Abstract
The persistent presence of rheumatoid factors (RFs) in the circulation is a characteristic phenomenon in patients with rheumatoid arthritis (RA). Recent data indicate that RFs associated with seropositive RA are derived from terminally differentiated CD20−, CD38+ plasma cells (PCs) present in synovial fluids of the inflamed joints. These cells were shown to secrete RFs actively and are thought to originate from germinal centre (GC)-like structures present in the inflamed synovium. To obtain a representative image of the structural properties of IgM and IgG RFs associated with RA, phage antibody display libraries were constructed from CD38+ PCs isolated from the inflamed joints of RF-seropositive patients with RA. Subsequently, human IgG Fc-binding monoclonal phage antibodies were selected and analysed. The data suggest that RA-associated RFs are encoded by a diverse set of VL and a more restricted set of VH regions. VH gene family usage of PC-derived IgM- and IgG-RFs was found to be restricted to the VH1 and 3 gene families, with a preference for VH3, and many different VL genes were shown to contribute to RF specificity. Clonally related VH as well as VL sequences were identified, based on the presence of identical CDR3 regions and shared somatic mutations. In this B cell selection process base-pair substitutions as well as deletions of triplets in CDR regions, leaving the transcripts in frame, were involved. Together, these data provide further evidence for an Ag-driven immune response in the terminal differentiation into RF-producing PCs in patients with RA, including expansion of clonally related B cells, selection and isotype switching, all hallmarks of a GC reaction.
Keywords: rheumatoid arthritis, synovial fluid plasma cells, phage display, rheumatoid factor activity, sequence analysis
Introduction
Rheumatoid factors (RFs) are antibodies directed against the Fc part of autologous IgG. Their persistent presence in the circulation is a characteristic phenomenon in patients with rheumatoid arthritis (RA) and high titres were shown to correlate with more severe disease [1,2]. RFs in RA are believed to contribute to local inflammation by immune complex formation and complement activation [3]. Recent data indicate that RFs associated with RA are derived from terminally differentiated CD20−, CD38+ plasma cells (PCs) present in synovial fluids (SFs) from inflamed joints of RF-seropositive patients with RA [4,5]. These cells were shown to secrete RFs actively [4] and are thought to originate from germinal centre (GC)-like structures present in the inflamed synovium [6–10]. The GC-like structures seem to provide a microenvironment in which selection of B cells occurs.
Most data concerning the functional and structural characteristics of locally produced RFs are obtained by studying the RA synovial B cell repertoire by cloning or somatic heterohybridization of Epstein-Barr virus (EBV)-infected cell lines [7,11–11]. However, a major limitation of these techniques is that EBV fails to transform and immortilize proliferating B cells or in vivo secreting PCs [15]. Furthermore, EBV immortalization targets less than 1% of the B cell fraction and EBV cloning and somatic heterohybridization are inefficient in humans [16,17]. So, it is debatable whether the RFs of EBV-transformed B cells can be considered representatives of the RFs produced in vivo. The data from EBV-transformed hybridoma cell lines reveal that in RA synovium a substantial quantity of the RF-encoding genes is somatically mutated and somatic mutation has been taken to indicate an antigen-driven response [6, 7, 11, 12, 13, 14]. An alternative, the generation of complementary DNA libraries from unselected cells extracted from RA synovial tissue allows only structural analysis of the V genes found [18–20]. This approach does not permit to directly correlate antibody sequence with RF activity.
To obtain a representative image of the structural properties of IgM- and IgG RFs produced we studied the RF repertoire of terminally differentiated CD38+ PCs present at the site of inflammation of RF-seropositive RA patients by the phage antibody display library technique [21,22]. Others have shown that monoclonal autoantibodies generated by combinatorial libraries resemble the serum autoantibodies of, e.g. patients with autoimmune thyroid disease or primary biliary cirrhosis in terms of affinity, specificity and epitopes recognized [22]. Separate heavy (H) and light (L) chain variable (V) region libraries constructed from amplified VH and VL genes expressed in B cells were combined in a phagemid vector and expressed as gene III fusion proteins on the surface of filamentous phage particles. Subsequently, human IgG Fc-binding monoclonal phage antibodies (moPhabs) were selected and characterized. Here we demonstrate that V genes of Fc-binding moPhabs constructed from SF PCs display the imprints of an antigen-dependent process of somatic hypermutation and clonal selection.
Materials And Methods
Study subjects
Heparinized SF was obtained from two RA patients with increased serum RF titres who were seen at the Department of Rheumatology of the Leiden University Medical Centre and fulfilled the criteria of the American Rheumatism Association for RA [23]. Patient G was a 56-year-old-male with a 13-years history of RA who was being treated with a disease-modifying antirheumatic drug. Patient V was a 71-year-old-male with a 30-year-old-history of RA who was being treated with a nonsteroidal anti-inflammatory drug only. The serum IgM-RF titres of patients G and V were at the time of sampling 20 and 91 IU/ml, respectively (normal values of IgM-RF in serum: ≤2·5 IU/ml [24]).
Isolation of CD38+ B cells
Synovial fluid mononuclear cells (SFMC) were purified by Ficoll-Hypaque (Pharmacia Biotech AB, Uppsala, Sweden; ρ= 1·077 g/ml) density gradient centrifugation and frozen in liquid nitrogen. SFMC were thawed and stained with the following phycoerythrin-conjugated monoclonal antibodies (Becton Dickinson, Mountain View, CA, USA): anti-CD3, anti-CD14, anti-CD16 and anti-CD56. B cells were isolated by negative selection using a cell sorter (FACStar, Becton Dickinson). Obtained cells contained ≥95% CD19+ lymphocytes as determined by fluorescence activated cell sorter analysis (data not shown). Subsequently, for positive selection of CD38+ B cells phycoerythrin-conjugated anti-CD38 (Becton Dickinson) and a cell sorter were used.
First strand cDNA synthesis
Total RNA was extracted from CD38+ B cells (patient G: 4·0 × 104 cells; patient V: 3·5 × 104 cells) using the RNAzol B kit (Campro Scientific, Veenendaal, the Netherlands) and 1/20 volume of the RNA solution was reverse-transcribed with an oligo-(dT)12−18 primer (0·5 µg) to first strand cDNA in a final volume of 20 µl containing 50 mm KCl, 10 mm Tris-HCl pH 8·3, 5 mm MgCl2, 1·25 mm of each dNTP, 10 mm DTT, 20 U Rnasin (Promega, Madison, WI, USA) and 20 U M-MLV-RT (GibcoBRL, Breda, the Netherlands). The reaction mixture was incubated at 42°C for 1 h. Subsequently, the enzyme was inactivated at 65°C for 10 min.
Library construction
Amplification of VH and VL regions was accomplished in a two step procedure by polymerase chain reaction (PCR). Sequences of all primers used for amplification are shown in Table 1[25–27]. In order to preferentially amplify VH regions from IgM- or IgG-expressing CD38+ B cells, a set of 5′ VH primers specific for each of the six human VH gene families in combination with a 3′ Cµ or Cγ primer specific for all four human IgG subclasses was used. For amplification of VL regions, 5′ Vκ or Vλ primers were used in combination with a 3′ Cκ or Cλ primer, respectively. PCR amplifications of cDNA samples (1 µl each) were followed with seminested PCR reactions. For re-PCR, 1/50 volume of the first PCR reaction product was amplified with 5′ primers that introduced NcoI and SacI restriction sites to the VH and VL regions, respectively. Similarly, 3′ primers specific for the six human JH segments were employed to introduce a XhoI restriction site to the VH regions and 3′ primers specific for four Jκ (Jκ1-Jκ4) and five Jλ (Jλ1-Jλ5) gene segments were used to introduce a NotI restriction site to VL regions. Reactions, preceded with an incubation at 95°C for 12 min, were carried out for 35 (first PCR) or 30 (re-PCR) cycles (30 s at 94°C, 40 s at 55 (first PCR) or 57 (re-PCR)°C and 50 s at 72°C) on a thermal cycler (Perkin-Elmer, Norwalk, CT, USA). Subsequently, the samples were incubated at 72°C for 7 min. All PCR reactions were performed in a volume of 25 µl containing 50 mm KCl, 10 mm Tris-HCl pH 8·3, 2 (first PCR) or 1·5 (re-PCR) mM MgCl2, 250 µm of each dNTP, 10 pmol of each primer and 0·5 U Ampli Taq Gold DNA polymerase (Perkin-Elmer). Amplified DNA was analysed on a 2% agarose gel.
