Abstract
Two principal types of stress protein, heat shock proteins (hsps) and metallothionein (MT), are induced in cells responding to a variety of stresses. They play an important role in protecting cells from these stresses. However, many reports indicate that antibodies to hsps are present in human serum and are associated with several autoimmunity diseases. Metals, which are commonly allergenic to humans, induce both MT and hsp70 (one of the hsps family). Until now, there has been no report of any antibody to MT in human serum. In the present study, serum samples from healthy controls (Group I), and patients suffering from atopic dermatitis without (Group II) or with (Group III) metal allergy, were measured for antibodies to MT and hsp70, using an enzyme-linked immunosorbent assay (ELISA). Metal allergy was confirmed by patch testing. We first found that antibody to MT exists in human serum. We also found a high positive frequency of antibody to MT (51·3%) and to hsp70 (43·6%) in the sera of Group III, compared to those of Group I (3·8% and 5·1%) or Group II (6·4% and 5·1%). Furthermore, there was a strong positive correlation between antibody to MT and antibody to hsp70 in Group III (P = 0·0013), but not in Group I and Group II. Our results indicate that antibody to MT exists in human serum, as do antibodies to hsps, and suggest that elevated levels of MT and hsp70 antibodies are associated with metal allergy in atopic patients.
Keywords: antibody to hsp70, antibody to metallothionein, metal allergy, patch testing
Introduction
Metallothionein (MT) is a low-molecular mass (6–7 kD), cysteine-rich intracellular protein with a high affinity for metals. It is induced by environmental agents including heavy metals [1–4] and oxidative stress [5] or by inflammatory cytokines, such as interleukin-1 (IL-1) and IL-6 [6,7]. MT plays an important role in protecting cells from stresses such as those caused by metals. There are four isoforms of MT (I-IV). MT-I and MT-II are expressed in all tissues. Protection against metal toxicity has been attributed primarily to them. MT-III is localized mainly in the brain [8] and plays a role in zinc homeostasis in neurones [9], whereas MT-IV is localized in stratified squamous epithelium [10]. The function of MT-IV remains unclear.
Another class of proteins induced by stress is heat shock proteins (hsps). They are found in both prokaryotic and eukaryotic cells, and are induced by stresses both environmental (e.g. heat shock, heavy metals, or oxidants) or physiologic (e.g. infections, inflammation, or ischaemia) to protect cells from these stresses (reviewed in [11,12]). They are also expressed under nonstress conditions, playing essential roles in protein metabolism, including functions in protein folding, membrane translocation, and degradation of misfolded proteins (reviewed in [13,14]). Different families (low molecular weight, 60, 70, 90, 110 kD) of hsps can be distinguished on the basis of their molecular weight. However, many reports indicate that there are antibodies against different members of the hsp family in the sera of healthy subjects and patients with certain autoimmune diseases. In fact, antibody to hsp70 occurs not only in systemic lupus erythematosus but also in healthy subjects [15,16].
We assumed, therefore, that antibody to MT is also present in human serum, as are antibodies to hsps, particularly in cases with metal allergy. The aim of our study was to examine this hypothesis and to investigate the correlation between antibody to MT and antibody to hsp70.
Subjects And Methods
Subjects
Healthy blood donors were used as healthy controls (Group I, 24 male and 54 female; mean age, 38·9 years). Patients with atopic dermatitis from the Department of Dermatology (Yokohama City University School of Medicine) and Nakayama Dermatology Clinic, between March 2000 and February 2002, were selected as subjects for this study. Patients suffering from autoimmune diseases (e.g. lupus erythematosus) or other severe illness were excluded from the study. Serum samples were taken from all subjects and stored at − 80°C until they were assayed. After serum was taken, all subjects received patch testing to determine whether they had metal allergy. Patients suffering from atopic dermatitis without metal allergy were classified as Group II (24 male and 54 female; mean age, 39·0 years), while those with metal allergy were allocated to Group III (12 male and 27 female; mean age, 39·5 years). The Local Ethics Committee approved the study and all subjects gave informed consent.
