE, represented by the horizontal bars, of triplicate determinations. (b) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by adding increasing concentrations of rhIL-6 to rhsIL-6R (100 ng/ml) (
;), or of rhsIL-6R (100 ng/ml) and rhIL-6 (3 ng/m)(h) and then added to wells coated with the indicated mAb. j None. The IL-6/sIL-6R complex bound to gp130FLAG was revealed with a mAb to the FLAG epitope. Results are shown as total O.D., as means + SE, represented by the horizontal bars, of triplicate determinations. (b) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by adding increasing concentrations of rhIL-6 to rhsIL-6R (100 ng/ml) ( ) or to a normal human serum (s) in the presence of gp130FLAG (250 ng/ml). Reaction mixtures were incubated at 37°C for 1 h and then tested, as described in the methods section, in the assay for IL-6/gp130 activity using the mAb 22·1 to the sIL-6R for capture. Results are shown as specific O.D., as means + SE, represented by the vertical bars, of triplicate determinations. (c) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by reading the A405 after a 30 minute (j), a 2-h (▴) and a 6-h (d) incubation with the substrate p-NPP. Results are shown as specific O.D., as means and SE, represented by the vertical bars, of triplicate determinations.
) or to a normal human serum (s) in the presence of gp130FLAG (250 ng/ml). Reaction mixtures were incubated at 37°C for 1 h and then tested, as described in the methods section, in the assay for IL-6/gp130 activity using the mAb 22·1 to the sIL-6R for capture. Results are shown as specific O.D., as means + SE, represented by the vertical bars, of triplicate determinations. (c) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by reading the A405 after a 30 minute (j), a 2-h (▴) and a 6-h (d) incubation with the substrate p-NPP. Results are shown as specific O.D., as means and SE, represented by the vertical bars, of triplicate determinations.
Determination of serum levels of soluble gp130
Soluble gp130 levels were measured using a commercial immunoassay (R & D System, Minneapolis, MN, USA) according to the instructions provided by the manufacturer. Serum samples were tested at 1:100 dilution. The detection limit of the assay is 0·25 ng/ml.
Immunoprecipitation
20 µl of Protein A-Sepharose (Pharmacia, Uppsala, Sweden) were incubated overnight at 4°C with 5 µg of a rabbit polyclonal antihuman CRP (Calbiochem, San Diego, CA, USA) and with 5 µg of hCRP (Calbiochem) in 150 mm NaCl, 10 mm CaCl2, 2 mm MgCl2, pH 7·5 (CRP buffer). After washing with CRP buffer, 500 ng of rhIL-6 was added and incubated overnight at 4°C. After 3 washes with CRP buffer, the immunoprecipitate was dissolved in 15 µl of reducing loading buffer, electrophoresed in a 12% SDS/PAGE, and blotted on a PVDF membrane (Bio-Rad, Hercules, CA). The presence of IL-6 was revealed with the biotinylated antihIL-6 mAb 34·1.
Anti-IL-6 autoantibodies
125I-rIL6 (250 dpm/pg) and unlabelled rIL-6 (Amersham, Birkerød, Denmark) were chromatographed on Sephadex G-75 superfine (Pharmacia, Hillerød, Denmark) in order to maximize specific activity. AutoAb-IL-6 binding activity was assessed after incubating 125I-rIL6400 pg/ml in diluted serum 10% (v/v), 1 h at room temperature followed by an overnight incubation at 4°C and judged as the amount of 125I-rIL6 present in high molecular weight fractions after molecular size chromatography on Sephadex G-75. Saturable binding was further confirmed by affinity chromatography on protein-G Sepharose CL-4B (Pharmacia) as described [26]. In order to exclude interfering factors in the samples, a blood donor derived autoantibody to IL-6 at a 1:10·000 dilution (a dilution binding approximately 25% of the tracer) was added. Recovery of this antibody in all samples always exceeded 95%.
Statistical analysis
Data were analysed with Wilcoxon test for paired samples, Mann–Whitney U-test for unpaired samples, and Spearman's correlation test, as appropriate. A P-value <0·05 was considered statistically significant.