Table 1.
Oligonucleotide primers used to construct phage display libraries
| First PCRs | |||
| Human VH back primers without restriction sites | |||
| VH1BACK | CAGGTGCAGCTGGTGCAGTCTGG | ||
| VH2BACK | CAGGTCAACTTAAGGGAGTCTGG | ||
| VH3BACK | GAGGTGCAGCTGGTGGAGTCTGG | ||
| VH4BACK | CAGGTGCAGCTGCAGGAGTCGGG | ||
| VH5BACK | GAGGTGCAGCTGTTGCAGTCTGC | ||
| VH6BACK | CAGGTACAGCTGCAGCAGTCAGG | ||
| Human Cµ and Cγ forward primer | |||
| Cµ | TGGAAGAGGCACGTTCTTTTCTTT | ||
| Cγ | GTCCACCTTGGTGTTGCTGGGCTT | ||
| Human Vκ back primer with SacI restriction site | |||
| Vκ-SacI | GA(A/C/T)AT(C/T)GAGCTCAC(A/C/G/T)CAGTCTCC | ||
| Human Cκ forward primer | |||
| Cκ | AGACTCTCCCCTGTTGAAGCTCTT | ||
| Human Cλ back primers with SacI restriction sites | |||
| Vλ1-SacI | TCCCAGTCTGAGCTCACGCAGCCGCCCTC | ||
| Vλ2-SacI | TCCTCCTATGAGCTCACTCAGCCACCCT | ||
| Vλ3-SacI | TCCTCCTATGAGCTCACTCAGCCACCCT | ||
| Human Cλ forward primer | |||
| Cλ | TGAAGATTCTGTAGGGGCCACTGTCTT | ||
| Re-PCRs† | |||
| Human VH back primers with NcoI restriction sites | |||
| VH1-NcoI | GTCCTCGCAACTGCGGCCCAGCCGGCCATGGCCCAGGTGCAGCTGGTGCAGTCTGG | ||
| VH2-NcoI | GTCCTCGCAACTGCGGCCCAGCCGGCCATGGCCCAGGTCAACTTAAGGGAGTCTGG | ||
| VH3-NcoI | GTCCTCGCAACTGCGGCCCAGCCGGCCATGGCCCAGGTGCAGCTGGTGGAGTCTGG | ||
| VH4-NcoI | GTCCTCGCAACTGCGGCCCAGCCGGCCATGGCCCAGGTGCAGCTGCAGGAGTCGGG | ||
| VH5-NcoI | GTCCTCGCAACTGCGGCCCAGCCGGCCATGGCCCAGGTGCAGCTGTTGCAGTCTGC | ||
| VH6-NcoI | GTCCTCGCAACTGCGGCCCAGCCGGCCATGGCCCAGGTACAGCTGCAGCAGTCAGG | ||
| Human JH forward primers with XhoI restriction sites | |||
| JH(1–3)-XhoI | GCCTCCACCTCTCGAGTGA(A/G)GAGACGGTGACCA(G/T)(G/T)GT(G/C)CC | ||
| JH(3–6)-XhoI | CCTCCACCTCTCGAGACGGTGACCAGGGT(C/T)CCTTG | ||
| Human Jκ forward primer with NotI restriction site | |||
| Jκ(1–4)-NotI | TTCTCGACTTGCGGCCGCACGTTTGATCTCCACCTTGGTCCC | ||
| Human Jλ forward primers with NotI restriction sites | |||
| Jλ1-NotI | GCAATGGTAGCGGCCGCACCTAGGACGGTGACCTTGGTCCC | ||
| Jλ(2,3)-NotI | GCAATCGTAGCGGCCGCACCTAGGACGGTCAGCTTGGTCCC | ||
| Jλ(4,5)-NotI | GCAATCGTAGCGGCCGCACCTAAAACGGTGAGCTGGGTCCC | ||
Family based primers were used to amplify VH and VL regions. Primers for re-PCR were constructed to introduce NcoI and XhoI restriction sites and SacI and NotI restriction sites (underlined) at the 5′and 3′ends of the VH and VL segments, respectively.
The same Vκ and Vλ primers were used in the first PCR and re-PCR.
H and L chain PCR products were separately cloned in phagemid vector pHEN-LINK using NcoI and XhoI or SacI and NotI restriction sites, respectively (For construction of phage display libraries, the pHEN1 vector [27] was modified to allow separate cloning of VH and VL regions. From a construct in pHEN1 containing the linkersequence and a Vκ3 light chain sequence [26], the VL region was excised by SacI and NotI restriction enzyme digestion and replaced by a 40-bp stuffer sequence from pBluescript (Stratagene, La Jolla, CA, USA) with the same restriction sites, resulting in pHEN-LINK). Subsequently, the L chain sequences were inserted in the SacI/NotI sites of the plasmids containing the H chain libraries. The resultant phagemids were electroporated into Escherichia coli (E. coli) strain TG1. The unamplified combinatorial libraries comprised the number of individual clones with both VH and VL inserts of the correct size as shown in Table 2.
Table 2.
Libraries constructed
| Insert percentage (%) | |||||
|---|---|---|---|---|---|
| RA patient | Number of CD38+ SF B cells | Library | Number of individual clones | VH | VL |
| G | 4·0 × 104 | Gm† | 7·5 × 105 | 100 | 100 |
| Gg† | 1·2 × 106 | 90 | 100 | ||
| V | 3·5 × 104 | Vm | 1·1 × 106 | 90 | 90 |
| Vg | 1·1 × 106 | 100 | 100 | ||
m and g indicate that the H chain V regions of the library are derived from IgM- or IgG-expressing B cells, respectively.
Library screening
Panning, phage selection and amplification procedures were essentially as described in detail elsewhere [27]. Briefly, the libraries were rescued individually using helper phage VCS-M13 (Stratagene, Westburg bv, Leusden, the Netherlands). The Fc-binding phages in the libraries were enriched by panning on human IgG Fc (HuIgG Fc; Cappel, Durham, NC, USA)-coated surfaces. For this purpose immunotubes (Nunc Maxisorp, GibcoBRL) were coated overnight at room temperature with HuIgG Fc fragments at a concentration of 10 µg/ml in a carbonate buffer, pH 9·6. After blocking with 0·1% (v/v) Tween 20 in PBS, each tube was incubated at room temperature for 2·5 h with 5 × 1012 phages. The phage solution was subsequently removed, tubes were washed 10 times with PBS, 0·1% (v/v) Tween 20 and 10 times with PBS (each washing step was performed by pouring buffer in and out immediately). Bound phages were eluted from the tube by 100 mm Triethylamine (Aldrich-Chemie, Steinheim, Germany). The eluted material was immediately neutralized by adding 1 m Tris.HCl, pH 7·4. Eluted phages were used to infect log-phase E. coli XL1-Blue cells [26]. After each round of selection, phages were rescued from single ampicilin-resistant colonies of infected XL1-Blue cells. Binding to HuIgG Fc was verified by ELISA, using bacterial supernatants containing monoclonal phage antibodies (moPhabs) or moPhabs which were purified and concentrated by polyethylene glycol/NaCl precipitation and resuspended in PBS containing 1% (w/v) BSA.