Patch testing
Patch testing was carried out by applying the metal series patch test allergens (type 9) on the back for two days, using Miniplaster® (Torii, Japan), a classic vinyl tape with six cloth discs. The use of aluminium chambers was avoided, because some metal allergens, especially mercury bichloride, have been reported to produce hydrochloric acid by reactions with aluminium [17]. The reagents used were 2% copper sulphate (CuSO4) in aqueous solution (aq), 1% palladium chloride (PdCl2) aq, 0·4% potassium dichromate (K2Cr2O7) aq, 2 and 5% nickel sulphate (NiSO4) aq, 2% cobalt chloride (CoCl2) aq, 0·05 and 0·1% mercury chloride (HgCl2) aq, 1% tin chloride (SnCl4) aq, 1% cadmium sulphate (CdSO4) aq, 0·2% hydrogen tetrachloroaurate (HAuCl4) aq, 0·5% hydrogen hexachloroplatinate (H2PtCl6) aq, 2% iron chloride (FeCl3) aq, 1% indium chloride (InCl3) aq, 1% iridium chloride (IrCl4) aq, 1% molybdenum chloride (MoCl5) aq, 2% silver bromide (AgBr) in petrolatum (pet.), 1% antimony chloride (SbCl3) pet., 2% zinc chloride (ZnCl2) pet. and 2% manganese chloride (MnCl2) pet. The patch tests were read on the second, third and seventh days according to the recommendation of the International Contact Dermatitis Research Group (ICDRG) guidelines. These guidelines are as follows: negative (–), erythema only? (+), palpable erythema (+), erythema plus papules or vesicles (+ +), blister (+ + +). Reactions of (+) or more were diagnosed as metal allergy.
Antibodies to MT and hsp70
Antibodies to MT and hsp70 in sera were determined by an ELISA method. Microtiter plates (Nunc, Copenhagen, Denmark) were coated with 100 µl of 8 µg/ml purified MT (containing MT I and II, purchased from Sigma Chemical Co., St. Louis, MO), or 4 µg/ml of recombinant human hsp70 (StressGen, Victoria, Canada), in coating buffer (carbonate buffer, pH 9·8). The plates were incubated at 4°C overnight, then blocked with 1% human serum albumin (Sigma) in phosphate-buffered saline (PBS) at room temperature (RT). Diluted sera (1 : 50) were added to the wells in duplicate and the plates were incubated at RT for 1·5 h. The plates were then washed with washing buffer (PBS/0·05% Tween-20), and diluted (1 : 1000, in PBS with 1% human serum albumin) horseradish peroxidase (HRP)-conjugated antihuman IgG monoclonal antibody (PharMingen, San Diago, CA, USA) was added to the wells and incubated for 1 h at RT. The plates were again washed. Thereafter tetramethylbenzidine (TMB) and hydrogen peroxide substrate reagent (PharMingen) were added to the wells. After incubation at RT for 30 min, the reaction was stopped by 1 m phosphoric acid (H3PO4). The absorbance was measured within 30 min at 450 nm by a microplate reader (Bio-Rad model 550: Bio-Rad Laboratories, Hercules, CA).
All samples were prescreened, and samples of highest known level were assigned a concentration of 1000 arbitrary units/ml (AU/ml). The standard dose–response curves of antibody to MT and antibody to hsp70 were generated by using a serial dilution of these samples. The concentrations of antibodies in this samples were these calculated from curves. After correction for background, absorbances exceeding 0·3 were regarded as positive for antibodies to MT (45 AU/ml) and hsp70 (55 AU/ml).
Statistical analysis
Statistical analyses were performed using StatView software for Macintosh. Categorical data were compared by the χ2 and Fisher's exact test. Correlation between the levels of two antibodies was analysed by nonparametric Spearman test. The relation between the levels of the two antibodies was calculated by regression analysis. A value of P < 0·05 was considered to indicate a significant difference in all statistical analyses.
Results
Antibodies to MT and hsp70 were found not only in healthy controls but also in patients suffering from atopic dermatitis. Metal allergy was confirmed by patch testing. There were no differences in the positive frequency of the two antibodies between healthy controls (Group I) and patients suffering from atopic dermatitis without metal allergy (Group II). However, significantly high positive frequencies of antibodies to MT and hsp70 were found in the patients suffering from atopic dermatitis with metal allergy (Group III), compared with those of Group I or Group II (Table 1), and high titres of both antibodies were seen in Group III (Fig. 1).
Table 1.
