Abstract
Intravesical BCG therapy is effective in the treatment of superficial bladder cancer. Both clinical and experimental results suggest a role for cytokines and delayed-type hypersensitivity (DTH) in BCG-induced antitumour immunity. We characterized the modulatory effects of BCG on bladder cytokine expression and determined the relationship between DTH and BCG antitumour activity. The bladders of mice were instilled with BCG through a catheter. Bladder tissue RNA and urine were collected for evaluation of cytokine expression using reverse transcriptase-polymerase chain reaction (RT-PCR) and/or ELISA. IFN-γ and TNF-α, the two major cytokines associated with DTH, were efficiently induced by BCG. IL10, an important down-regulator of DTH, was also induced by BCG. Constitutive levels of IL4 and IL5 were observed, but neither IL4 nor IL5 were modulated by BCG. Similar results were observed in the kinetic analysis of urinary cytokines in patients after intravesical BCG therapy. Production of Th1 (T helper type 1) cytokines (IFN-γ, IL2 and IL12) preceded that of the Th2 (T helper type 2) cytokine IL10. A tendency toward higher ratios of IFN-γ versus IL10 for BCG responders also was observed. In animal studies the absence of IL10 abrogated either by antibody inhibition or the use of genetically modified, IL10 deficient (IL10–/–) mice resulted in enhanced DTH responses. Under conditions of enhanced DTH, a significant enhancement in antitumour activity was observed. These data demonstrate that DTH and its associated mononuclear infiltration and cytokine production are important to the antitumour activity of intravesical BCG therapy, and suggest that effects to diminish IL10 production may have therapeutic value.
Keywords: BCG, DTH, cytokine, antitumour immunity
Introduction
Mycobacterium bovis bacillus Calmette-Guérin (BCG) has been shown in prospective randomized trials to be the most effective treatment for superficial bladder cancer, and is considered by many to be the most successful clinical application of cancer immunotherapy. While the clinical effectiveness is well established, the immunological basis of BCG-induced antitumour activity remains poorly defined.
BCG therapy for superficial bladder cancer consists of the instillation of approximately 5 × 108 colony forming units (CFU) through a catheter into the lumen of the bladder once a week for a minimum of six consecutive weeks. BCG retention after instillation has been shown to be dependent on bacterial attachment to fibronectin [1,2]. Attachment was linked to matrix fibronectin within the bladder and also to epithelial cells and tumour cells via a fibronectin bridge [3,4]. Fibronectin-mediated BCG attachment was demonstrated to be a necessary event for the activation of immunity to BCG and for the induction of antitumour activity [5].
The immunological effector events associated with the BCG induced antitumour response are less well defined. Reports demonstrate that T lymphocytes are necessary for antitumour activity [6–8]. Both CD4+ and CD8+ T cells were required for the effective elimination of orthotopic bladder tumours after BCG treatment [6]. While T lymphocytes were necessary for antitumour activity, protective immunity to tumour associated antigens was not observed [6]. These data suggest that T lymphocytes reactive with BCG antigens may be important in the antitumour response.
Indirect evidence supporting a role for immunity to BCG in antitumour activity was obtained from peripheral blood lymphocytes (PBLs) of treated patients [9]. When exposed to BCG in vitro, patient PBLs expressing the markers CD8 and CD56 were activated to mediate lysis of bladder tumour cells. Activation of lytic activity required CD4+ T cells, antigen presenting cells, and the production of both IFN-γ and IL2. These BCG activated killer (BAK) cells were not lytic for the NK sensitive K562 cells [9,10]. In this regard studies on effector mechanisms of BCG-mediated antitumour activity showed that natural killer (NK) cells were not the primary effector cells [11]. Depletion of NK cells had no effect on the BCG induced antitumour response.
Clinical data consistent with an important role for a BCG directed T cell response have been reported. These studies revealed a significant correlation between cutaneous DTH reactivity to BCG antigens (purified protein derivative) and tumour free status [12]. Furthermore, retrospective analysis of factors associated with an effective clinical response showed a significant correlation between tumour free status and prior exposure to BCG [12]. Although only suggestive, these clinical data link BCG-induced immunity to antitumour activity and suggest that clinical antitumour responses are associated with a Th1 DTH response to BCG antigens.
Immunohistochemical characterization of post-BCG biopsy specimens and evaluation of the cytokines released in urine after BCG therapy support a role for a Th1 response in BCG therapy [13, 14, 15, 16, 17, 18]. Granulomatous inflammation was observed in most bladder biopsy specimens evaluated after BCG treatment [12]. The predominant T cell infiltrate in these biopsies was shown to be cells expressing the CD4 marker [19]. Characterization of cell surface markers on epithelial cells in post-BCG biopsies showed epithelial cell expression of HLA-DR and ICAM [18]. Cytokines including IFN-γ and IL2, which are consistent with a Th1 response, were observed in urine after BCG treatment [15–17]. Moreover, Th1 cytokines in urine specimens correlated with successful BCG-mediated antitumour activity [16]. Taken together these data suggest a link between DTH and BCG-induced antitumour activity. In this study we tested the hypothesis that the DTH response to BCG is important in BCG-mediated antitumour activity.
Materials And Methods
Bacteria
A lyophilized preparation of Pasteur Strain BCG was obtained from Armand Frappier (Quebec, Canada) and diluted in phosphate buffered saline (0·1 m, PBS) to designated doses of CFU for use in most experiments. A Pasteur strain of live BCG (MV261 BCG) that had been previously transfected with the kanamycin resistance plasmid was used for induction of urinary cytokines [20,21]. The latter was kept in log rate growth at 37°C in 7H9 Middlebrook broth (Difco, Detroit, MI, USA) containing 0·5% BSA and 0·05% Tween 80 (Sigma Chemicals, St. Louis, MO, USA) under conditions of continuous shaking. Log phase cultures of viable BCG were quantified using the absorbance at 600 nm (1 A600 unit = 2·5 × 107 CFU). Previous experiments had shown that responses to this BCG were very similar to those obtained using commercial lyophilized preparations.
Mice
Female C57BL/6 mice (6–8 weeks of age at the time of study initiation) were obtained from the National Cancer Institute. Mice were allowed free access to food and water.
Treatment regimen
Mice were anaesthetized with Nembutal (intraperitoneal, 0·05 µg/g animal weight). Anaesthetized mice underwent electrocautery and BCG (107 CFU) were instilled into the bladder via catheter in a volume of 0·1 ml of PBS as previously described [22]. The urethral openings were then temporarily ligated with 2–0 silk suture and the mice anaesthetized as needed to ensure a two-hour contact time of the BCG with the bladder epithelium. Bladders were harvested at 2 h, 24 h, and 7 days after the first BCG-instillation, and at 2 h and 24 h after each successive weekly treatment through five weeks. Groups of 5 mice were treated weekly in this manner for 5 weeks. Control groups were treated in a similar manner but only 0·1 ml of PBS was instilled into the bladder.