Enzyme-linked immunosorbent assay (ELISA)
Binding of moPhabs to HuIgG Fc was assessed by ELISA using plates (Titertek, Flow Laboratories, Zwanenburg, the Netherlands) coated overnight at room temperature with HuIgG Fc fragments (10 µg/ml in a carbonate buffer, pH 9·6). A monoreactive moPhab directed against group B Streptococcae (kind gift of Dr J. de Kruif, Department of Immunology, University Hospital Utrecht, Utrecht, the Netherlands) or a representative moPhab which does not bind to HuIgG Fc was used as a negative control. MoPhabs binding to antigen were detected using horseradish peroxidase (HRP)-conjugated sheep anti-M13 antibody and 2′,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) substrate (Detection Module Recombinant Phage Antibody System, Pharmacia Biotech AB) according to the manufacturer's recommendations with the modification that PBS containing 0·05% (v/v) Tween 20 and 1% (v/v) newborn bovine serum (Flow Laboratories, Irvine, Scotland) was used for blocking. The colour reaction was read at 415 nm in an ELISA reader (EL 312e Bio-kinetics Reader, Bio-Tek Instruments, Inc., Winooski, VT, USA).
DNA fingerprinting of clones
The diversity of moPhabs with HuIgG Fc-binding activity was assessed by MvaI DNA fingerprinting of clones. The scFv insert was amplified by PCR using primers M13 reverse (5′-AAC AGC TAT GAC CAT G-3′) and PHENSEQ (5′-CTA TGC GGC CCC ATT CA-3′) [28]. Reactions, preceded with an incubation at 95°C for 12 min, were carried out for 30 cycles (60 s at 94°C, 60 s at 55°C and 120 s at 72°C) on a thermal cycler. Subsequently, the samples were incubated at 72°C for 7 min. All PCR reactions were performed in a volume of 25 µl containing 50 mm KCl, 10 mm Tris-HCl pH 8·3, 2 mm MgCl2, 250 µm of each dNTP, 10 pmol of each primer and 0·5 U Ampli Taq Gold DNA polymerase (Perkin-Elmer). Amplified DNA was digested with the frequent-cutting enzyme MvaI (MBI Fermentas, Amherst, NY, USA) and analysed on a 3% agarose gel.
Soluble scFv production
Soluble single chain (sc) Fv fragments were produced in E. coli amber nonsuppressor strain SF110, modified to contain the F′ episome of E. coli XL1-Blue, as described [26,27], likely resulting in the production of a mixture of scFv monomers and dimers [29]. Integrity and quantity of monoclonal scFv fragments was assessed by Western blotting using antimyc mAb 9E10 (CRL-1729, ATCC, Rockville, MD) which recognizes the C-terminal peptide tag and rabbit antimouse Ig-HRP antibodies (DAKO, Glostrup, Denmark). Blots were assayed using the enhanced chemiluminescence (ECL) detection system (Amersham, Little Chalfont, UK). Binding of soluble monoclonal scFv fragments to HuIgG Fc was determined by ELISA. ScFv fragments bound to antigen were detected using the mouse antimyc mAb 9E10, a rabbit antimouse Ig-HRP antibody (DAKO) and ABTS. The colour reaction was read at 415 nm in an ELISA reader.
Nucleic acid sequence analysis
Nucleotide sequence analysis of H and L chain variable regions from phage library-derived clones was performed on a LiCor infrared automated DNA Sequencer, using a dye-labelled M13 reverse primer (5′-GGA TAA CAA TTT CAC ACA GG-3′), and on an ABI 310 Genetic Analyser (Perkin Elmer) using PHENSEQ and LINKSEQ (5′-CGA TCC GCC ACC GCC AGA G-3′) primers [28]. To determine the most homologueous germline genes for the cloned V regions, sequences were aligned online with the V BASE sequence directory available at the MRC in Cambridge (Tomlinson et al. MRC Centre for Protein Engineering, Cambridge, UK; http://www.mrc-cpe.cam.ac.uk/imt-doc/vbase-home-page.html). For analysis of the number of replacement (R) and silent (S) mutations, the regions encoded by the primer used for PCR amplification and the complementarity-determining region 3 (CDR3) and JH/JL regions were excluded.
Results
Generation of libraries
Phage antibody display libraries were constructed using CD38+ B cells isolated from SFMCs of 2 RF-seropositive RA patients (G and V). L chain V regions were combined with the corresponding H chain V regions from IgM- or IgG-expressing B cells in phagemid vector pHEN-LINK, resulting in 4 combinatorial libraries (Gm, Gg, Vm, Vg) (Table 2). The libraries contained the numbers of individual clones as indicated (Table 2), of which at least 90% had both VH and VL inserts, as determined by restriction analysis of 10 randomly chosen clones of each library (Table 2).
Enrichment of HuIgG Fc-binding Phabs by panning
The obtained libraries (Vm and Vg) were rescued by superinfection with helper phage and HuIgG Fc-binding Phabs were selected by panning on human IgG Fc-coated surfaces. Eluted phages were used to infect E. coli and the libraries were again rescued with helper phage. Subsequently, the phage antibody particles were subjected to a second round of selection. Up to 4 rounds of rescue-selection-infection were performed. After each round of selection, phages were prepared from 94, randomly chosen, individual ampicillin resistant clones of each library and analysed for binding to HuIgG Fc by ELISA. After 3 rounds of selection, the frequencies of phages binding to HuIgG Fc in the Vm and Vg libraries were 5·3% and 86%, respectively. After 4 rounds of selection, the frequency of HuIgG Fc-binding phages in the Vm library was increased to 67% (Table 3). HuIgG Fc-binding moPhabs of the libraries, obtained after different rounds of selection, were analysed for diversity using MvaI DNA fingerprinting (data not shown). MoPhabs of clones that differed in their MvaI restriction patterns were tested for binding to solid-phase HuIgG Fc fragments in ELISA (Fig. 1a). Also monoclonal soluble scFv fragments were produced (Gg library) and tested (Fig. 1b).
Table 3.
Frequency of binding clones determined during successive rounds of panning on HuIgG Fc-coated surfaces
| Rounds of selection | ||||
|---|---|---|---|---|
| Library | 1 | 2 | 3 | 4 |
| Vm | 0/94 | 1/94 | 5/94 | 63/94 |
| Vg | 2/94 | 11/94 | 81/94 | |
Fig. 1.
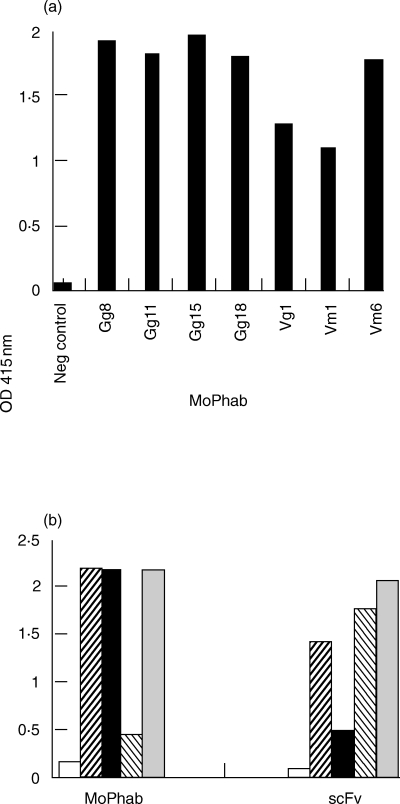
HuIgG Fc-binding reactivity of positive clones. (a) The binding to solid-phase HuIgG Fc of seven representative moPhabs (1011 phage particles/well) was assessed by ELISA. A monoreactive moPhab directed against group B Streptococcae was used as a negative control. (b) The reactivity of representative moPhabs (1012 phage particles/well) and their corresponding soluble monoclonal scFv fragments (1 : 2 diluted periplasmic scFv fragment preparations) towards HuIgG Fc was determined by ELISA. Gg24 was used as a representative clone which does not bind to HuIgG Fc. □ Gg24,  Gg19, ▪ Gg20,
Gg19, ▪ Gg20,  Gg21,
Gg21,  Gg22.