Results
Development of an assay for the detection of IL-6 available for binding gp130
Recombinant hIL-6 (rhIL-6) and rhsIL-6R were preincubated in the presence of a recombinant soluble gp130 tagged at the C-terminus with the FLAG epitope (gp130FLAG). For capture of the IL-6/sIL-6R complex bound to gp130FLAG, we evaluated two different mAb to the hsIL-6R, which do not inhibit IL-6 binding to IL-6R, as well as biological effects of IL-6 in vitro[33, and not shown]. A mAb to the FLAG epitope was used to detect the IL-6/sIL-6R/gp130FLAG complex. An evident increase in optical density was observed when IL-6 and sIL-6R were added together with gp130FLAG, while no increase in optical density was observed when IL-6 was not included in the reaction mixture or when an irrelevant antibody was used for capture (Fig. 1a). These results are consistent with the ability of IL-6R to associate with gp130 only in the presence of IL-6. Because of the highest signal with mAb 22·1 to IL-6R for capture, this mAb was used in subsequent experiments. Addition of increasing concentrations of rhIL-6 to rhIL-6R or to a normal human serum resulted in similar dose–response curves (Fig. 1b). Dose–response curves of the reference serum obtained at different times of incubation with the substrate are shown in Fig. 1c. These results show that this assay detects the binding to gp130FLAG of the IL-6/sIL-6R complex, formed by either recombinant or natural sIL-6R, and therefore can be used to estimate the amount of serum IL-6 which is both complexed with the sIL-6R and available for binding to gp130.
IL-6/gp130 binding activity in sera from patients with s-JIA
Sera from healthy controls had little if any detectable IL-6/sIL-6R complex available for binding to gp130FLAG (specific optical density 0·012 ± 0·010), consistent with the absence of detectable levels of IL-6, as measured by the B9 cells, and of IL-6/sIL-6R complex in these samples. On the contrary, the majority of sera from patients with s-JIA showed measurable optical density in the assay (specific optical density 0·232 ± 0·337) with a highly significant difference from healthy controls (P < 0·001). The amount of IL-6 available for binding to gp130 (IL-6/gp130 binding activity) present in sera was extrapolated from a standard curve obtained by adding increasing concentrations of rhIL-6 to a reference control serum, as described in the method section. In sera from 22 patients with s-JIA, the IL-6/gp130 binding activity (1·63 ± 3·49 ng/ml) was similar to the amount of IL-6 measured by the B9 cells in the same samples (1·45 ± 3·47 ng/ml), and significantly lower (P < 0·001 by Wilkoxon matched pair test) than the levels of IL-6 estimated to be present in the circulating IL-6/sIL-6R complex (10·61 ± 14·97 ng/ml) (Fig. 2). Further supporting the strict relationship between the amount of IL-6 available for binding to gp130 and its biological activity, the amount of IL-6 estimated by the IL-6/gp130 binding activity assay was strictly correlated with the amount of IL-6 measured by the HGF assay (Rs = 0·777, P < 0·0001). These results show that a great portion of the serum IL-6/sIL-6R complex is not available for binding to gp130, therefore suggesting that it is not biologically active.
Fig. 2.

Comparison of the levels of IL-6 estimated by the B9 cell assay, the IL-6/gp130 binding activity assay and the immunoassay for the IL-6/sIL-6R complex in s-JIA sera.
Measurement of serum IL-6 levels with human cells
To confirm that the great portion of the circulating IL-6/sIL-6R was not biologically active, we measured serum IL-6 levels in representative samples with two additional bioassays employing: (a) the human XG-1 cell line which, as the B9 cell line, derives from the B cell lineage (b) an assay of acute phase protein production in the human hepatoma cells Hep3b. IL-6 levels measured with the XG-1 cells in a total of 8 sera (0·79 ± 1·24 ng/ml) were comparable with those measured with the B9 assay (0·98 ± 1·12 ng/ml) and with those estimated by the IL-6/gp130 binding activity assay (1·10 ± 1·17 ng/ml), but significantly lower (P = 0·01) than those estimated to be present in the IL-6/sIL-6R complex (11·48 ± 9·94 ng/ml) (Fig. 3a for 4 representative samples). Similar results were obtained in another set of samples when serum IL-6 levels estimated by the SEAP/CRP assay in Hep3B cells (0·38 ± 0·37 ng/ml) were compared with those obtained with B9 cells (0·40 ± 0·23 ng/ml), with the IL-6/gp130 binding activity assay (0·43 ± 0·42 ng/ml), and with the immunoassay for the IL-6/sIL-6R complex (3·02 ± 3·1 ng/ml) (Fig. 3b). These results show that the great part of the circulating IL-6/sIL-6R complex is not biologically active on cells of different species and of different tissue origin and, together with the results presented in the previous paragraph, suggest the presence of factor(s) interfering with the binding of the IL-6/sIL-6R complex to gp130.
Fig. 3.