RNA isolation
Bladders were harvested, minced, and snap frozen in liquid nitrogen. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction, described by Chomczynski & Sacchi [23], was followed. Bladder tissue was homogenized in a 1·5-ml of denaturing solution D (4 m guanidinium thiocyanate; 25 mm sodium citrate, pH 7·0; 0·5% Sarcostyle; and 0·1 m 2-mercaptoethanol). The solution was vortexed for 10 s and cooled on ice for 10 min Supernatants were collected by centrifuged in a microfuge at 10 000 r.p.m. at 4°C for 2 min and phenol-chloroform-isoamyl alcohol extracted three times. RNA was precipitated at 4°C. The pellet was washed in 100% ETOH and resuspended on 0·2 ml water. RNA was stored at −70°C until needed.
RT-PCR
The reverse transcriptase (RT) reaction was carried out as outlined by Watson & Milbrandt [24]. 0·5 µg RNA was added to 200 ng of oligo DT17 in 11 µl and heated at 68°C for 5 min, then cooled to 27°C. The RT reaction mix consisted of 50 mm Tris-HCl (pH 8·3), 50 mm KCl, 10 mm MgCl2, 1 mm DTT, 10 µg/ml BSA, 1 mm dNTPs, 10 units RNAS, and 10 units AMV reverse transcriptase in 20 µl total volume, and was added to the RNA DT17 solution. Reactions were carried out at 42°C for 12 h. The RT reaction was diluted fivefold with H2O and boiled for 5 min The cDNA was stored at − 20°C for subsequent PCR analysis. The PCR reaction mixture contained 10 µl of 10× storage buffer A (Promega Corporation, Madison, WI, USA), 2·5 units of Taq DNA Polymerase (Promega), 200 µm dNTP, 1·5 mm MgCl2, 1 µm 5′ oligonucleotide primers, 1 µm 3′ oligonucleotide primers. Aliquots were denatured at 94°C for 2 min, and amplified for 30 cycles in a DNA Thermocycler (Perkin-Elmer Corp., Norwalk, CT). Each cycle consisted of denaturation at 94°C for 1 minute, annealing at 50°C for 1 minute and extension at 72° for 1 minute. The sequences of cytokine specific primer pairs, 5′ and 3′, were as follows:
PCR product analysis
Samples were analysed by Southern blotting. Gels were denatured in 1·5 m NaCl and 0·5 N NaOH for 45 min, then neutralized in 1 m TRIS and 1·5 m NaCl (PH 7·4) for 45 min Southern Transfer was carried out overnight (10–14 h) in a 20× SSC transfer buffer. The nitrocellulose filter was washed in 6× SSC for 5 min and dried for 30 min at 80°C in a vacuum oven. Prehybridization was carried out at 42°C for 1–2 h in 6× SSPE, 10× Denhardt’s, 0·5% SDS, 100 µg of herring sperm DNA (Promega), and 50% formamide. Hybridization was done overnight (10–14 h) with random primed DNA α-32P dCTP cytokine probes. Probed blots were washed with 6× SSPE/0·1% SDS for 15 min twice at 22°C, I× SSPE/0·5% SDS for 15 min two times at 37°C, and 0·1× SSPE/1·0% SDS for 30 min at 42°C. Exposures at −70°C were then made and the autoradiographs developed.
Bladder tissue IL10 ELISA
Bladders were harvested 24 h after BCG treatment, weighed and homogenized in PBS containing 0·1% Tween 20. The extract was sonicated and centrifuged for 5 min at 13 000 g. Supernatants were removed and measured for IL10 by an ELISA as previously described [25]. Briefly, a 50% ammonium sulphate precipitate of an ascetic preparation of anti-IL10 IgG1 capture antibody (JES5·2A5, DNAX, Inc., Palo Alto, CA, USA) was prepared. The antibody was diluted in carbonate buffer, pH 9·0, and added to microtiter wells at a concentration of 5 µg/well and incubated at 4°C overnight. Plates were washed with PBS (0·1 m, pH 7·0) containing 0·1% Tween 20. Wells were blocked with PBS containing 1% bovine serum albumen (BSA) for 2 h at room temperature. Plates were washed and bladder extracts and recombinant IL10 standards were added and incubated overnight at 4°C. Plates were washed and IL10 was detected with a biotinylated anti-IL10 (1·0 µg/ml, IgM, SXC-1; DNAX, Inc.). Wells were developed with a 1 : 500 dilution of avidin-alkaline phosphatase (Sigma Chemicals). Plates were read on an ELIAS plate reader at OD 405 nm.
Urine collection and urinary cytokine ELISA
Mouse urine was collected by placing mice in metabolic cages for 15 h after BCG treatment. Urine was collected in a recovery tube containing 0·1 ml/mouse of a 10-fold urine stabilizer solution (2 m Tris-HCl; pH 7·6, 5% BSA, 0·1% sodium azide, plus 1/2 COMPLETE protease inhibitor tablet (Roche Molecular Biochemicals, Indianapolis, IN, USA)). Mouse produced an average of 0·5–1·0 ml of urine/mouse during this time. After removed of any solid debris, the urine was stored at −70°C until batch ELISA cytokine measurements were performed.
Human urine was collected from patients with superficial bladder cancer in accordance with the approved institutional review board guidelines. Urine was stabilized before freezing by the addition of a 10-fold concentrated buffer containing 2 m Tris-HCl (pH 7·6), 5% BSA, 0·1% sodium azide, and the following protease inhibitors (Sigma): aprotinin, pepstatin, leupeptin at 0·01 mg/ml, and 4-(2-aminoethyl) benzenesulphonyl fluoride (AEBSF) at 0·1 mg/ml. Samples were routinely stored at −70°C before batch analysis for cytokines by ELISA.
Paired mouse and human antibodies were obtained from Endogen (Cambridge, MA, USA) for IFN-γ and from PharMingen (San Diego, CA, USA) for TNF-α and IL10. ELISAs were performed in a sandwich format according to the manufacturer's instructions. Cytokine concentrations were calculated in standard mass/volume format using standard curves derived from purified recombinant cytokines.
Measurement of DTH in mouse footpads
Mice were immunized to BCG by subcutaneous injection of 1·0 mg heat killed BCG 7 days prior to testing. DTH was measured by injection of 5 µg PPD (Aventis Pasteur, Swift Water, PA, USA) in the hind footpad. The opposite footpad was injected with the PPD diluent, PBS and used as a control. Footpad thickness was measured with a dial gauge caliper at the times indicated in the figures. In experiments where anti-IL10 was administered, anti-IL10 (JES5·2A5) treatment was initiated 2 days prior to challenge and continued throughout the experiment. JES5·2A5 was injected intraperitoneally at a concentration of 200 µg/mouse. Neutralization of IL10 was confirmed by ELISA.
Antitumour effects of BCG
Mice were immunized against BCG as described for the DTH experiments above. Seven days later mice were anaesthetized, catheterized and bladders were cauterized as described above. Immediately after cautery 5 × 104 MB49 bladder tumour cells were instilled into the bladder as previously described [22,26]. Twenty-four hours later BCG (1 × 106 or 1 × 107 CFU) were instilled into the bladder via a catheter. Mice were subsequently treated at weekly intervals as described above. Two days prior to tumour implantation anti-IL10 and isotype antibody control treatment were initiated as described above for the determination of the effects of anti-IL10 on DTH. Group size for all therapy studies was 10 mice/group.