Comparison of positive rates of antibody to MT (Ab to MT) and antibody to hsp70 (Ab to hsp70)
| Ab to MT | Ab to hsp 70 | |||
|---|---|---|---|---|
| Subjects | No. positive | (%) | No. positive | (%) |
| Group I (n = 78) | 3 | (3·8) | 4 | (5·1) |
| Group II (n = 78) | 5 | (6·4) | 4 | (5·1) |
| Group III (n = 39) | 20 | (51·3)*† | 17 | (43·6)*† |
Group I, healthy controls; Group II, patients suffering from atopic dermatitis without metal allergy; Group III, patients suffering from atopic dermatitis with metal allergy.
P < 0·0001, compared with Group I, and
P < 0·0001, compared with Group II, significant differences on χ2 and Fisher's exact test.
Fig. 1.
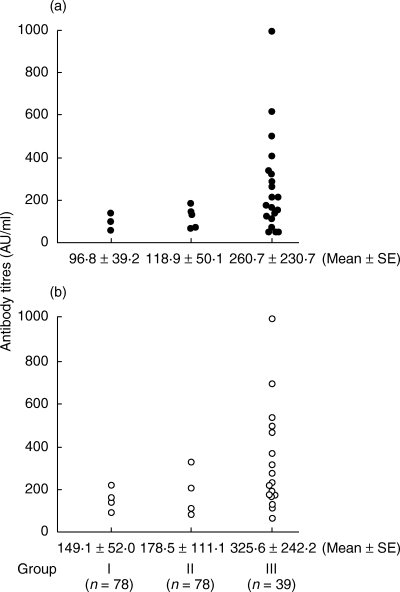
Antibodies to (a) MT (•) and (b) hsp70 (○) in sera were measured by ELISA method. Scattered plots showed positive antibody levels.
Numerous metals and their salts are known to be allergens in human. In this study, the results of patch testing of 39 patients showed hypersensitive reactions to different metals; the most common metal allergen was nickel (11 patients), and the next was mercury (5 patients).
The correlation between antibody to MT and antibody to hsp70 was calculated. There was a significant positive correlation between the two antibodies in Group III, and a significant negative correlation in Group I and II (Fig. 2). These results indicate that serum antibodies to MT and hsp70 are both present in cases of metal allergy.
Fig. 2.
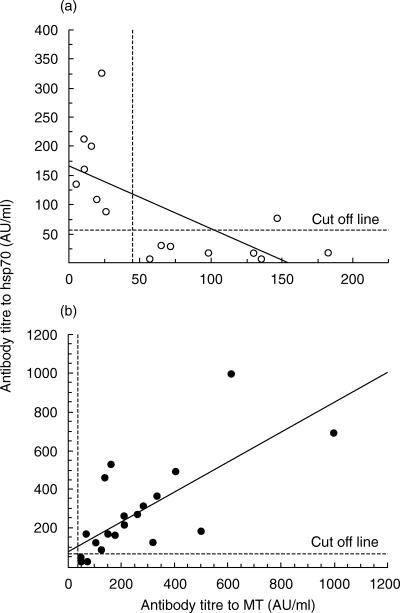
The relation between antibody to MT and antibody to hsp70 was calculated by regression analysis. Each plot represented one subject that showed positive to MT and/or hsp70. Correlation between two antibodies was analysed by nonparametric Spearman test. (a) Group I plus Group II (r* = −0·660, P = 0·0053). (b) Group III (r = 0·719, P = 0·0013).*Correlation coefficient.
Discussion
Exposure to metal compounds, including occupational and environmental pollutants, food contaminants and drugs, can give rise to adverse reactions mediated by the immune system. Metal compounds have attracted increasing attention for the last two decades. It is well known that numerous metals and their salts are allergens. Two pathogenically different modes of action have to be considered (reviewed in [18]). First, the metal compounds or their metabolites may exert toxic effects on the immune system as the target organ and cause a general dysfunction of immune response. Second, metal salts are able to elicit specific effects mediated by the immune system through the specific antigen receptor on T and B cells. Moreover, metal ions may alter the physiological processing and presentation of self-proteins or peptides and thus cause activation of autoreactive T cells (reviewed in [19]). Among various metals, nickel is the commonest allergen in humans. Most nickel exposure occurs through accessories and daily necessities. Investigative results among Finnish University students in 1995 showed that nickel allergy was encountered in 39% of all female students and in 42% of those with pierced skin [20]. A clear relation between ear piercing and nickel sensitization has been found in several studies [21,22]. Mercury is another common metallic allergen; it used to be a component of several drugs and still is an ingredient of preservatives, disinfectants, and ointments. The main exposure of the general population results from mercury as a major constituent of dental amalgams. In the metallic state, mercury reacts with eccrine sweat on the skin (reviewed in [23]), and with the oral mucosa, to form allergenic ions, which account for mercury-induced lichenoid lesions [24]. In fact, it has been reported that patients with dental restoration materials represent a subgroup with a high frequency of metal allergy [25]. Until now, there has been no effective curative medicine for this kind of allergy. What is necessary is to determine the allergens as fast as possible and to avoid exposure.