Gg22.
Sequence analysis of HuIgG Fc-binding scFv fragments
In order to demonstrate diversity, a total of 38 HuIgG Fc-binding clones from the libraries were used for nucleotide sequence analysis of VH and VL gene inserts. Twenty-one of these clones were previously analysed for diversity using MvaI DNA fingerprinting (data not shown) and 17 clones were randomly picked from HuIgG Fc-binding clones. Nucleotide sequences were compared to the V BASE germline sequence directory, a data base containing all known human V, D and J germline genes. The percentage of identity compared to the most homologueous germline gene was determined and DH and J segments were assigned when possible.
All VH regions analysed from the Vm library were in germline or in near-germline whereas the 2 identical VH regions from the Gm library harboured 13 mutations (Table 4). The 12 sequenced VH genes of the 2 libraries could be assigned to 3 different germline counterparts belonging to the VH1 (1x) and VH3 (4x) gene families. The VH regions analysed from the Gg library harboured somatic mutations, ranging from 3 to 13 mutations. In these VH regions, the overall ratio's of R and S mutations in CDR and framework regions (FRs) were 4·0 and 3·0, respectively. The heavy chain V regions from the Vg library of patient V contained less mutations compared to those of the corresponding library of patient G (Table 4). The 26 analysed VH genes of the Gg and Vg libraries could be assigned to 5 different germline counterparts belonging to the VH1 (3x) and VH3 (7x) gene families. Germline DH genes could be assigned in 29 out of 38 sequences (Table 4). All VD gene segments were joined to a JH3, JH4, JH5 or JH6 joining segment (Table 4). Comparison of H chain sequences revealed that 12 of the 18 clones derived from the Gg library (1) are encoded by the same DP-77/D3-3/JH4d rearrangement (2) bear identical CDR3 regions and (3) share somatic mutations compared to the DP-77 germline gene (Table 4 and Fig. 2). Interestingly, the VH regions of the Gg6 and Gg11 clones show a complete deletion of a codon at position 52a in the CDR2 region (Fig. 2). A statistical analysis of these VH genes was performed as described by Chang and Casali [31]. Consistent with selection by Ag, all VH genes displayed higher and lower numbers of R mutations in the CDR and FR, respectively, than those theoretically expected (Table 5). Also the probability that excess or scarcity of R mutations in CDRs or FRs resulted from chance only was calculated (Table 5). The VH gene of Gg6 and Gg11 was under negative pressure to mutate the FR structure and was under positive pressure to mutate the CDR structure (Table 5).
Table 4.
Molecular analysis of VH genes of monoclonal phageAbs selected for HuIgG Fc-binding
| Clone | VH family | Germline gene | Homology (%) | D gene | JH gene | CDR3 (AA) | CDR3 (AA) | CDR1 + 2 (R/S)* | FR1-3 (R/S)* | Total number of mutations |
|---|---|---|---|---|---|---|---|---|---|---|
| Gm1; Gm2 | 1 | DP-10 | 95·2 | na† | 6b | 15 | EGGGVAVAGYYGMDV | 2/1 | 3/5 | 13 |
| Gg10 | 3 | DP-77 | 98·9 | na | 4d | 13 | VDSSGGFGLEMDV | 2/0 | 1/0 | 3 |
| Gg1; Gg2; Gg5; Gg12; Gg13; Gg16; Gg17 | 3 | DP-77 | 98·9 | D3-3 | 4d | 18 | FPPGRFLEWLFPVWYFDY | 2/0 | 1/0 | 3 |
| Gg9 | 3 | DP-77 | 98·5 | D3-3 | 4d | 18 | FPPGRFLEWLFPVWYFDY | 2/0 | 2/0 | 4 |
| Gg3; Gg4 | 3 | DP-77 | 98·2 | D3-3 | 4a/4d | 18 | FPPGRFLEWLFPVWYFDY | 2/1 | 1/1 | 5 |
| Gg6; Gg11 | 3 | DP-77/HHG4 | 98·5 | D3-3 | 4a/4d | 18 | FPPGRFLEWLFPVWYFDY | 2/0‡ | 1/1 | 4 |
| Gg7; Gg8; Gg15; Gg18 | 1 | VHGL1·2 | 95·2 | na | 3b | 13 | GRRGSGGYFLVDI | 3/1 | 5/1 | 13 |
| Gg14 | 3 | DP-58 | 95·9 | na | 3b | 18 | PPVGYGSGNYPAFKTFNI | 3/2 | 4/2 | 11 |
| Vm1 | 3 | DP-35 | 100 | na | 3b | 15 | DPILPVAGTSDAFDI | 0/0 | 0/0 | 0 |
| Vm2; Vm7; Vm10 | 3 | DP-51 | 100 | D6-19 | 3b | 12 | GHSSGWYEAFDI | 0/0 | 0/0 | 0 |
| Vm3; Vm4; Vm5; Vm8; Vm9 | 3 | DP-51 | 99·6 | D6-19 | 3b | 12 | GHSSGWYEAFDI | 0/0 | 0/1 | 1 |
| Vm6 | 3 | DP-51 | 100 | D6-19 | 3b | 12 | GHSSGWYQAFDI | 0/0 | 0/0 | 0 |
| Vg1; Vg7; Vg8 | 3 | DP-35 | 94·8 | D7-27 | 5a | 12 | ERSWGSPSGDLI | 3/2 | 5/3 | 14 |
| Vg3; Vg4; Vg5; Vg6 | 1 | DP-14 | 100 | D5-5/D5-18 | 6b | 17 | GPADTANAPYYYCGMDV | 0/0 | 0/0 | 0 |
| Vg2 | 1 | DP-14 | 99·6 | D5-5/D5-18 | 6b | 17 | GPADTANAPYYYCGMDV | 0/0 | 1/0 | 1 |
Heavy chain D region could not be assigned;
Total number of replacement (R) and silent (S) mutations in CDR1 and CDR2 or framework (FR) (FR1, FR2 and FR3) regions of analysed moPhabs taking into consideration only the consequence of mutations at the level of the codon.
Does not include the deletion of the triplet at position 52a (fourth codon of CDR2).
Fig. 2.
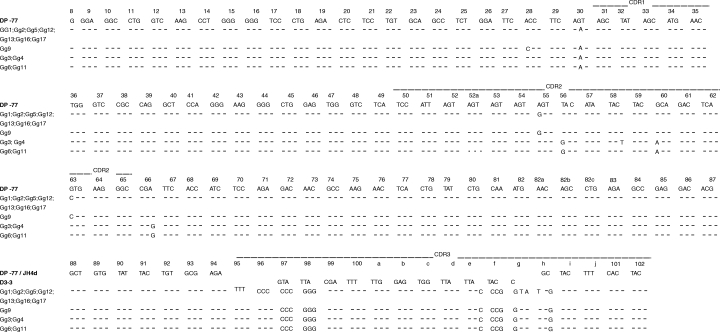
VH region nucleotide sequences of HuIgG Fc-binding clones of RA patient G and corresponding germline gene segments. Position of identity is indicated by a dash, deletion by a dot. Numbering is according to Kabat et al.[30].
Table 5.