Results
Cytokine induction by intravesical BCG administration
Initial experiments were performed to characterize the cytokine profile induced by intravesical instillation of BCG in the bladders of naive mice and mice immunized with either subcutaneous or intravesical BCG. Bladders were removed at the time intervals shown, snap frozen, and assessed for cytokine mRNA by RT-PCR or proteins by ELISA. Both naive and immune mice expressed equivalent levels of IL4 and IL5 mRNA (Fig. 1a). These data suggest that both IL4 and IL5 are constitutively expressed in the bladder of adult mice, and that neither were significantly modulated by BCG. IFN-γ, a major Th1 cytokine, was not observed in naïve mice treated with diluent, but was present 24 h after BCG treatment (Fig. 1b). In immune mice no IFN-γ mRNA was observed in the absence of BCG stimulation but consistent with previous reports on contact sensitivity, IFN-γ, was substantially elevated at the both 2 and 24 h time points after BCG treatment [27]. The expression of TNF-α was similar to that of IFN-γ except that TNF-α was observed in the absence of BCG stimulation in immune mice (Fig. 1b). Consistently, urine of naïve mice showed a gradually increased pattern of IFN-γ following intravesical BCG treatments (Fig. 2). Kinetic analysis of clinical patients’ voided urine collected after intravesical BCG therapy likewise demonstrated a precedence of Th1 cytokine production (IFN-γ, IL2 and IL12) to cytokine production (IL10) often linked to a Th2 response (Fig. 3). This process was accelerated in patient number 2, who had previously been exposed to tuberculosis. A tendency of a higher ratio of IFN-γ versus IL10 was also observed for BCG responders (Fig. 4). These data reveal that BCG immunization via the bladder mucosa is consistent with activation of a Th1 response as has been previously described for other immunization routes [28]. Moreover, these data support the hypothesis that BCG-induced DTH may be associated with antitumour activity as has been previously implied by correlative clinical data [12].
Fig. 1.
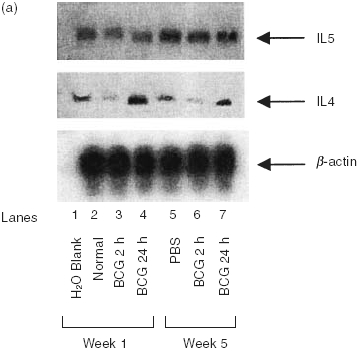
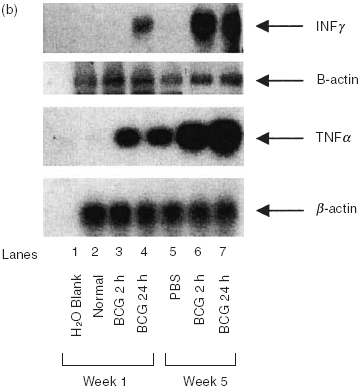
Semi-quantitative RT-PCR for cytokines in bladder tissue treated with BCG. BCG (107 cfu) were instilled via catheter into the bladder and retained for 2 h (see Materials and methods). Bladders were obtained 24 h later. RNA was isolated, reverse transcribed, and specific cytokine primers, as outlined in Materials and methods, were used for amplification. (a) Analysis of Type II cytokine profiles in bladder tissue. (b) Analysis of Type I cytokine profiles in bladder tissue. Bladders from 5 mice were combined for RNA isolation. Each experiment was performed a minimum of 3 times.
Fig. 2.
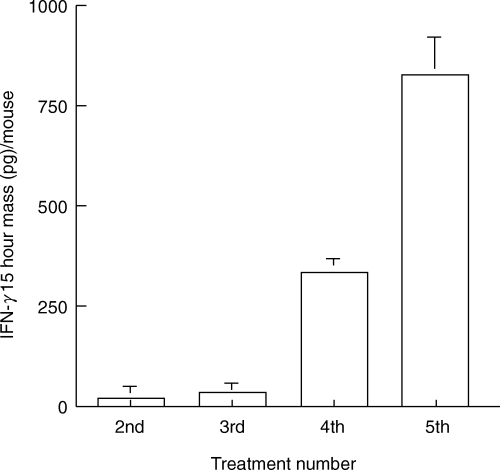
Urinary IFN-γ production after intravesical BCG treatment in mice. C57BL/6 mice were treated intravesically every other day with 2·5 × 106 CFU/dose of MV261 BCG. After each treatment, urine was collected and urinary IFN-γ mass per mouse for the 15-h collection was recorded. N= 2–4 mice per time point. Values represent the mean ± SD from two independent determinations.
Fig. 3.
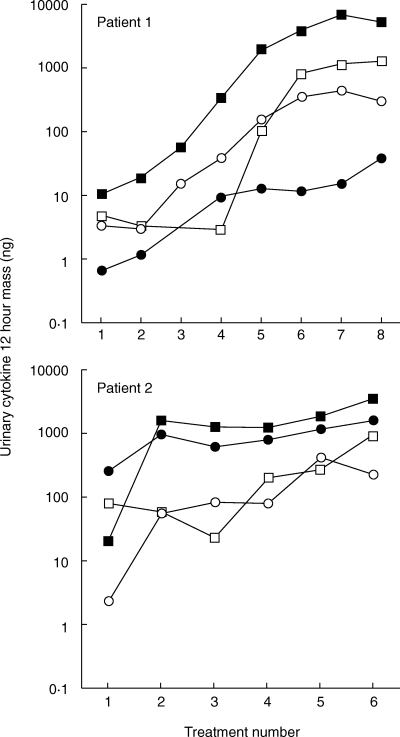
Urinary cytokine production in two representative bladder cancer patients during induction course of intravesical BCG therapy. Patients were treated with BCG once a week (8 treatments for patient 1 and 6 treatments for patient 2), and urine was collected for the first 12 h after each BCG treatment and assayed for cytokines IFN-γ (▪), IL12 (•), IL2 (○) and IL10 (□).
Fig. 4.
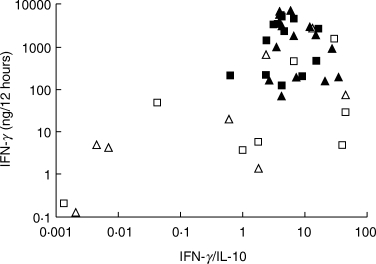
Urinary cytokine results for two 12-h collections per patient after the final (□,▪) or next to final (▵,▴) BCG instillations. Fourteen patients were complete clinical responders (▪,▴) and eight failed BCG with recurrent disease within 12 months of completing treatment (□,▵).