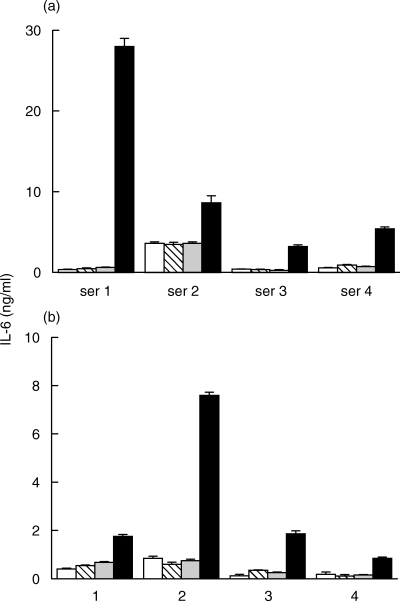
Comparison of the IL-6 levels estimated (a) by the human myeloma XG-1 cells (h) or (b)by the human hepatoma Hep 3b cells (h) with those estimated by the murine hybridoma B9 cells ( ), by the IL-6/gp130 binding activity assay (
), by the IL-6/gp130 binding activity assay ( ) and the immunoassay for the IL-6/sIL-6R complex (j) in representative sera from patients with s-JIA (4 out of 15 tested in the XG-1 assay and 4 out of 8 tested in the Hep 3b assay). Results are shown as means + SE, represented by the vertical bars, of triplicate determinations.
) and the immunoassay for the IL-6/sIL-6R complex (j) in representative sera from patients with s-JIA (4 out of 15 tested in the XG-1 assay and 4 out of 8 tested in the Hep 3b assay). Results are shown as means + SE, represented by the vertical bars, of triplicate determinations.
Sera from s-JIA patients inhibit the binding of the IL-6/sIL-6R complex to gp130
In order to verify the possible presence of factor(s) interfering with the binding of the IL-6/sIL-6R complex to gp130, control sera and sera from s-JIA patients were incubated in the presence of exogenous rhIL-6, thus allowing the formation of the IL-6/sIL-6R complex, and then the amount of IL-6/gp130 binding activity generated was evaluated. In order to obtain similar baseline conditions for the controls and s-JIA sera, this experiment was performed using s-JIA sera with a low endogenous IL-6/gp130 binding activity: baseline (i.e. in the absence of exogenous IL-6) specific optical densities were 0·041 ± 0·011 and 0·054 ± 0·024 for controls and s-JIA sera, respectively. Following the addition of 4 ng/ml of IL-6, the amount of IL-6/gp130 binding activity generated in s-JIA sera (1·65 ± 0·29 ng/ml) was significantly lower (P = 0·015) than that generated in control sera (4·28 ± 0·83 ng/ml) (Fig. 4a). Similar results were obtained following addition of 1 ng/ml of IL-6 (data not shown). We next addressed whether the addition of increasing amounts of sera from patients with s-JIA to a normal control serum incubated with exogenous rhIL-6 affected the IL-6/gp130 binding activity. Also in this experiment s-JIA sera with low endogenous IL-6/gp130 binding activity were used. As shown in Fig. 4b, the addition of increasing amount of sera from patients with s-JIA resulted in a dose-dependent inhibition of the IL-6/gp130 binding activity. The presence of sizeable inhibition at low percentages of s-JIA serum appears to rule out that the results reported in Fig. 4a might be secondary to low levels, or absence, of a factor, such as, for example, low free sIL-6R, necessary for the formation of the complex. All together, these results show that sera from patients with active s-JIA contain a factor, or factors, that inhibit the binding of the IL-6/sIL-6R complex to gp130.
Fig. 4.

s-JIA sera interfere with the binding of the IL-6/sIL-6R complex to gp130. (a) Amount of IL-6/gp130 binding activity generated in controls and s-JIA sera following exogenous addition of IL-6. Sera were preincubated with 4 ng/ml of recombinant human IL-6 and then tested in the IL-6/gp130 binding activity assay. Results are shown as means + SE, represented by the vertical bars, of triplicate determinations. The horizontal dotted line indicates the 4 ng/ml amount of IL-6 that was exogenously added. (b) Dose dependent inhibition of the IL-6/gp130 binding activity obtained adding increasing percentages of sera of patients with active s-JIA (s-JIA Pool) to a normal control serum incubated with 1 ng/ml of rhIL-6.
Lack of evidence that soluble gp130, autoantibodies to IL-6 or C-reactive protein represent the factors interfering with the binding to gp130
Since, as previously mentioned sgp130, immunoglobulins and CRP were among the IL-6-associated proteins in human serum, we investigated their potential role as the interfering factors. In agreement with a previous study [35], serum levels of sgp130 were found to be comparable between s-JIA patients (n = 20) and controls (n = 13) (363·5 ± 88·6 and 389·6 ± 57·7 ng/ml, respectively). (Fig. 5a). In individual s-JIA samples, the levels of sgp130 were not correlated with the degree of discrepancy between the assay for IL-6/sIL-6R complex and the assay for the the IL-6/gp130 binding activity. Moreover, antibodies to IL-6 or to the sIL-6R failed to coimmunoprecipitate sgp130 from s-JIA sera (not shown).