A specific set of proteins, called stress proteins, are induced, or their cellular synthesis enhanced, in response to a variety of stresses. Generally, there are two types of stress protein, metallothionein (MT) and heat shock proteins (hsps). MT is a cysteine-rich intracellular protein with a high affinity for metals. It is induced by stresses due to heavy metals [1–4], and has important cytoprotective effects against their toxins. It has been reported that MT represents a constitutive mechanism of defense expressed by different types of cells in the skin, which is triggered by contact with metallic compounds at patch-test sites [26].
The hsps are induced by stresses due to metals and other causes such as heat shock, oxidants, and infections (reviewed in [11,12]). They play an important role in protecting cells from these stresses. However, many reports indicate that there are antibodies against different members of the hsp family in the sera of healthy subjects and patients with certain autoimmune diseases. Immune responses against hsps can be cross-reactive among bacterial and human species and are capable of recognizing both foreign and self-hsps (reviewed in [11,27]). It has been suggested that healthy subjects may use this capacity to respond to self-hsp determinants and help eliminate infected autologous cells (reviewed in [27]). However, the amount of antibodies to hsps in the serum may also be elevated in patients with certain autoimmune diseases. In fact, antibody to hsp70 occurs not only in systemic lupus erythematosus but also in healthy subjects [15,16]. However, no data are currently available on the autoimmune response against hsps in patients with metal allergy.
The present research therefore was carried out to assess whether antibody to hsp70 is related to metal allergy, as hsp70 is induced by metals. Furthermore, we speculated that antibody to MT may also be present in human serum, like antibodies to hsps, and compared the positivity of antibodies to hsp70 and MT, between patients suffering from atopic dermatitis, with or without metal allergy. As our hypothesis, antibody to MT is also present in the serum. Moreover, significantly high positive rates of antibodies to MT and hsp70 are found in the sera of patients with metal allergy, but not in those without metal allergy. There is also a strong positive correlation between the two antibodies in cases of metal allergy, but not in other cases. These results suggest that elevated levels of MT and hsp70 antibodies are associated with metal allergy in atopic patients. There are many possible mechanisms triggering the breakdown of immunotolerance against MT or hsp70, and subsequent development of an anti-MT or hsp70 autoimmune response. First, extensive destruction of cells due to chronic exposure to metals can result in cell death and release of induced MT or hsp70. This can lead to bypassing of immunotolerance and to the onset of an autoimmune response. Second, metals bind with MT or affect hsp70, and this results in structural alteration and produces an immunogen. Further research is needed to investigate the exact mechanisms. Clearly, more studies are necessary to investigate the roles of antibody to MT in metal allergy and other autoimmune diseases.
Acknowledgments
This work was supported in part by a grant and aid from Sanstar Inc (Japan).