Enhancement of DTH response to BCG by abrogating IL10
In order to evaluate the role of DTH in BCG-induced antitumour activity, experiments were designed to determine the effects of modulating DTH on the antitumour response. Previous studies showed that IL10 is an important regulator of DTH [25]. Since the patients’ urine cytokines suggest an inverse relationship between IFN-γ and IL10, abrogation of IL10 may provide an approach to modulating BCG-induced DTH in the bladder. To test this hypothesis, we initiated experiments to determine the effects of IL10 neutralization on BCG-induced antitumour activity. First, mice were treated with anti-IL10 neutralizing antibody (GES 2·4) to determine whether BCG-induced IL10 could be neutralized in bladder tissue. Mice were immunized with BCG and 7 days later challenged with BCG intravesically. Two days prior to BCG challenge, mice were treated with anti-IL10 or control antibody [25]. Anti-IL10 treatment efficiently reduced IL10 to background levels (Fig. 5). Next, we determined the effects of anti-IL10 on DTH using the mouse footpad challenge model. Mice were immunized with BCG and 7 days later challenged with 5 µg purified protein derivative (PPD). The data show that treatment with anti-IL10 significantly prolonged the DTH response to PPD as measured by footpad swelling (Fig. 6a). Similar studies also were performed in genetically modified mice lacking IL10 (IL10–/–). As expected, the DTH response in the IL10 null mice was enhanced (Fig. 6b). In the IL10–/– mice both the degree of footpad swelling and the duration of the response were increased. Histological assessment of footpads showed mononuclear infiltration consistent with DTH as previously described (data not shown) [25].
Fig. 5.
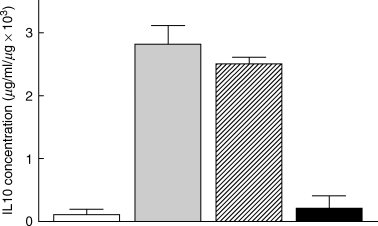
Neutralization of bladder IL10 after BCG treatment. Mice were treated with anti-IL10 or control antibody 48 h prior to BCG instillation. Twenty-four hours after BCG treatment, bladders were collected, weighed and homogenized. The extract was assayed for IL10. Three mice were assayed in each group. The experiment was performed twice. □ Diluent,  BCG,
BCG,  Isotype control, ▪ Anti-IL 10.
Isotype control, ▪ Anti-IL 10.
Fig. 6.
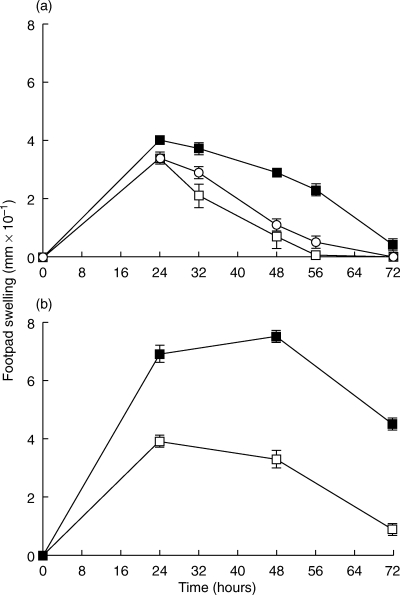
Effect of IL10 on delayed type hypersensitivity (DTH). Mice were immunized with BCG 7 days prior to challenge. (a) Effect of anti-IL10 on DTH. Anti-IL10 treatment was initiated 2 days before footpad challenge with 5 µg purified protein derivative (PPD); ○ Untreated, □ Isotype control, ▪ Anti-IL10. (b) DTH response in IL10–/– mice. Each group contained 3 mice and each experiment was performed at least twice. Data are reported as the mean of 3 mice from a representative experiment. □ Heterozygote, ▪ IL10 knockout.
Further studies were performed to determine whether IL10 modulated DTH in the bladder after BCG treatment. DTH was monitored by histological assessment of mononuclear infiltration 24 h after treatment in naive and BCG immune mice. Inflammation was compared in mice receiving BCG after subcutaneous immunization 7 days before challenge or after 5 weekly treatments. The data show a strong DTH response in mice immune to BCG, with the inflammation in IL10–/– mice extending into the muscle and perivesical fat (Table 1, Fig. 7). Minimal to no inflammation was observed in diluent-treated bladder specimens or bladder specimens from naive normal or IL10 null mice treated with BCG (Table 1, Fig. 7). Inflammation was similar in bladder specimens from mice immunized subcutaneously and challenged by a single BCG instillation 7 days later and mice receiving 5 weekly instillations of BCG.
Table 1.
Bladder inflammation induced by BCG treatment in normal C57BL/6 and IL10–/– mice*
| Normal C57BL/6 Mice | IL10–/– Mice | |||||||
|---|---|---|---|---|---|---|---|---|
| Sequential intravesical BCG† | Single intravesical BCG‡ | Sequential intravesical BCG† | Single intravesical BCG‡ | |||||
| Treatment | Naive | Immune | Naive | Immune | Naive | Immune | Naive | Immune |
| PBS | 0 | 0 | 0 | 0 | + | + | 0 | + |
| BCG | NT | + + | + | + + | NT | + + + | + | + + + |
Scoring system: 0 no inflammation, + mild inflammation, + + moderate inflammation, + + + severe mononuclear inflammation.
Mice were treated weekly for 5 weeks with 107 CFU BCG or PBS. Sequential treatment with BCG primes mice; thus BCG treatment of naive mice after the 5th treatment was not tested (NT).
Mice were immunized with BCG 7 days prior to intravesical treatment.
Fig. 7.
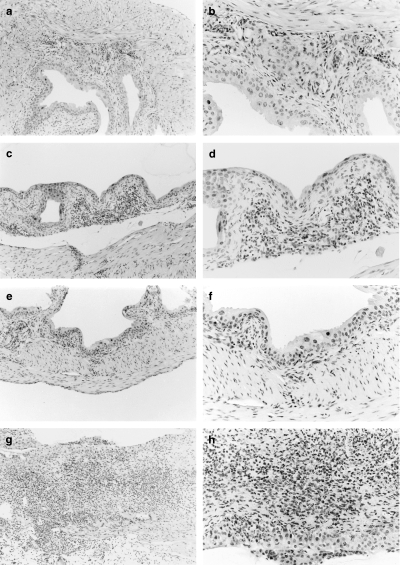
Histological evaluation of delayed type hypersensitivity (DTH) response in bladders of C57BL/6 and IL10–/– mice. Mice were immunized 7 days before challenge with BCG or diluent. Two mice were evaluated in each group. Representative histology is reported for each group. Bladders were removed 24 h after treatment. See also, Table 1. (a) BCG immune C57BL/6 mice treated with PBS (magnification ×10); (b) 20× magnification of (a); (c) BCG immune C57BL/6 mice treated with BCG (magnification ×10); (d) 20× magnification of (c); (e) BCG immune IL10–/– mice treated with PBS (magnification ×10); (f) 20× magnification of (e); (g) BCG immune IL10–/– mice treated with BCG (magnification ×10); (h) 20× magnification of (g).