R and S mutations in VH and VL genes of monoclonal phageAbs selected for HuIgG Fc-binding
| CDR1 + 2 | FR1-3 | ||||||
|---|---|---|---|---|---|---|---|
| Clone | Germline gene | R/S* | Expected R mutations† | P-value‡ | R/S* | Expected R mutations† | P-value‡ |
| VH gene | |||||||
| Gg1; Gg2; Gg5; Gg12; Gg13; Gg16; Gg17 | DP-77 | 2/0 | 0·6 | 0·097 | 1/0 | 1·7 | 0·328 |
| Gg9 | DP-77 | 2/0 | 0·8 | 0·155 | 2/0 | 2·2 | 0·365 |
| Gg3; Gg4 | DP-77 | 2/1 | 1·0 | 0·206 | 1/1 | 2·8 | 0·107 |
| Gg6; Gg11 | DP-77/HHG4 | 5/0§ | 1·4 | 0·004 | 1/1 | 3·9 | 0·029 |
| VL gene | |||||||
| Gg4 | IGLV3S2 | 3/0 | 1·3 | 0·092 | 1/3 | 4·0 | 0·025 |
| Gg5 | IGLV3S2 | 2/2 | 1·6 | 0·291 | 2/3 | 5·1 | 0·032 |
| Gg9 | IGLV3S2 | 7/1 | 2·7 | 0·008 | 4/3 | 8·5 | 0·014 |
| Gg10 | IGLV3S2 | 4/0 | 2·5 | 0·145 | 7/3 | 8·0 | 0·183 |
| Gm1 | IGLV3S2 | 7/0 | 4·0 | 0·053 | 9/6 | 12·5 | 0·055 |
| Gm2 | DPK22 | 2/0 | 0·7 | 0·130 | 2/0 | 2·3 | 0·359 |
| Gg18 | DPK22 | 5/0 | 1·3 | 0·003 | 1/1 | 4·0 | 0·024 |
Observed total number of replacement (R) and silent (S) mutations in CDR1 and CDR2 or framework (FR) (FR1, FR2 and FR3) regions of analysed moPhabs assuming that, in codons with 2 or 3 point mutations, the nucleotide exchanges occurred independently. Each mutation was classified as R or S in comparison to the germline sequence of the codon.
Calculated number of expected R mutations in V gene CDRs or FRs based on chance only [31].
Calculation (according to the binomial distribution model) of the probability p that excess or scarcity of R mutations (in Ig V gene CDRs or FRs) resulted from change only [31].
Deletion of triplet included.
The VL regions from the libraries of patient G harboured somatic mutations, ranging from 0 to 22 mutations. In these VL regions, the overall R/S ratio's in CDR and FR regions were 7·8 and 1·6, respectively. In line with the VH regions, VL genes from the libraries of patient V seemed to display less mutations compared to those of patient G. The 38 VL sequences from the libraries of the patients G and V were derived from 11 different germline genes belonging to the Vλ1 (11×), Vλ2 (1×), Vλ3 (11×), Vκ3 (3×) and Vκ4 (2×) family germline gene segments (Table 6). Comparison of L chain sequences revealed that some clones derived from the libraries of patient G are encoded by the same IGLV3S2/Jλ3b or IGLV3S2/Jλ2 or λ3a rearrangement, bear identical or almost identical CDR3 regions and share somatic mutations compared to the IGLV3S2 germline gene (Table 6 and Fig. 3). Also one VL region (clone Gg18) shows a complete deletion of a codon (at position 95a of the CDR3 region) compared to the clonally related VL region of Gm2 (Fig. 4). Statistical analysis of these VL genes revealed that, similar to the VH genes, all genes diplayed higher and lower numbers of R mutations in the CDR and FR, respectively, than those theoretically expected. The VL genes of Gg4, Gg5, Gg9 and Gg18 were under negative pressure to mutate the FR structure. The VL genes of Gg9 and Gg18 were under positive pressure to mutate the CDR structure (Table 5).
Table 6.
Molecular analysis of VL genes of monoclonal phageAbs selected for HuIgG Fc-binding
| Clone | VL family | Germline gene | Homology (%) | JL gene | CDR3 (AA) | CDR3 (AA) | CDR1 + 2 (R/S)* | FR1-3 (R/S)* | Total number of mutations |
|---|---|---|---|---|---|---|---|---|---|
| Gg4 | λ3 | IGLV3S2 | 97·0 | λ3b | 11 | QVWDSSSDHWV | 3/0 | 1/3 | 7 |
| Gg5 | λ3 | IGLV3S2 | 96·2 | λ3b | 11 | QVWDSSSDHWV | 2/2 | 2/3 | 9 |
| Gg9 | λ3 | IGLV3S2 | 93·6 | λ3b | 11 | QVWDSSSDHPM | 5/1 | 4/3 | 15 |
| Gg10 | λ3 | IGLV3S2 | 94·1 | λ2/λ3a | 9 | QAWDSSTVV | 2/0 | 5/2 | 14 |
| Gm1 | λ3 | IGLV3S2 | 90·7 | λ2/λ3a | 9 | QAWDSSTGV | 5/0 | 7/5 | 22 |
| Gm2 | κ3 | DPK22 | 98·4 | κ1 | 10 | QQYGGSRTWT | 2/0 | 2/0 | 4 |
| Gg18 | κ3 | DPK22 | 97·1 | κ1 | 9 | QQYGSSPGT | 4/0 | 1/1 | 7 |
| Gg11 | λ2 | V1-2 | 100 | λ1 | 10 | SSYAGSNNYV | 0/0 | 0/0 | 0 |
| Gg1 | λ1 | DPL2 | 94·2 | λ1 | 11 | ATWDDSLNGYV | 5/2 | 5/1 | 14 |
| Gg3; Gg7; Gg8; Gg15 | λ1 | DPL7/DPL8 | 100 | λ1 | 12 | QSYDSSLRGSKV | 0/0 | 0/0 | 0 |
| Gg6 | λ1 | DPL7/DPL8 | 99·6 | λ1 | 12 | QSYDSSLRGSKV | 1/0 | 0/0 | 1 |
| Gg12 | λ1 | DPL7/DPL8 | 99·2 | λ1 | 11 | QSYDSSLSLYV | 0/0 | 2/0 | 2 |
| Gg2 | λ1 | DPL7/DPL8 | 98·8 | λ3b | 10 | QSYDSSLNWV | 2/0 | 1/0 | 3 |
| Gg13 | λ3 | DPL23 | 98·7 | λ2/λ3a | 9 | QAWDSSTAV | 2/0 | 1/0 | 3 |
| Gg16 | κ4 | DPK24 | 99·2 | κ4 | 9 | QQYYSTPLT | 2/0 | 0/0 | 2 |
| Gg14 | κ4 | DPK24 | 98·8 | κ4 | 9 | QQYYSTSLT | 3/0 | 0/0 | 3 |
| Gg17 | λ1 | DPL7/DPL8 | 98·8 | λ3b | 11 | ATWDDSLSGPV | 1/0 | 0/2 | 3 |
| Vm2; Vm7 | λ3 | IGLV3S2 | 100 | λ3b | 12 | QVWDSSSDHPWV | 0/0 | 0/0 | 0 |
| Vg8 | λ3 | IGLV3S2 | 99·6 | λ2/λ3a | 11 | QVWDRNTDEGV | 0/0 | 1/0 | 1 |
| Vm1 | κ3 | DPK22 | 96·7 | κ4 | 9 | QHYGSSPLP | 5/0 | 1/2 | 8 |
| Vg1 | λ1 | DPL3 | 99·6 | λ3b | 10 | QSYDSSLSGV | 0/0 | 0/1 | 1 |
| Vm3; Vm4; Vm5; Vm8; Vm9; Vg2 | λ1 | DPL5 | 100 | λ3b | 11 | GTWDSSLSSWV | 0/0 | 0/0 | 0 |
| Vg3; Vg4 | λ1 | DPL5 | 99·6 | λ3b | 11 | GTWDSSLSSWV | 0/0 | 1/0 | 1 |
| Vg6 | λ1 | DPL7/DPL8 | 100 | λ3b | 10 | QSYDSSLSGV | 0/0 | 0/0 | 0 |
| Vm10 | λ1 | DPL7/DPL8 | 99·6 | λ3b | 10 | QSYDSSLSGV | 0/0 | 0/1 | 1 |
| Vm6 | λ3 | V2-19 | 99·6 | λ3b | 10 | YSAADNNPWV | 0/0 | 1/0 | 1 |
| Vg5 | λ3 | V2-14 | 94·9 | λ1 | 9 | QAWDSSTYV | 0/0 | 4/5 | 12 |
| Vg7 | λ3 | V2-14 | 95·3 | λ2/λ3a | 11 | QVWDRNTDEGV | 4/0 | 2/3 | 11 |
Total number of replacement (R) and silent (S) mutations in CDR1 and CDR2 or framework (FR) (FR1, FR2 and FR3) regions of analysed moPhabs taking into consideration only the consequence of mutations at the level of the codon.