Fig. 5.

Serum levels of soluble gp130 (a) and autoantibodies to IL-6 (b) in patients with systemic JIA (s-JIA) (open bar in panel a) and in healthy controls (CTRL) (hatched bar in panel a). Sgp130 levels were measured by ELISA as described in the method section. For the determination of antibodies to IL-6, a reference Ig preparation with high levels of autoantibodies to hIL-6 (IgG anti-IL-6), or sera from patients with s-JIA (n = 11) and from controls (n = 8) were incubated with 125I-rIL-6 and then subjected to chromatography on Sephadex G-75 as described in the method section. Results are expressed as the ratio between bound and total added 125I-rIL-6. Results are shown as means + SD, represented by the vertical bars.
As shown in Fig. 5b, while a reference Ig preparation with high levels of autoantibodies to IL-6 was able to immunoprecipitate radiolabelled IL-6, none of the sera from s-JIA patients showed significant immunoprecipitation over background level. The absence of detectable autoantibodies to IL-6 is in agreement with the study from Keul et al. [35].
Markedly increased serum levels of CRP occurs in s-JIA. In this series of patients serum CRP levels ranged from 19 to 75 mg/l, while levels in controls were <3 mg/l. In coimmuneprecipitation experiments employing recombinant human IL-6 and purified human CRP, IL-6 was found only in the immunoprecipitate obtained in the presence of CRP (Fig. 6a). When IL-4 was used instead of IL-6, no immunoprecipitate was present (not shown). These results show that CRP is able to bind directly rhIL-6. However, addition of exogenous purified CRP to normal human serum did not interfere with generation of IL-6/gp130 binding activity (Fig. 6b) or with IL-6 activity on XG-1 cells (Fig. 6c). All together these findings appear to exclude that sgp130, autoantibodies to IL-6 or CRP could be the serum factors interfering with the binding of the IL-6/sIL-6R complex to gp130.
Fig. 6.
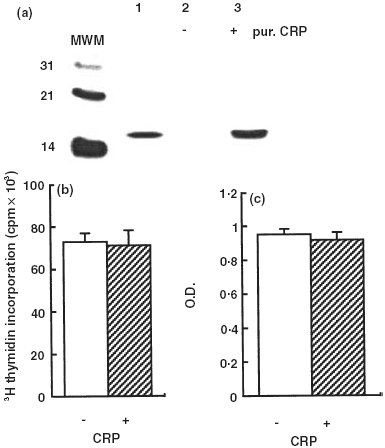
CRP binds IL-6 without interfering with its biological activity. (a) IL-6 is coimmunoprecipitated with CRP. Recombinant human IL-6 (500 ng) was incubated with an anti-CRP polyclonal antibody conjugated to Protein A-Sepharose in the absence (lane 2) or in the presence of 5 µg of human purified CRP (lane 3). Lane 1 shows recombinant human IL-6. The immunoprecipitates were subjected to SDS-PAGE and Western Blotting, and the presence of IL-6 was revealed with a mAb to hIL-6. MWM, molecular weight markers. (b) CRP does not affect the biological activity of IL-6 on XG-1 cells. A control human serum was preincubated with rhIL-6 (100 pg/ml) for 1 h at 37°C in the absence or in the presence of human purified CRP (100 µg/ml) and then tested on the XG-1 cells. Results are expressed as mean + SE, represent by the vertical bars, of triplicate determinations. (c) CRP does not affect the binding of the IL-6/sIL-6R complex to gp130. rhIL-6 (5 ng/ml) was added to a normal control serum in the absence or in the presence of exogenous CRP (100 µg/ml) and then tested in the IL-6/gp130 binding activity assay. Results are expressed as mean + SE, represent by the vertical bars, of triplicate determinations.
Discussion
We previously reported that sera from patients with s-JIA contain high levels of IL-6/sIL-6R complex and that the great portion of this complex is not biologically active in the B9 cell assay [18]. By means of two additional bioassays, employing XG-1 and Hep-3B cells, we confirm that the amount of biologically active IL-6 in s-JIA sera is approximately 10-fold lower than that detected in circulating IL-6/sIL-6R complex, therefore showing that this discrepancy is not dependent on the species or tissue origin of the cells employed for the measurement of serum IL-6. We also found that the great part of the IL-6 present in the IL-6/sIL-6R complex is not detected by a commercially available ELISA for IL-6 (data not shown).