Enhancement of BCG antitumour activity by enhancing DTH response
The above studies showed that the elimination of IL10 resulted in an enhancement of the DTH response to BCG in both the bladder and footpad. If the DTH response to BCG is linked to therapeutic outcome, the enhanced DTH response in IL10 null mice should also result in improved antitumour activity. Thus, we sought to test the effects of IL10 depletion on BCG-induced antitumour activity in an orthotopic bladder cancer model. Dose–response studies were first performed to assess the effect of BCG dose on the induction of antitumour activity. The data shown in Fig. 8 represent mean values of 4 independent experiments. The optimal antitumour activity is achieved at a BCG dose of 107 CFU. BCG administered at CFU levels of 106 consistently showed depression of tumour growth but statistically significant inhibition of tumour growth in individual experiments was observed in 2/4 of the studies, indicating a consistent but intermediate effect at the 106 BCG dose. To assess the effect of DTH on BCG-induced antitumour activity, studies were performed on normal and IL10 deficient mice at a BCG dose of 106 CFU, where differences in efficacy could be detected (Fig. 9).
Fig. 8.
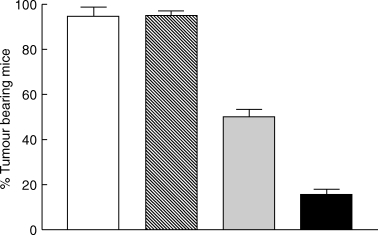
Dose dependent inhibition of orthotopic bladder tumour growth by intravesical BCG treatment. Data are reported as mean tumour outgrowth from 4 separate experiments. Each experiment used 10 mice/group (total of 40 mice evaluated in each group). □ PBS,  105 cfu,
105 cfu,  106 cfu, ▪ 107 cfu. Statistics, χ2: PBS versus 105 cfu, not significant (P = 0·5536); PBS versus 106 cfu, P= 0·0015; PBS versus 107 cfu, P < 0·0001; 106versus 107 cfu, P= 0·001
106 cfu, ▪ 107 cfu. Statistics, χ2: PBS versus 105 cfu, not significant (P = 0·5536); PBS versus 106 cfu, P= 0·0015; PBS versus 107 cfu, P < 0·0001; 106versus 107 cfu, P= 0·001
Fig. 9.
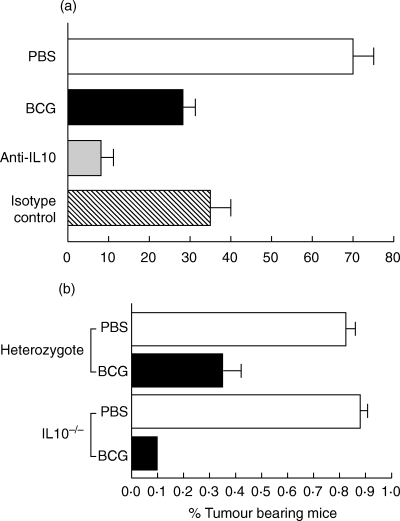
Effect of neutralization of IL10 on BCG-induced antitumour activity. (a) Effect of anti-IL10 in normal mice. Data are reported as mean of 3 separate experiments (10 mice/group/experiment; total of 30 mice/group evaluated). □ PBS, ▪ BCG,  Anti-IL 10,
Anti-IL 10,  Isotype control. Statistics (Pearson's χ2): BCG versus PBS, P= 0·0019; Isotype control versus PBS, P= 0·0097; Anti-IL10 versus PBS, P < 0·0001; Anti-IL10 versus BCG, P= 0·0528; Anti-IL10 versus Isotype Control, P= 0·0146; Anti-IL10 versus BCG + Isotype Control, P= 0·0167; BCG versus Isotype control, not significant (P = 5839). (b) Data are reported as averages of 2 separate experiments (10 mice/group/experiment). Statistics (Pearson's χ2): Heterozygote, BCG versus PBS, P= 0·0015; IL10–/–, BCG versus PBS, P < 0·0001; BCG heterozygote versus BCG IL10–/–, P= 0·0583.
Isotype control. Statistics (Pearson's χ2): BCG versus PBS, P= 0·0019; Isotype control versus PBS, P= 0·0097; Anti-IL10 versus PBS, P < 0·0001; Anti-IL10 versus BCG, P= 0·0528; Anti-IL10 versus Isotype Control, P= 0·0146; Anti-IL10 versus BCG + Isotype Control, P= 0·0167; BCG versus Isotype control, not significant (P = 5839). (b) Data are reported as averages of 2 separate experiments (10 mice/group/experiment). Statistics (Pearson's χ2): Heterozygote, BCG versus PBS, P= 0·0015; IL10–/–, BCG versus PBS, P < 0·0001; BCG heterozygote versus BCG IL10–/–, P= 0·0583.
Initially, mice were treated with anti-IL10 as described for the cytokine data generated in Fig. 5. Administration of anti-IL10 significantly (P = 0·0167, Pearson's χ2) enhanced the antitumour activity of BCG when compared to the isotype control group (Fig. 9a). To confirm the effects of IL10 on antitumour activity, similar experiments were performed on IL10 null mice (Fig. 9b). These data also show enhanced antitumour activity in IL10–/– mice treated with BCG, where 90% of mice were tumour free in both experiments compared with 60–70% tumour free status for heterozygotes. While the differences were consistent, statistical significance was approached but not achieved (P = 0·0583, Pearson's χ2). Taken together with the DTH data, these results link the intensity of the DTH response to BCG-induced antitumour activity.
Discussion
BCG therapy is the treatment of choice for superficial bladder cancer. BCG has been demonstrated in prospective randomized trials to be superior to chemotherapy treatments with thiotepa, doxorubicin, and in some reports mitomycin C [29–31]. Using current treatment standards, approximately 70% of patients treated with BCG are classified as complete responders [32–35]. Preliminary studies with modified treatment regimens increase the complete response rate to approximately 85%[36]. Because of the clinical utility of BCG for bladder cancer, we have pursued studies to characterize the mechanisms by which BCG mediate antitumour activity.
It is clear from previous reports that effective BCG-induced antitumour activity is immunologically mediated [1,7]. Studies in diverse animal models established a requirement for T cell immunity in BCG-mediated antitumour activity [6,8]. Studies in bladder tumour models showed that BCG therapy was not effective in athymic nude mice or mice depleted of either CD4 or CD8 T cells [6,8]. Although T lymphocytes were required for expression of an antitumour response, neither tumour specific cytotoxic T lymphocytes nor protection against a secondary tumour challenge were observed. These data suggest that the T cell response required for effective BCG-induced antitumour activity is directed toward BCG and not tumour associated antigens. This hypothesis is supported by studies in animal models and by correlative data from clinical investigations [6,37].