Fig. 3.
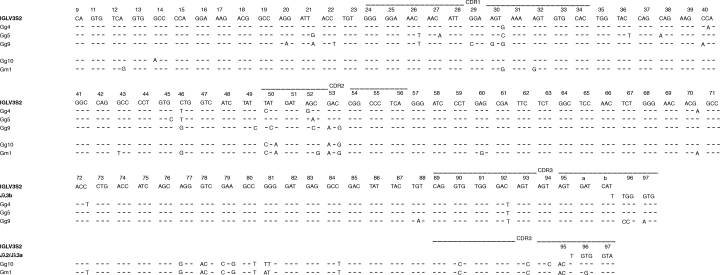
VL region nucleotide sequences of HuIgG Fc-binding clones of RA patient G and corresponding germline gene segments. Position of identity is indicated by a dash. Numbering is according to Kabat et al.[30].
Fig. 4.
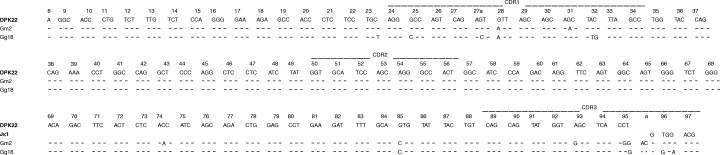
VL region nucleotide sequences of HuIgG Fc-binding clones of RA patient G and corresponding germline gene segments. Position of identity is indicated by a dash, deletion by a dot. Numbering is according to Kabat et al.[30].
Discussion
This report describes the V genes of moPhabs directed against HuIgG Fc which were constructed from terminally differentiated Ig-producing SF PCs derived from RF-seropositive patients with RA. The data reveal that RA-associated RFs are encoded by a diverse set of VL and a more restricted set of VH regions. VH gene family usage of PC-derived IgM- and IgG-RFs was found to be restricted to the VH1 and 3 gene families, with a preference for VH3, and many different VL genes were shown to contribute to RF specificity.
In the present study, clonally related VH as well as VL sequences were identified in the Gg library of patient G, based on the presence of identical CDR3 regions and shared somatic mutations. In this B cell selection process base-pair substitutions as well as deletions of triplets, leaving the transcripts in frame, were involved. Consistent with data of others [32–34], deletions, associated with somatic hypermutation, were found in CDR regions. The phenomenon was demonstrated to occur in VH and VL genes and is believed to provide a further mechanism for introducing structural diversity into antibodies [32,33]. Somatic mutation has occasionally been associated with increased affinity for Fc [7], as well as with changes in gross and fine specificity [35]. So, it seems that in this nonlymphoid tissue the GC-like structures provide a microenvironment in which selection of B cells occurs. However, we are aware that in combinatorial antibody libraries, cloned VH and VL genes are randomly combined and expressed as antibody fragments in bacteria. Thus, the VH/VL combinations analysed at the level of a moPhab may not be representative of the original combinations in vivo. However, an enrichment of autoantibody-specific PCs as applied in our procedure increases the chance of recovering original VH-VL paires [22,36]. Moreover, in this respect it is also relevant to note that substantial evidence is available that the H chain CDR3 region has a dominant role in both RF specificity and polyreactivity. In agreement with this we found that combinations of a single VH region with multiple VL regions yielded moPhabs with similar specificities. Consequently we believe that the VH usage of our Fc-binding moPhabs is indeed representative of the genuine RF repertoire but we cannot completely guarantee that the VL genes found represent the genuine RF VL genes.
Comparison with previously reported RFs obtained from RA synovial membranes with methods such as EBV cloning and somatic heterohybridization reveals similarity. RF from the synovial membrane of patients with RA predominantly used H chain segments encoded by genes from the VH3 family [37,38] and a substantial quantity of the RF-encoding genes was found to be somatically mutated [6, 7, 11, 12, 13, 14].
Harindranath et al.[39] has described the structural differences between ‘natural’ RFs occurring in healthy subjects and RFs of B cells derived from the PB compartment of patients with RA. Furthermore, V genes of IgM-RF-producing hybridomas obtained from PB of immunized healthy donors were compared with those established from RA synovial tissues [37,38]. The overall conclusion from the latter studies is that RF produced in the synovial tissue of patients with RA are encoded by a wider range of V genes than the RF in healthy immunized donors. Furthermore, there is an increase in the representation of VH3 genes in the synovial RF hybridomas. In addition, RF produced in RA synovia show evidence for affinity maturation [7, 37, 38, 40], isotype switch to IgG-RF [37,38] and exhibit differences in IgG binding specificity compared with RF from healthy immunized individuals [35]. These observations support the concept that there are two classes of RFs, physiological as part of the normal immune response and pathological associated with RA.
In patient G especially the DP-77/D3-3/JH4d rearrangement was frequently used. The intrinsic R/S ratio for random mutagenesis in CDRs and FRs of human VH and VL germline genes was found to be approximately 3 [41,42]. Upon Ag activation, in general, a strong counterselection of R mutations are observed in FRs, resulting in lower R/S-values, whereas the CDRs should be selected for affinity-increasing R mutations, resulting in higher R/S-values [42]. In the DP-77/D3-3/JH4d sequences the overall R/S ratio's in CDR and FR regions were 8·0 and 2·5, respectively, suggesting that clonal selection has occurred on the basis of Ag binding. The CDR3 regions of these clonally related sequences show evidence of extensive N region additions that are GC-rich and thought to be added through the activity of terminal deoxynucleotidyl transferase (TdT) [43, 44, 45, 46, 47, 48]. This phenomenon was previously demonstrated to occur in CDR3 regions of RFs [11,49]. The DP-77 gene segment was recently demonstrated to be involved in clonal expansion of RA synovial IgG1-RFs [50] and is the VH3 gene most frequently expressed, mostly unmutated, by peripheral IgM-RF+ RA B cells [51]. DP-35 and DP-58 were previously found to be gene segments that can potentially encode antibodies with RF activity [38, 40, 50]. A cDNA library generated from RA synovium revealed frequent utilization and clonal expansion of the DP-35 gene segment which is not frequently expressed in normal human antibody repertoires [20]. DP-10 has been reported to encode RF in patients with RA [38, 40, 52, 53, 54, 55, 56] and to be preferentially expressed in fetal liver during ontogeny of the antibody repertoire [57,58]. In both patients, Vλ1 and 3 rearranged with Jλ1 or 3b were preferentially used by the VL regions. Rearrangments of IGLV3S2/Jλ3b, IGLV3S2/Jλ2 or Jλ3a and DPL7/DPL8 with Jλ3b were often encountered and used in both patients. In the IGLV3S2/Jλ3b sequences of patient G the overall R/S ratio's in CDR and FR regions were 3·3 and 0·8, respectively. The overall R/S-values in CDR and FR regions of IGLV3S2/Jλ2 or Jλ3a rearrangements from patient G were 7/0 and 1·7, respectively. In the DPK22/κ1 sequences of the same patient the overall R/S-values in CDR and FR regions were found to be 6/0 and 3·0, respectively. These data indicate that also these clonally related VL sequences are the result of an Ag-driven response.
Somatically mutated V genes are also found among IgM-bearing B cells [42]. For example, the RA synovial tissue-derived IgM-RF RF-SJ1 was demonstrated to have accumulated a total of 16 substitutions in the H chain [7]. RF-SJ1 was shown to have 100-fold higher affinity for IgG than the clonally related RF close to germline. The VH region assigned to germline gene DP-10 derived from the Gm library of patient G harboured 13 mutations. However, the R/S-values in the CDR and FR regions of this H chain were 2·0 and 0·6, respectively, indicating that this rearrangement was not involved in an Ag-dependent GC reaction.