Other reports showed important discrepancies in the estimation of serum IL-6 between immunoassays and bioassays and between different immunoassays [20, 21, 36]. These discrepancies might be due to the interference of other ligand(s) bound to IL-6 and/or to different recognition by the mAb employed of post-translational modifications of IL-6. Levels of IL-6 recovered after spiking were found to be comparable between several immunoassays when normal serum was used [37], while relevant discrepancies were reported when IL-6 was measured in plasma of patients with sepsis [36]. In addition, immunoreactivity and biological activity of the large amounts of circulating IL-6 found in high molecular weight complexes differed depending on the size of the complexes and on the origin of samples (i.e. normal controls, patients with psoriasis, acute infections, or tumours) [20–22]. All together these observations suggest a complex, and not yet clarified, regulation of the transport and of the biological activity of IL-6 in human blood, possibly through the associations with ligands, for the great part yet unidentified, that appear to interfere with its bioactivity and immunoreactivity. In our opinion, the presently available evidence suggests that great caution should be exercised when interpreting absolute serum IL-6 concentrations in human studies, but do not allow to conclude on which is the most adequate and relevant method to measure IL-6. In this respect, it is worth to note that we have previously found that serum IL-6 levels measured in the IL-6/sIL-6R complex correlated with CRP concentrations more strictly than the IL-6 levels measured with B9 cells [18]. This better correlation may be explained by hypothesizing that, while the production of IL-6 may fluctuate (as for example occurs during a febrile peak [29]), the in vivo levels of IL-6/sIL-6R complex, albeit biologically inactive for the great part, may be more constant over time and therefore better reflect the production of IL-6 in the preceding hours than the measurement of bioactive IL-6 at a given time point.
IL-6 activities are dependent on the ability of the IL-6/IL-6R complex (either membrane or soluble form) to bind gp130. We developed an assay for the evaluation of the binding of the IL-6/sIL-6R complex to gp130, that allows to estimate the amount of serum IL-6 which is both complexed with the sIL-6R and available for binding to gp130. In agreement with the presence of biologically active IL-6 in s-JIA patients [18,29], we found that sera from s-JIA patients contained IL-6/sIL-6R complex able to bind gp-130. However, the amount of IL-6 available for binding to gp130 was found to be comparable to that measured by the bioassays, but 10-fold lower than that estimated to be present in circulating IL-6/sIL-6R complex. We found that s-JIA sera interfered with the binding of the IL-6/sIL-6R complex to gp130, suggesting the presence of IL-6 inhibitor(s). Modulation of bioavailability and of biological response by antagonizing proteins (i.e. soluble cytokine receptors or natural receptor antagonists) is a common principle in cytokine biology. However, so far IL-6 appears to be a notable exception. The sIL-6R is not an antagonist, but rather a cocytokine potentiating IL-6 bioactivities and increasing its half-life [11]. Other observations suggest the presence of circulating factors interfering with the biological activity of IL-6. High levels of immunoreactive IL-6 with no or little biological activity on B9 cells were reported in patients with acute infections, psoriasis, or solid tumours. Observations from other authors have lead to the identification of some of the IL-6-associated proteins in human serum, including sgp130, CRP and immunoglobulins [20–23], therefore suggesting that these may be potential candidates as the factor(s) interfering with the binding of the IL-6/sIL-6R complex to gp130 in s-JIA sera, as well as in other pathological conditions.
As previously mentioned, sgp130 has been reported to inhibit IL-6 bioactivities in vitro[24,25]. Serum sgp130 levels were similar between s-JIA sera and control sera, the latter not showing inhibition of the binding of the IL-6/sIL-6R complex to gp130. It is also worth to note that inhibition of IL-6 bioactivity by sgp130 has been reported to occur at concentration higher than 100 ng/ml in in vitro systems depending on the presence of s-IL-6R, while only marginal inhibition occurred on cell lines that expressed membrane IL-6R [24,25], such as the three cell lines employed in our study. Further supporting the conclusion that sgp130 is not the serum factor interfering with binding of the IL-6/sIL-6R complex to gp130, we did not find any correlation between serum sg130 levels and the discrepancy between the IL-6/sIL-6R complex and the IL-6/gp130 binding activity. Moreover, antibodies to IL-6 or to the sIL-6R failed to coimmunoprecipitate sgp130 from s-JIA sera, therefore excluding that a significant portion of the IL-6/sIL-6R complex was associated with sgp130. A novel truncated form of sgp130, termed gp130-RAPS, has been recently identified, and shown to be present in human serum and to inhibit IL-6 bioactivity in vitro[38]. Further studies are necessary to establish whether gp130-RAPS is present in s-JIA sera, and can be detected by the available immunoassays for gp130.