In animal studies it was observed that the elimination of tumour cells through a BCG-induced DTH response was independent of the development of tumour specific protective immunity [6]. These investigators concluded that the DTH response resulted in the activation of macrophages, which were responsible for the clearance of tumour cells at the site of BCG injection [38]. Consistent with the observations in animal models, previous clinical studies reported an association between BCG-induced DTH and patient tumour free status. Kelley et al.[12] observed a significant correlation between cutaneous skin test reactivity to purified protein derivative (PPD) and a tumour free response. In addition to the skin test data, histological characterization of biopsy specimens from BCG treated patients showed the presence of granulomatous inflammation in the majority of specimens. Again, a significant association between BCG-induced granuloma and tumour free status was observed. Furthermore, Luftenegger et al.[39] showed a significantly improved tumour free response in BCG treated patients who were PPD positive at the time of initiation of BCG treatment.
In our initial studies we wished to establish the character of the immune response induced by BCG via the intravesical route. Previous studies have shown that immunization by different routes generate diverse effector responses [27,40]. Moreover, it is known that mycobacteria can induce both Th1 and Th2 responses[16,41]. Although immune induction by BCG administration via other mucosal routes including oral, nasal, and rectal has been reported, evaluation of the responses induced after introduction of BCG through the bladder mucosa have not been defined. Our data show that intravesical instillation of BCG preferentially induces a Th1 response. Instillation of BCG into the bladder efficiently induced IFN-γ production in both naive and BCG immune mice. Consistently, in the treatment of clinical patients intravesical BCG favours Th1 cytokine induction (IFN-γ, IL2 and IL12) that precedes Th2 cytokine induction (IL10). A higher ratio of urinary IFN-γ versus IL10 for BCG responders was also observed. IL4, a Th2 cytokine, was not modulated by BCG stimulation, since this cytokine was observed in naive mice both with and without BCG stimulation. It has also not been observed in urine specimens obtained from BCG treated patients (our unpublished observations).
Clinical studies of others also demonstrated Th1 cytokine induction by BCG [13, 15, 17, 18]. These studies confirmed the presence of IFN-γ in post-BCG treatment urine samples and also showed the expression of the activation markers HLA DR and ICAM on epithelial cells [18]. Similar to our observations, Haaff et al.[42] observed increased levels of urine IL2 after BCG therapy. They observed that IL2 appeared in the urine 4–6 h after BCG treatment beginning with treatment 3–5, which is an expression pattern consistent with immune activation. Although not statistically significant, IL2 production correlated with tumour free status. De Reijke et al.[13] reported similar findings. Thus, the data reported herein from the murine orthotopic bladder tumour model are comparable with the cytokine profiles induced by BCG in clinical specimens. These data show that the Th1 environment previously suggested to be important to BCG-induced antitumour activity is present in the bladder after BCG treatment.
Consistent with our observations, studies assessing the immune response to BCG show that protective immunity for mycobacteria is characterized by the development of a Th1-driven DTH response [43]. A report by Appelberg et al.[44] using IFN-γ knockout mice demonstrated the requirement for IFN-γ in the control of mycobacterial infections. While IFN-γ is known to be necessary component in the antimycobacterial response, it is not sufficient for the complete elimination of the bacteria. Bloom et al.[45] provided strong support for a role for CD8+ T cells in immunity to mycobacteria by showing that mycobacterial infections were rapidly lethal in animals lacking CD8+ cells. These data demonstrate the requirement for both CD4+ and CD8+ T cells in mycobacterial immunity as was shown for tumour therapy [6].
In order to determine whether BCG-induced DTH is linked to antitumour activity, we utilized the regulatory effects of IL10 to modulate DTH as previously described [25]. Our data show that abrogation of IL10 activity, either by antibody neutralization or by the use of IL10 null mice, resulted in an enhancement of BCG-induced DTH. Enhanced DTH resulted in substantially increased mononuclear infiltration into BCG-treated bladder specimens. Utilizing the modulatory effects of IL10, we assessed the effects of enhanced BCG-induced DTH on antitumour activity. These studies showed that BCG induced significantly greater antitumour activity under conditions in which the DTH response was enhanced. These data provide direct evidence linking BCG-induced DTH to antitumour activity; however, the effector mechanism(s) remain to be elucidated.
The association between BCG-induced DTH and antitumour activity suggests that the antitumour response is a consequence of the anti-BCG response and further suggests that immune mediators produced by the response either directly or indirectly participate in antitumour activity. These observations are consistent with conclusions from previous studies that developed efficacy criteria for successful BCG therapy. They can be summarized as a requirement for an intact immune response, contact between BCG and the tumour, and the presence of a limited tumour burden [46]. Furthermore, the data are consistent with observations in other models in which IL10 modulation of Th1-driven autoimmune disease and antigen induced inflammatory diseases was observed [47,48].
The question remains as to the actual effector event or events. Since tumour and BCG must be in close association, cytokines released as byproducts of the immune response to BCG may either inhibit tumour proliferation or have direct cytotoxic effects on the tumour cells. Two candidate cytokines, IFN-γ and TNF-α, were identified in our studies as being expressed during BCG treatment. These cytokines have been consistently observed during BCG treatment and have been shown in a melanoma model to function in concert to inhibit tumour cell proliferation [49]. We have previously reported on the effects of these cytokines on bladder tumour proliferation for the MBT2 bladder tumour model [50]. In this study, we extended our observations to MB49 bladder tumour cells. Similarly, both IFN-γ and TNF-α, either as single agents or in combination, were reported to inhibit MB49 cell growth when the cytokine concentrations usually found in urine collected after BCG treatment were used.
These data suggest that the DTH response to BCG act indirectly through either BCG antigen recognition or activation of nonspecific lytic mechanisms. Previous studies have shown that bladder tumour cells express class II and can act as antigen presenting cells to CD4+ T cells [51,52]. Jakobson et al.[53] showed that CD4+ Th1 cells were capable of killing bladder tumour cells via fas dependent mechanisms. These investigators and others showed that MHC and other cell surface markers including fas were up-regulated by IFN-γ. In addition, studies by ourselves and others have shown that both IFN-γ and TNF-α enhanced the susceptibility of MB49 to fas mediated killing suggesting an important role for these cytokines [54,55]. Whether fas mediated killing of bladder tumour cells is associated with the antitumour response in vivo remains to be established.
An alternate cellular mechanism is the activation of nonspecific killer cells by the cytokines produced in response to BCG. It is clear from previous studies that macrophages and natural killer (NK) cells can be activated by Th1 cytokines. Previous studies by Ratliff and associates [11] using the MBT2 bladder cancer model demonstrated that NK cells are not the primary killer cells in BCG therapy. These investigators showed that the depletion of NK cells had no effect on BCG induced antitumour activity. In this regard, Bohle et al. demonstrated that a human CD8+, CD56+ T cell could be activated by BCG to mediate lysis of bladder tumour cells. The cells were termed BCG activated killer cells (BAK) and were shown to require IFN-γ, IL2 and IL12 for activation [9,10]. Interestingly, activation of BAK cell activity did not result in enhanced cytolysis of NK sensitive target cells. The participation of cells with these cell surface markers would be consistent with the data described above but direct evidence is needed to link BAK activity to BCG-mediated antitumour activity.
In conclusion, we show a direct link between BCG-induced DTH and the expression of antitumour activity in a bladder tumour model. The data further implicate IL10 as an important modulator of immune mediated events in vivo and suggest efforts to down-modulate this inhibitory cytokine may be of therapeutic value.