There are several examples where the V genes of RFs derived from RA synovial tissues are unmutated or only slightly mutated and it has been suggested that IgG binding is encoded in the germline repertoire [7, 11, 52, 59]. In line with these findings, the VH and VL gene segments from the libraries constructed from PCs of patient V harboured less mutations compared to those of patient G, suggesting that extensive somatic mutation is not required to generate disease-associated autoantibodies against IgG Fc.
This structural study of HuIgG Fc-binding moPhabs constructed from PCs isolated from the central site of inflammatory activity provides further evidence for an Ag-driven immune response in patients with RA, including expansion of clonally related B cells, selection and isotype switching, all hallmarks of a GC reaction.
Acknowledgments
We thank Dr J. de Kruif for support. This work was supported by the Dutch Arthritis Association (grant NR 842).
References
- 1.Withrington RH, Teitsson I, Valdimarsson H, Seifert MH. Prospective study of early rheumatoid arthritis. II. Association of rheumatoid factor isotypes with fluctuations in disease activity. Ann Rheum Dis. 1984;43:679–85. doi: 10.1136/ard.43.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zeben D, Hazes JMW, Zwinderman AH, Cats A, Van der Voort EAM, Breedveld FC. Clinical significance of rheumatoid factors in early rheumatoid arthritis. Ann Rheum Dis. 1992;51:1029–35. doi: 10.1136/ard.51.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan JH. Pathogenetic concepts and origins of rheumatoid factor in rheumatoid arthritis. Arthritis Rheum. 1993;36:1–6. doi: 10.1002/art.1780360102. [DOI] [PubMed] [Google Scholar]
- 4.Reparon-Schuijt CC, Van Esch WJE, Van Kooten C, Levarht EWN, Breedveld FC, Verweij CL. Functional analysis of rheumatoid factor-producing B cells from the synovial fluid of rheumatoid arthritis patients. Arthritis Rheum. 1998;41:2211–20. doi: 10.1002/1529-0131(199812)41:12<2211::AID-ART17>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Reparon-Schuijt CC, Van Esch WJE, Van Kooten C, Rozier BCD, Levarht EWN, Breedveld FC, Verweij CL. Regulation of synovial B cell survival in rheumatoid arthritis by VCAM-1 (CD106) expressed on fibroblast-like synoviocytes. Arthritis Rheum. 2000;43:1115–21. doi: 10.1002/1529-0131(200005)43:5<1115::AID-ANR22>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Hakoda M, Ishimoto T, Hayashimoto S, Inoue K, Taniguchi A, Kamatani N, Kashiwazaki S. Selective infiltration of B cells committed to the production of monoreactive rheumatoid factor in synovial tissue of patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1993;69:16–22. doi: 10.1006/clin.1993.1144. [DOI] [PubMed] [Google Scholar]
- 7.Randen I, Brown D, Thompson KM, et al. Clonally related IgM rheumatoid factors undergo affinity maturation in the rheumatoid synovial tissue. J Immunol. 1992;148:3296–301. [PubMed] [Google Scholar]
- 8.Olee T, Lu EW, Huang D-F, Soto-Gil RW, Deftos M, Kozin F, Carson DA, Chen PP. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992;175:831–42. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dechanet J, Merville P, Durand I, Banchereau J, Miossec P. The ability of synoviocytes to support terminal differentiation of activated B cells may explain plasma cell accumulation in rheumatoid synovium. J Clin Invest. 1995;95:456–63. doi: 10.1172/JCI117685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schröder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci USA. 1996;93:221–5. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victor KD, Randen I, Thompson K, Forre O, Natvig JB, Fu SM, Capra JD. Rheumatoid factors isolated from patients with autoimmune disorders are derived from germline genes distinct from those encoding the Wa, Po, and Bla cross-reactive idiotypes. J Clin Invest. 1991;87:1603–13. doi: 10.1172/JCI115174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascual V, Victor K, Randen I, Thompson K, et al. Nucleotide sequence analysis of rheumatoid factors and polyreactive antibodies derived from patients with rheumatoid arthritis reveals diverse use of VH and VL gene segments and extensive variability in CDR-3. Scand J Immunol. 1992;36:349–62. doi: 10.1111/j.1365-3083.1992.tb03108.x. [DOI] [PubMed] [Google Scholar]
- 13.Lu EW, Deftos M, Olee T, Huang D-F, Soto-Gil RW, Carson DA, Chen PP. Generation and molecular analyses of two rheumatoid synovial fluid-derived IgG rheumatoid factors. Arthritis Rheum. 1993;36:927–37. doi: 10.1002/art.1780360709. [DOI] [PubMed] [Google Scholar]
- 14.Ermel RW, Kenny TP, Chen PP, Robbins DL. Molecular analysis of rheumatoid factors derived from rheumatoid synovium suggests an antigen-driven response in inflamed joints. Arthritis Rheum. 1993;36:380–8. doi: 10.1002/art.1780360314. [DOI] [PubMed] [Google Scholar]
- 15.Casali P, Notkins AL. Probing the human B-cell repertoire with EBV. Polyreactive antibodies and CD5+ B lymphocytes. Ann Rev Immunol. 1989;7:513–35. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 16.Moynier M, Abderrazik M, Rucheton M, Combe B, Sany J, Brochier J. The B cell repertoire in rheumatoid arthritis. I. Frequency of EBV-inducible circulating precursors producing autoantibodies. J Autoimmunity. 1991;4:631–49. doi: 10.1016/0896-8411(91)90182-c. [DOI] [PubMed] [Google Scholar]
- 17.Martínez-Maza O, Britton S. Frequencies of the separate human B cell subsets activatable to Ig secretion by Epstein-Barr virus and pokeweed mitogen. J Exp Med. 1983;157:1808–14. doi: 10.1084/jem.157.6.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridges SL, Jr, Lee SK, Koopman WJ, Schroeder HW., Jr Analysis of immunoglobulin gamma heavy chain expression in synovial tissue of a patient with rheumatoid arthritis. Arthritis Rheum. 1993;36:631–41. [PubMed] [Google Scholar]
- 19.Lee SK, Bridges SL, Jr, Koopman WJ, Schroeder HW., Jr The immunoglobulin kappa light chain repertoire expressed in the synovium of a patient with rheumatoid arthritis. Arthritis Rheum. 1992;35:905–13. doi: 10.1002/art.1780350809. [DOI] [PubMed] [Google Scholar]
- 20.Clausen BE, Bridges SL, Jr, Lavelle JC, Fowler PG, Gay S, Koopman WJ, Schroeder HW., Jr Clonally-related immunoglobulin VH domains and nonrandom use of DH gene segments in rheumatoid arthritis synovium. Mol Med. 1998;4:240–57. [PMC free article] [PubMed] [Google Scholar]
- 21.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol. 1994;12:433–55. doi: 10.1146/annurev.iy.12.040194.002245. [DOI] [PubMed] [Google Scholar]
- 22.Rapoport B, Portolano S, McLachlan SM. Combinatorial libraries: new insights into human organ-specific autoantibodies. Immunol Today. 1995;16:43–9. doi: 10.1016/0167-5699(95)80070-0. [DOI] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Otten HG, Daha MR, De Rooij HH, Breedveld FC. Quantitative detection of class-specific rheumatoid factors using mouse monoclonal antibodies and the biotin/streptavidin enhancement system. Br J Rheum. 1989;28:310–6. doi: 10.1093/rheumatology/28.4.310. [DOI] [PubMed] [Google Scholar]
- 25.Marks JD, Tristem M, Karpas A, Winter G. Oligonucleotide primers for polymerase chain reaction amplification of human immunoglobulin variable genes and design of family-specific oligonucleotide probes. Eur J Immunol. 1991;21:985–91. doi: 10.1002/eji.1830210419. [DOI] [PubMed] [Google Scholar]