References
- 1.Rodilla V, Miles AT, Jenner W, Hawksworth GM. Exposure of cultured human proximal tubular cells to cadmium, mercury, zinc and bismuth: toxicity and metallothionein induction. Chem Biol Interact. 1998;14:71–83. doi: 10.1016/s0009-2797(98)00059-3. [DOI] [PubMed] [Google Scholar]
- 2.Schurz F, Sabater-Vilar M, Fink-Gremmels J. Mutagenicity of mercury chloride and mechanisms of cellular defence: the role of metal-binding proteins. Mutagenesis. 2000;15:525–30. doi: 10.1093/mutage/15.6.525. [DOI] [PubMed] [Google Scholar]
- 3.Zalups RK, Koropatnick J. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J Pharmacol Exp Ther. 2000;295:74–82. [PubMed] [Google Scholar]
- 4.Tandon SK, Singh S, Prasad S, Mathur N. Hepatic and renal metallothionein induction by an oral equimolar dose of zinc, cadmium or mercury in mice. Food Chem Toxicol. 2001;39:571–7. doi: 10.1016/s0278-6915(00)00167-8. [DOI] [PubMed] [Google Scholar]
- 5.Bauman JW, Liu J, Liu YP, Klaassen CD. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991;110:347–54. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- 6.De SK, McMaster MT, Andrews GK. Endotoxin induction of murine metallothionein gene expression. J Biol Chem. 1990;265:15267–74. [PubMed] [Google Scholar]
- 7.Kondo Y, Kuo SM, Lazo JS. Interleukin-1 beta-mediated metallothionein induction and cytoprotection against cadmium and cis-diamminedichloroplatinum. J Pharmacol Exp Ther. 1994;270:1313–8. [PubMed] [Google Scholar]
- 8.Palmiter RD, Findley SD, Whitmore TE, Durnam DM. MT-III, a brain-specific member of the metallothionein gene family. Proc Natl Acad Sci USA. 1992;89:6333–7. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masters BA, Quaife CJ, Erickson JC, Kelly EJ, Froelick GJ, Zambrowicz BP, Brinster RL, Palmiter RD. Metallothionein III is expressed in neurons that sequester zinc in synaptic vesicles. J Neurosci. 1994;14:5844–57. doi: 10.1523/JNEUROSCI.14-10-05844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, Palmiter RD. Induction of new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry. 1994;33:7250–9. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann SH. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–36. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 12.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–4. [PubMed] [Google Scholar]
- 13.Rothman JE. Polypeptide chain binding proteins. catalysts of protein folding and related processes in cells. Cell. 1989;59:591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 14.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–9. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 15.Kindas-Mugge I, Steiner G, Smolen JS. Similar frequency of autoantibodies against 70-kD class heat-shock proteins in healthy subjects and systemic lupus erythematosus patients. Clin Exp Immunol. 1993;92:46–50. doi: 10.1111/j.1365-2249.1993.tb05946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 17.Kubo Y, Nonaka S, Yoshida H. False positive reaction to patch testing with aqueous mercuric chloride in an aluminum Finn Chamber. Contact Dermatitis. 1992;26:136–7. doi: 10.1111/j.1600-0536.1992.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 18.Schuppe HC, Ronnau AC, Von Schmiedeberg S, Ruzicka T, Gleichmann E, Griem P. Immunomodulation by heavy metal compounds. Clin Dermatol. 1998;16:149–57. doi: 10.1016/s0738-081x(97)00194-6. [DOI] [PubMed] [Google Scholar]
- 19.Goldman M, Druet P, Gleichmann E. TH2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 20.Mattila L, Kilpelainen M, Terho EO, Koskenvuo M, Helenius H, Kalimo K. Prevalence of nickel allergy among Finnish University students in 1995. Contact Dermatitis. 2001;44:218–23. doi: 10.1034/j.1600-0536.2001.044004218.x. [DOI] [PubMed] [Google Scholar]
- 21.Dotterud LK, Falk ES. Metal allergy in north Norwegian schoolchildren and its relationship with ear piercing and atopy. Contact Dermatitis. 1994;31:308–13. doi: 10.1111/j.1600-0536.1994.tb02025.x. [DOI] [PubMed] [Google Scholar]
- 22.Meijer C, Bredberg M, Fischer T, Widström L. Ear piercing and nickel and cobalt sensitization, in 520 young Swedish men doing compulsory military service. Contact Dermatitis. 1995;32:147–9. doi: 10.1111/j.1600-0536.1995.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 23.Flint GN. A metallurgical approach to metal contact dermatitis. Contact Dermatitis. 1998;39:213–21. doi: 10.1111/j.1600-0536.1998.tb05912.x. [DOI] [PubMed] [Google Scholar]
- 24.Laine J, Kalimo K, Happonen RP. Contact allergy to dental restorative materials in patients with oral lichenoid lesions. Contact Dermatitis. 1997;36:141–6. doi: 10.1111/j.1600-0536.1997.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 25.Marcusson JA. Contact allergies to nickel sulfate, gold sodium thiosulfate and palladium chloride in patients claiming side-effects from dental alloy components. Contact Dermatitis. 1996;34:320–3. doi: 10.1111/j.1600-0536.1996.tb02215.x. [DOI] [PubMed] [Google Scholar]
- 26.Santucci B, Amantea A, Giuliano MC, Valenzano C, Cristaudo A. Expression of metallothioneins-I and – II isoforms at positive patch-test sites. Contact Dermatitis. 2000;43:103–6. doi: 10.1034/j.1600-0536.2000.043002103.x. [DOI] [PubMed] [Google Scholar]
- 27.Young RA. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–20. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]