Acknowledgments
This work was supported by National Cancer Institute grant CA44426 and National Institutes of Health grant R29CA64230.
References
- 1.Ratliff TL. Bacillus Calmette-Guérin (BCG). mechanism of action in superficial bladder cancer. Urol. 1991;37:8–11. doi: 10.1016/0090-4295(91)80127-s. [DOI] [PubMed] [Google Scholar]
- 2.Zhao W, Schorey JS, Groger R, Allen PM, Brown EJ, Ratliff TL. Characterization of the fibronectin binding motif for a unique mycobacterial fibronectin attachment protein, FAP. J Biol Chem. 1999;274:4521–6. doi: 10.1074/jbc.274.8.4521. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MA, Brown EJ, Ritchey JK, Ratliff TL. Modulation of fibronectin-mediated Bacillus Calmette-Guérin attachment to murine bladder mucosa by drugs influencing the coagulation pathways. Cancer Res. 1991;51:3726–32. [PubMed] [Google Scholar]
- 4.Coplen DE, Brown EJ, McGarr J, Ratliff TL. Characterization of fibronectin attachment by a human transitional cell carcinoma line, T24. J Urol. 1991;145:1312–5. doi: 10.1016/s0022-5347(17)38621-4. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Schorey JS, Bong-Mastek M, Ritchey J, Brown EJ, Ratliff TL. Role of a bacillus Calmette-Guérin fibronectin attachment protein in BCG-induced antitumor activity. Int J Cancer. 2000;86:83–8. doi: 10.1002/(sici)1097-0215(20000401)86:1<83::aid-ijc13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol. 1993;150:1018–23. doi: 10.1016/s0022-5347(17)35678-1. [DOI] [PubMed] [Google Scholar]
- 7.Ratliff TL. Role of the immune response in BCG for bladder cancer. Eur Urol. 1992;21:17–21. doi: 10.1159/000474916. [DOI] [PubMed] [Google Scholar]
- 8.Ratliff TL, Gillen DP, Catalona WJ. Requirement of a thymus dependent immune response for BCG-mediated antitumor activity. J Urol. 1987;137:155–8. doi: 10.1016/s0022-5347(17)43909-7. [DOI] [PubMed] [Google Scholar]
- 9.Bohle A, Thanhauser A, Ulmer AJ, Ernst M, Flad HD, Jocham D. Dissecting the immunobiological effects of bacillus Calmette-Guérin (BCG) in vitro: evidence of a distinct BCG-activated killer (BAK) cell phenomenon. J Urol. 1993;150:1932–7. doi: 10.1016/s0022-5347(17)35941-4. [DOI] [PubMed] [Google Scholar]
- 10.Thanhauser A, Bohle A, Flad HD, Ernst M, Mattern T, Ulmer AJ. Induction of bacillus-Calmette-Guérin-activated killer cells from human peripheral blood mononuclear cells against human bladder carcinoma cell lines in vitro. Cancer Immunol Immunother. 1993;37:105–11. doi: 10.1007/BF01517042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratliff TL, Shapiro A, Catalona WJ. Inhibition of murine bladder tumor growth by bacillus Calmette-Guérin: lack of a role of natural killer cells. Clin Immunol Immunopathol. 1986;41:108–15. doi: 10.1016/0090-1229(86)90055-3. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DR, Haaff EO, Becich M, Lage J, Bauer WC, Dresner SM, Catalona WJ, Ratliff TL. Prognostic value of PPD skin test and granuloma formation in patients treated with intravesical bacillus Calmette-Guérin. J Urol. 1986;135:268–71. doi: 10.1016/s0022-5347(17)45605-9. [DOI] [PubMed] [Google Scholar]
- 13.De Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette-Guérin therapy for superficial bladder cancer. processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 14.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Intravesical Evans strain BCG therapy. quantitative immunohistochemical analysis of the immune response within the bladder wall. J Urol. 1992;147:1636–42. doi: 10.1016/s0022-5347(17)37668-1. [DOI] [PubMed] [Google Scholar]
- 15.Bohle A, Nowc C, Ulmer AJ, Musehold J, Gerdes J, Hofstetter AG, Flad HD. Elevations of cytokines interleukin-1, interleukin-2 and tumor necrosis factor in the urine of patients after intravesical bacillus Calmette-Guérin immunotherapy. J Urol. 1990;144:59–64. doi: 10.1016/s0022-5347(17)39366-7. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell MA, Chen X, DeWolf WC. Maturation of the cytokine immune response to BCG in the bladder: implications for treatment schedules. J Urol. 1996;155:1030A. [Google Scholar]
- 17.Prescott S, James K, Hargreave TB, Chisholm GD, Smyth JF. Radio-immunoassay detection of interferon-gamma in urine after intravesical Evans BCG therapy. J Urol. 1990;144:1248–51. doi: 10.1016/s0022-5347(17)39713-6. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AM, Alexandroff AB, Kelly RW, Skibinska A, Esuvaranathan K, Prescott S, Chisholm GD, James K. Changes in urinary cytokines and soluble intercellular adhesion molecule-1 (ICAM-1) in bladder cancer patients after Bacillus Calmette-Guérin (BCG) immunotherapy. Clin Exp Immunol. 1995;99:369–75. doi: 10.1111/j.1365-2249.1995.tb05560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peuchmaur M, Benoit G, Viellefond A, Chevalier A, Lemaigre G, Martin ED, Jardin A. Analysis of mucosal bladder leukocyte subpopulations in patients treated with intravesical bacillus Calmette-Guérin. Urol Res. 1989;17:299–303. doi: 10.1007/BF00262987. [DOI] [PubMed] [Google Scholar]
- 20.Stover CK, de la Cruz VF, Fuerst TR, et al. New use of BCG for recombinant vaccines. Nature. 1991;351:456–60. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell MA, Aldovini A, Duda RB, Yang H, Szilvasi A, Young RA, DeWolf WC. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro A, Kelley DR, Oakley DM, Catalona WJ, Ratliff TL. Technical factors affecting the reproducibility of intravesical mouse bladder tumor implantation during therapy with bacillus Calmette-Guérin. Cancer Res. 1984;44:3051–4. [PubMed] [Google Scholar]
- 23.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 24.Watson MA, Milbrandt J. Expression of the nerve growth factor-regulated NGFI-A and NGFI-B genes in the developing rat. Development. 1990;110:173–83. doi: 10.1242/dev.110.1.173. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson TA, Dube P, Griffith TS. Regulation of contact hypersensitivity by interleukin 10. J Exp Med. 1994;179:1597–604. doi: 10.1084/jem.179.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D, Bohle A. Optimizing syngeneic orthotopic murine bladder cancer (MB49) Cancer Res. 1999;59:2834–7. [PubMed] [Google Scholar]
- 27.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–98. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 28.Kramnik I, Radzioch D, Skamene E. T-helper 1-like subset selection in Mycobacterium bovis bacillus Calmette-Guérin-infected resistant and susceptible mice. Immunol. 1994;81:618–25. [PMC free article] [PubMed] [Google Scholar]