- 26.De Kruif J, Boel E, Logtenberg T. Selection and application of human single chain Fv antibody fragments from a semi-synthetic phage antibody display library with designed CDR3 regions. J Mol Biol. 1995;248:97–105. doi: 10.1006/jmbi.1995.0204. [DOI] [PubMed] [Google Scholar]
- 27.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–97. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- 28.Hoogenboom HR, Winter G. By-passing immunisation. Human antibodies from synthetic repertoires of germline VH gene segments rearranged in vitro. J Mol Biol. 1992;227:381–8. doi: 10.1016/0022-2836(92)90894-p. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths AD, Malmqvist M, Marks JD, et al. Human anti-self antibodies with high specificity from phage display libraries. EMBO. 1993;12:725–34. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. 5. Bethesda, MD: US Department of Health and Human Services; 1991. NIH Publications No. 91–3242. [Google Scholar]
- 31.Chang B, Casali P. The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol Today. 1994;15:367–73. doi: 10.1016/0167-5699(94)90175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson PC, De Bouteiller O, Liu Y-J, Potter K, Banchereau J, Capra JD, Pascual V. Somatic hypermutation introduces insertions and deletions into immunoglobulin V genes. J Exp Med. 1998;187:59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Wildt RMT, Van Venrooij WJ, Winter G, Hoet RMA, Tomlinson IM. Somatic insertions and deletions shape the human antibody repertoire. J Mol Biol. 1999;294:701–10. doi: 10.1006/jmbi.1999.3289. [DOI] [PubMed] [Google Scholar]
- 34.Goossens T, Klein U, Küppers R. Frequent occurrence of deletions and duplications during somatic hypermutation: Implications for oncogene translocations and heavy chain disease. Proc Natl Acad Sci USA. 1998;95:2463–8. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonagura VR, Agostino N, Børretzen M, Thompson KM, Natvig JB, Morrison SL. Mapping IgG epitopes bound by rheumatoid factors from immunized controls identifies disease-specific rheumatoid factors produced by patients with rheumatoid arthritis. J Immunol. 1998;160:2496–505. [PubMed] [Google Scholar]
- 36.Winter G, Milstein C. Man-made antibodies. Nature. 1991;349:293–9. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- 37.Thompson KM, Børretzen M, Randen I, Førre Ø, Natvig JB. V-gene repertoire and hypermutation of rheumatoid factors produced in rheumatoid synovial inflammation and immunized healthy donors. Ann NY Acad Sci. 1995;764:440–9. doi: 10.1111/j.1749-6632.1995.tb55861.x. [DOI] [PubMed] [Google Scholar]
- 38.Mageed RA, Børretzen M, Moyes SP, Thompson KM, Natvig JB. Rheumatoid factor autoantibodies in health and disease. Ann NY Acad Sci. 1997;815:296–311. doi: 10.1111/j.1749-6632.1997.tb52071.x. [DOI] [PubMed] [Google Scholar]
- 39.Harindranath N, Goldfarb IS, Ikematsu H, Burastero SE, Wilder RL, Notkins AL, Casali P. Complete sequence of the genes encoding the VH and VL regions of low- and high-affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int Immunol. 1991;3:865–75. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Børretzen M, Chapman C, Natvig JB, Thompson KM. Differences in mutational patterns between rheumatoid factors in health and disease are related to variable heavy chain family and germ-line gene usage. Eur J Immunol. 1997;27:735–41. doi: 10.1002/eji.1830270323. [DOI] [PubMed] [Google Scholar]
- 41.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–11. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 42.Klein U, Goossens T, Fischer M, Kanzler H, Braeuninger A, Rajewsky K, Küppers R. Somatic hypermutation in normal and transformed human B cells. Immunol Rev. 1998;162:261–80. doi: 10.1111/j.1600-065x.1998.tb01447.x. [DOI] [PubMed] [Google Scholar]
- 43.Deibel MR, Riley LK, Coleman MS, Cibull ML, Fuller SA, Todd E. Expression of terminal deoxynucleotidyl transferase in human thymus during ontogeny and development. J Immunol. 1983;131:195–200. [PubMed] [Google Scholar]
- 44.Desiderio SV, Yancopoulos GD, Paskind M, Thomas E, Boss MA, Landau N, Alt FW, Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984;311:752–5. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- 45.Gauss GH, Lieber MR. Mechanistic constraints on diversity in human V (D) J recombination. Mol Cell Biol. 1996;16:258–69. doi: 10.1128/mcb.16.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basu M, Hegde MV, Modak MJ. Synthesis of compositionally unique DNA by terminal deoxynucleotidyl transferase. Biochem Biophys Res Commun. 1983;111:1105–12. doi: 10.1016/0006-291x(83)91413-4. [DOI] [PubMed] [Google Scholar]
- 47.Kirkham PM, Schroeder HW., Jr Antibody structure and the evolution of immunoglobulin V gene segments. Semin Immunol. 1994;6:347–60. doi: 10.1006/smim.1994.1045. [DOI] [PubMed] [Google Scholar]
- 48.Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991;147:1720–9. [PubMed] [Google Scholar]
- 49.Randen I, Pascual V, Victor K, Thompson KM, Førre Ø, Capra JD, Natvig JB. Synovial IgG rheumatoid factors show evidence of an antigen-driven immune response and a shift in the V gene repertoire compared to IgM rheumatoid factors. Eur J Immunol. 1993;23:1220–5. doi: 10.1002/eji.1830230604. [DOI] [PubMed] [Google Scholar]
- 50.Williams DG, Moyes SP, Mageed RA. Rheumatoid factor isotype switch and somatic mutation variants within rheumatoid arthritis synovium. Immunology. 1999;98:123–36. doi: 10.1046/j.1365-2567.1999.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He X, Goronzy JJ, Zhong W, Xie C, Weyand CM. VH3-21 B cells escape from a state of tolerance in rheumatoid arthritis and secrete rheumatoid factor. Mol Med. 1995;1:768–80. [PMC free article] [PubMed] [Google Scholar]
- 52.Pascual V, Randen I, Thompson K, Sioud M, Forre O, Natvig J, Capra JD. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. J Clin Invest. 1990;86:1320–8. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borretzen M, Randen I, Natvig JB, Thompson KM. Structural restriction in the heavy chain CDR3 of human rheumatoid factors. J Immunol. 1995;155:3630–7. [PubMed] [Google Scholar]
- 54.Fang Q, Kannapell CC, Gaskin F, Solomon A, Koopman WJ, Fu SM. Human rheumatoid factors with restrictive specificity for rabbit immunoglobulin G. Auto- and multi-reactivity, diverse VH gene segment usage and preferential usage of VλIIIb. J Exp Med. 1994;179:1445–56. doi: 10.1084/jem.179.5.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen PP, Liu MF, Glass CA, Sinha S, Kipps TJ, Carson DA. Characterization of two immunoglobulin VH genes that are homologous to human rheumatoid factors. Arthritis Rheum. 1989;32:72–6. doi: 10.1002/anr.1780320112. [DOI] [PubMed] [Google Scholar]
- 56.Mantovani L, Wilder RL, Casali P. Human rheumatoid B-1a (CD5+ B) cells make somatically hypermutated high affinity IgM rheumatoid factors. J Immunol. 1993;151:473–88. [PMC free article] [PubMed] [Google Scholar]
- 57.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791–3. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- 58.Schroeder HW, Jr, Wang JY. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci USA. 1990;87:6146–50. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deftos M, Olee T, Carson DA, Chen PP. Defining the genetic origins of three rheumatoid synovium-derived IgG rheumatoid factors. J Clin Invest. 1994;93:2545–53. doi: 10.1172/JCI117265. [DOI] [PMC free article] [PubMed] [Google Scholar]