Extending the observation by May et al. [20] that CRP was associated to IL-6 in serum, we found that rhIL-6 was coimmunoprecipitated with purified CRP, showing the ability of CRP to directly bind IL-6. This is in agreement with the well-known capacity of CRP to bind several proteins [39,40]. However, CRP was not able to interfere with the binding of the IL-6/sIL-6R complex to gp130, nor to inhibit IL-6 bioactivity on XG-1 cells, therefore ruling out the hypothesis that CRP is the serum factor interfering with IL-6 bioactivity.
Antibodies to IL-6 have been found in normal controls and at higher levels in systemic sclerosis and in rheumatoid arthritis [26, 27, 41]. In agreement with a recent study [35], we found that sera from s-JIA patients do not contain significant levels of antibodies to IL-6, ruling out antibodies to IL-6 as the serum factor that interferes with the binding to gp130 of the IL-6/sIL-6R complex. To the best of our knowledge autoantibodies to the IL-6R have never been described. Incidentally Western blotting analysis did not reveal the presence in s-JIA sera of immunoglobulins binding to the sIL-6R (not shown). Tanaka et al. [38] have recently described the presence in patients with rheumatoid arthritis of autoantibodies to the previously mentioned gp130-RAPS. However, these autoantibodies recognize an epitope which is present on gp130RAPS, but not on sgp130. Moreover, autoantibodies to gp130-RAPS behave as IL-6 agonist by neutralizing the IL-6 inhibitory effect of gp130-RAPS [38], therefore ruling out their possible role as IL-6 inhibitor. To the best of our knowledge, no other reports on the presence of autoantibodies to gp130 are present in the literature.
Potential candidates are also represented by other cytokines that use gp130 as a part of their receptor complex. Oncostatin M has been shown to inhibit, albeit at high concentrations, the in vitro formation of the ternary complex comprising IL-6, sIL-6R and gp130 [42]. No reports are available on a similar effect of leukaemia inhibitory factor (LIF) or IL-11. However, it is worth to note that, while other cytokines of the gp130 family, such as IL-11, LIF and Oncostatin M, are present in synovial fluids of patients with chronic arthritides, their levels are undetectable in serum [43,44]. Specifically regarding s-JIA, we found that serum LIF levels were undetectable (unpublished observations).
It has been recently reported that alternative splicing of the IL-6 mRNA leads to the generation of an IL-6 protein lacking exon-4. This protein has been shown to bind to the IL-6R, but not to gp130 [45]. Although further studies are necessary to determine whether this form of IL-6 lacking exon 4 is present in human serum, the preliminary available data on its functions make it a possible candidate as a factor able to interfere with binding to gp130 acting as a competitive antagonist of the full length cytokine.
In addition to s-JIA, increased production of IL-6 has been shown to play a pathogenic role in several human diseases including B cell neoplasia, osteoporosis and chronic inflammatory diseases [1, 2, 3, 4, 5, 6, 7, 8,46]. Therefore, neutralization of IL-6 represents an attractive therapeutic option. Administration of a single monoclonal antibody to IL-6 does not lead to efficient neutralization of its biological activities in vivo, due to the stabilization of IL-6 in monomeric complexes leading to accumulation of the cytokine in the circulation [47]. Monoclonal antibodies to the IL-6R or IL-6 receptor antagonists are the currently available experimental alternative therapeutic approaches [34,48]. A potential limitation to their use is represented by the possible immunogenicity of these nonself proteins that may hamper the long-term efficacy of these treatments. Together with previously mentioned observations from other groups, our results support the conclusion that a natural inhibitor of IL-6 is present in serum. Identification of this or these natural inhibitor(s) may not only provide a better knowledge of the regulation of the in vivo bioavailability of IL-6, but also a new therapeutic tool for its neutralization.