- 29.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guérin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–9. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Pineiro JA, Jimenez-Leon J, Martinez-Pineiro LJr, Fiter L, Mosteiro JA, Navarro J, Garcia-Matres MJ, Carcamo P. Bacillus Calmette-Guérin versus doxorubicin versus thiotepa: a randomized prospective study in 202 patients with superficial bladder cancer. J Urol. 1990;143:502–6. doi: 10.1016/s0022-5347(17)40002-4. [DOI] [PubMed] [Google Scholar]
- 31.Malmstrom P. Improved patient outcomes with BCG immunotherapy vs. chemotherapy – Swedish and worldwide experience. Eur Urol. 2000;37:16–20. doi: 10.1159/000052377. [DOI] [PubMed] [Google Scholar]
- 32.Kavoussi LR, Torrence RJ, Gillen DP, Hudson MA, Haaff EO, Dresner SM, Ratliff TL, Catalona WJ. Results of 6 weekly intravesical bacillus Calmette-Guérin instillations on the treatment of superficial bladder tumors. J Urol. 1988;139:935–40. doi: 10.1016/s0022-5347(17)42722-4. [DOI] [PubMed] [Google Scholar]
- 33.De Jager R, Guinan P, Lamm D, et al. Long-term complete remission in bladder carcinoma in situ with intravesical Tice bacillus Calmette-Guérin. Overview analysis of six phase II clinical trials. Urology. 1991;38:507–13. doi: 10.1016/0090-4295(91)80166-5. [DOI] [PubMed] [Google Scholar]
- 34.Herr HW, Pinsky CM, Whitmore WF. Long-term effect of intravesical bacillus Calmette-Guérin on flat carcinoma in situ of the bladder. J Urol. 1986;135:265–7. doi: 10.1016/s0022-5347(17)45604-7. [DOI] [PubMed] [Google Scholar]
- 35.Lamm DL. Long-term results of intravesical therapy for superficial bladder cancer. Urol Clin North Am. 1992;19:573–80. [PubMed] [Google Scholar]
- 36.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guérin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–9. [PubMed] [Google Scholar]
- 37.Herr HW, Whitmore WF. Ureteral carcinoma in situ after successful intravesical therapy for superficial bladder tumors. incidence, possible pathogenesis and management. J Urol. 1987;138:292–4. doi: 10.1016/s0022-5347(17)43123-5. [DOI] [PubMed] [Google Scholar]
- 38.Snodgrass MJ, Hanna MGJ. Ultrastructural studies of histiocyte–tumor cell interactions during tumor regression after intralesional injection of Mycobacterium bovis. Cancer Res. 1973;33:701–16. [PubMed] [Google Scholar]
- 39.Luftenegger W, Ackermann DK, Futterlieb A, Kraft R, Minder CE, Nadelhaft P, Studer UE. Intravesical versus intravesical plus intradermal bacillus Calmette-Guérin: a prospective randomized study in patients with recurrent superficial bladder tumors. Randomized Controlled Trial. J Urol. 1996;155:483–7. doi: 10.1016/s0022-5347(01)66427-9. [DOI] [PubMed] [Google Scholar]
- 40.Ibsen MW, Bakken V, Jonsson R, Hordnes K. Immune responses in mice after gastric and subcutaneous immunization with BCG. Scand J Immunol. 1997;46:274–80. doi: 10.1046/j.1365-3083.1997.d01-116.x. [DOI] [PubMed] [Google Scholar]
- 41.Zlotta AR, Drowart A, Huygen K, et al. Humoral response against heat shock proteins and other mycobacterial antigens after intravesical treatment with bacille Calmette-Guérin (BCG) in patients with superficial bladder cancer. Clin Exp Immunol. 1997;109:157–65. doi: 10.1046/j.1365-2249.1997.4141313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haaff EO, Catalona WJ, Ratliff TL. Detection of interleukin 2 in the urine of patients with superficial bladder tumors after treatment with intravesical BCG. J Urol. 1986;136:970–4. doi: 10.1016/s0022-5347(17)45142-1. [DOI] [PubMed] [Google Scholar]
- 43.Ladel CH, Daugelat S, Kaufmann SH. Immune response to Mycobacterium bovis Calmette-Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–84. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 44.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and – dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–71. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stenger S, Mazzaccaro RJ, Uyemura K, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–7. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 46.Zbar B, Bernstein I, Bartlett GL, Hanna MG, Rapp HJ. Immunotherapy of cancer: regression of intradermal tumors and prevention of growth of lymph node metastases after intralesional injection of living Mycobacterium bovis. J Natl Cancer Inst. 1972;49:119–30. [PubMed] [Google Scholar]
- 47.Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, Cheever AW, Sher A. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice. cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun. 2001;69:4232–41. doi: 10.1128/IAI.69.7.4232-4241.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ito A, Bebo BFJ, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–52. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto T, O'Donnell MA, Szilvasi A, Yang H, Duda RB. Bacillus Calmette-Guérin plus interleukin-2 and/or granulocyte/macrophage-colony-stimulating factor enhances immunocompetent cell production of interferon-γ, which inhibits B16F10 melanoma cell growth in vitro. Cancer Immunol Immunother. 1996;42:280–4. doi: 10.1007/s002620050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahnson RR, Ratliff TL. In vitro and in vivo anti-tumor activity of recombinant mouse tumor necrosis factor (TNF) in a mouse bladder tumor (MBT-2) J Urol. 1990;144:172–5. doi: 10.1016/s0022-5347(17)39404-1. [DOI] [PubMed] [Google Scholar]
- 51.Lattime EC, Gomella LG, McCue PA. Murine bladder carcinoma cells present antigen to BCG-specific CD4+ T-cells. Cancer Res. 1992;52:4286–90. [PubMed] [Google Scholar]
- 52.Patard JJ, Chopin DK, Boccon-Gibod L. Mechanisms of action of bacillus Calmette-Guérin in the treatment of superficial bladder cancer. World J Urol. 1993;11:165–8. doi: 10.1007/BF00211413. [DOI] [PubMed] [Google Scholar]
- 53.Jakobson E, Jonsson G, Bjorck P, Paulie S. Stimulation of CD40 in human bladder carcinoma cells inhibits anti-Fas/APO-1 (CD95)-induced apoptosis. Int J Cancer. 1998;77:849–53. doi: 10.1002/(sici)1097-0215(19980911)77:6<849::aid-ijc9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 54.O'Donnell MA, Szilvasi A, Luo Y, DeWolf WC. Fas mediated killing of transitional cell carcinoma. J Urol. 1996;155:567A. [Google Scholar]
- 55.Miller MI, Klein LT, Ikeguchi E, Buttyan R, Katz A, Raffo J, Olsson C, Connor JP. Anti fas antibody mediated apoptosis in bladder tumor cells: a potential intravesical therapeutic agent. J Urol. 1996;155:569A. [Google Scholar]