References
- 1.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 2.Hilbert DM, Kopf M, Mock BA, Köhler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasm. J Exp Med. 1995;182:243–8. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallek M, Leif Bergsagel P, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91:3–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interelukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74:1360–7. [PubMed] [Google Scholar]
- 5.Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, Kishimoto T, Yoshizaki K. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. [PubMed] [Google Scholar]
- 6.Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin-6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–87. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Taga T, Hibi M, Hirata Y, Yamasaki K, Yasukawa K, Matsuda T, Hirano T, Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer gp130. Cell. 1989;58:573–81. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–54. [PubMed] [Google Scholar]
- 11.Peters M, Jacobs S, Ehlers M, et al. The function of the soluble interleukin 6 (IL-6) receptor in vivo: sensitization of human soluble IL-6 receptor transgenic mice towards IL-6 and prolongation of the plasma half-life of IL-6. J Exp Med. 1996;183:1399–406. doi: 10.1084/jem.183.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300:281–90. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura T, Udagawa N, Takahashi N, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci USA. 1993;90:11924–8. doi: 10.1073/pnas.90.24.11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mihara M, Moriya Y, Kishimoto T, Ohsugi Y. Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor. Br J Rheumatol. 1995;34:321–5. doi: 10.1093/rheumatology/34.4.321. [DOI] [PubMed] [Google Scholar]
- 15.Tajima S, Tsuji K, Ebihara Y, et al. Analysis of interleukin 6 receptor and gp130 expressions and proliferative capability of human CD34+ cells. J Exp Med. 1996;184:1357–64. doi: 10.1084/jem.184.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6:315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 17.Klouche M, Bhakdi S, Hemmes M, Rose-John S. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J Immunol. 1999;163:4583–9. [PubMed] [Google Scholar]
- 18.De Benedetti F, Massa M, Pignatti P, Albani S, Novick D, Martini A. Serum soluble IL-6 receptor and IL-6/soluble IL-6 receptor complex in systemic juvenile rheumatoid arthritis. J Clin Invest. 1994;93:2114–9. doi: 10.1172/JCI117206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martini A, De Benedetti F. Is systemic juvenile rheumatoid arthritis an interleukin 6 mediated disease? J Rheumatol. 1998;25:203–7. [PubMed] [Google Scholar]
- 20.May LT, Viguet H, Kenney JS, Ida N, Allison AC, Sehgal PB. High levels of ‘complexed’ interleukin-6 in human blood. J Biol Chem. 1992;267:19698–704. [PubMed] [Google Scholar]
- 21.May LT, Patel K, Garcia D, Ndubuisi MI, Ferrone S, Mittelman A, Mackiewicz A, Sehgal PB. Sustained high levels of circulating chaperoned interleukin-6 after active specific cancer immunotherapy. Blood. 1994;84:1887–95. [PubMed] [Google Scholar]
- 22.Ndubuisi MI, Patel K, Rayanade RJ, Mittelman A, May LT, Sehgal PB. Distinct classes of chaperoned IL-6 in human blood: differential immunological and biological availability. J Immunol. 1998;160:494–501. [PubMed] [Google Scholar]
- 23.Jostock T, Mullberg J, Ozbek S, et al. Soluble gp130 is a natural inhibitor of soluble interleukin-6 receptor transsignalling response. Eur J Biochem. 2001;268:160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 24.Narazaki M, Yasukawa K, Saito T, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82:1120–6. [PubMed] [Google Scholar]
- 25.Jostock T, Mullberg J, Ozbek S, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transignalling responses. Eur J Biochem. 2001;268:160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 26.Hansen MB, Svenson M, Abell K, Yasukawa K, Diamant M, Bendtzen K. Influence of interleukin-6 (IL-6) autoantibodies on IL-6 binding to cellular receptors. Eur J Immunol. 1995;25:348–54. doi: 10.1002/eji.1830250207. [DOI] [PubMed] [Google Scholar]
- 27.Takemura H, Suzuki H, Yoshizaki K, Ogata A, Yuhara T, Akama T, Yamane K, Kashiwagi H. Anti-interleukin-6 autoantibodies in rheumatic diseases. Arthritis Rheum. 1992;35:940–3. doi: 10.1002/art.1780350814. [DOI] [PubMed] [Google Scholar]
- 28.Petty RE, Southwood TR, Baum J, et al. Revision of the proposed classification criteria for juvenile idiopathic arthritis. Durban 1997. J Rheumatol. 1998;25:1991–4. [PubMed] [Google Scholar]
- 29.De Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, Martini A. Correlation of serum interleukin 6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34:1158–63. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XG, Bataille R, Jourdan M, Saeland S, Banchereau J, Mannoni P, Klein B. Granulocyte-macrophage colony-stimulating factor synergizes with interleukin-6 in supporting the proliferation of human myeloma cells. Blood. 1990;76:2599–605. [PubMed] [Google Scholar]
- 31.Gregory B, Savino R, Ciliberto G. A fast and sensitive colorimetric assay for IL-6 in hepatoma cells based on the production of a secreted form of alkaline phosphatase (SEAP) J Immunol Meth. 1994;170:47–56. doi: 10.1016/0022-1759(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 32.Paonessa G, Graziani R, De Serio A, et al. Two distinct and independent sites on IL-6 trigger gp130 dimer formation and signaling. EMBO J. 1995;14:1942–51. doi: 10.1002/j.1460-2075.1995.tb07186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novick D, Engelmann H, Revel M, Leitner O, Rubinstein M. Monoclonal antibodies to the soluble human IL-6 receptor. affinity purification, ELISA, and inhibition of ligand binding. Hybridoma. 1991;10:137–46. doi: 10.1089/hyb.1991.10.137. [DOI] [PubMed] [Google Scholar]
- 34.Savino R, Ciapponi L, Lahm A, et al. Rational design of receptor super-antagonist of human interleukin-6. EMBO J. 1994;13:5863–70. doi: 10.1002/j.1460-2075.1994.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keul R, Heinrich PC, Muller-Newen G, Muller K, Woo P. A possible role for soluble IL-6 receptor in the pathogenesis of systemic onset juvenile chronic arthritis. Cytokine. 1998;10:729–34. doi: 10.1006/cyto.1997.0343. [DOI] [PubMed] [Google Scholar]
- 36.Ledur A, Fitting C, David B, Hamberger C, Cavaillon JM. Variable estimates of cytokine levels produced by commercial ELISA kits: results using international cytokine standards. J Immunol Meth. 1995;186:171–9. doi: 10.1016/0022-1759(95)00184-c. [DOI] [PubMed] [Google Scholar]
- 37.Krakauer T. Variability in the sensitivity of nine enzyme-linked immunosorbant assays (ELISAs) in the measurement of human interleukin 6. J Immunol Meth. 1998;219:161–7. doi: 10.1016/s0022-1759(98)00138-0. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Kishimura M, Ozaki S, Osakada F, Hashimoto H, Okubo M, Murakami M, Nakao K. Cloning of novel soluble gp130 and detection of its neutralizing autoantibodies in rheumatoid arthritis. J Clin Invest. 2000;106:137–44. doi: 10.1172/JCI7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepys MB, Booth SE, Tennent GA, Butler PJG, Williams DG. Binding of pentraxins to different nuclear structures: C-reactive protein binds to small nuclear ribonucleoprotein particles, serum amyloid P component binds to chromatin and nucleoli. Clin Exp Immunol. 1994;97:152–7. doi: 10.1111/j.1365-2249.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du Clos TW, Marnell L, Zlock LR, Burlingame RW. Analysis of the binding of C-reactive protein to chromatin subunits. J Immunol. 1991;146:1220–5. [PubMed] [Google Scholar]
- 41.Suzuki H, Takemura H, Yoshizaki K, et al. IL-6-anti-IL6 autoantibody complexes with IL-6 activity in sera from some patients with systemic sclerosis. J Immunol. 1994;152:935–42. [PubMed] [Google Scholar]
- 42.Sporeno E, Paonessa G, Salvati AL, Graziani R, Delmastro P, Giliberto G, Toniatti C. Oncostatin M binds directly to gp130 and behaves as an interleukin-6 antagonist on a cell line expressing gp30 but lacking functional oncostatin M receptors. J Biol Chem. 1994;269:10991–5. [PubMed] [Google Scholar]
- 43.Okamoto H, Yamamura M, Morita Y, Harada S, Makino H, Ota Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and Oncostatin-M in rheumatoid arthritis. Arthritis Rheum. 1997;40:1096–105. doi: 10.1002/art.1780400614. [DOI] [PubMed] [Google Scholar]
- 44.De Benedetti F, Pignatti P, Gerloni V, et al. Differences in synovial fluid cytokine levels between juvenile and adult rheumatoid arthritis. J Rheumatol. 1997;24:1403–9. [PubMed] [Google Scholar]
- 45.Bihl MP, Ruediger JJ, Leufgen H, Hermann MJ, Perruchoud AP, Roth M, Tamm M. IL-6. potential new function and regulation through alternative splicing. Eur Respir J. 2001;18(Suppl.):476s. [Google Scholar]
- 46.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 47.Lu YZ, Brailly H, Wijdenes J, Bataille R, Rossi JF, Klein B. Measurement of whole body interleukin-6 (IL-6) production: prediction of the efficacy of anti-IL-6 treatments. Blood. 1995;86:3123–7. [PubMed] [Google Scholar]
- 48.Sato K, Tsuchiya M, Saldanha J, Koishihara Y, Ohsugi Y, Kishimoto T, Bendig MM. Reshaping a human antibody to inhibit the interleukin 6 dependent tumor cell growth. Cancer Res. 1993;53:851–8. [PubMed] [Google Scholar]


