Abstract
This paper considers both monocytes and peripheral blood lymphocytes as potential targets for maternal immunological modulation in pregnancy. Peripheral blood mononuclear cells (PBMCs) from non-pregnant and normal pregnant donors were stimulated in vitro, and cytokine production detected intracellularly by flow cytometry. It was found that monocyte production of TNF-α was unaltered in pregnancy, while production of IL-12 was significantly enhanced. In contrast, production of the Th1 type cytokine IFN-γ was suppressed in the lymphocyte subsets: CD4+ T helper cells and CD56+ NK cells. Production of the Th2 type cytokine IL-4 in CD4+ cells was not significantly altered in pregnancy. These data suggest that the concept that pregnancy is a ‘Th2 phenomenon’ cannot be generalized to the function of all aspects of maternal cellular immunity as, paradoxically, circulating monocytes are ‘primed’ to produce the Th1 cytokine IL-12. Furthermore, these data support the hypothesis that components of maternal innate immunity are activated in normal pregnancy.
Keywords: human pregnancy, interleukin 12, monocytes, Th1/Th2
Introduction
It has been recognized for some time that the systemic maternal immune system is altered in some way in normal pregnancy [1,2], and the prevailing framework is that pregnancy is a ‘Th2 phenomenon’ [3]. In other words, maternal immune function is biased towards Th2 type (antibody-mediated) responses. Experimental evidence for this hypothesis is still limited as far as human pregnancy is concerned. Most workers have focused on the production of the cytokines IL-4 (Th2 type) and IFN-γ (Th1 type) by stimulated lymphocytes in vitro, looking for a shift towards Th1 in cases of pregnancy failure [4]. In normal pregnancy the IL-4 : IFN-γ production ratio has been shown to be increased when cytokines are detected extracellularly [5] or intracellularly in T cells [6].
Lymphocytes, however, are only one component of the immune system. Monocytes play a key role in any immune response by processing antigen, producing cytokines, so determining the nature of the subsequent lymphocyte response. Less is known about circulating monocytes in normal human pregnancy, although we [7] and other investigators [8,9] have reported evidence that they are activated. Activated monocytes produce a range of cytokines, some of which are of immediate relevance to the Th1 : Th2 paradigm. TNF-α is a proinflammatory cytokine which has been associated with Th1 type responses in animals, although less so in humans [10]. IL-12, on the other hand, is primarily a monocyte product which is a defining cytokine of a Th1 type response [11]. It stimulates NK cells and T lymphocytes, induces the production of IFN-γ and enhances cell-mediated immunity.
Therein lies an apparent paradox that if pregnancy is a ‘Th2 phenomenon’, production of Th1 type cytokines including both IFN-γ and IL-12 would be predicted to be suppressed, while if monocytes are activated in pregnancy, IL-12 (and TNF-α) production would be enhanced. It is therefore of interest that while IL-12 and TNF-α are not normally present in the blood of non-pregnant women, both the p40 subunit of IL-12 [12] and low levels of TNF-α [13] have been reported in blood from normal non-labouring pregnant women.
More useful functional data require an accurate assessment of the source of cytokine production, and this can be achieved even in heterogeneous cell populations such as peripheral blood leucocytes. After stimulation in vitro, flow cytometry has been used to detect intracellular cytokines in lymphocytes [14–16] and, to a lesser extent, in monocytes [17,18]. We have recently reported similar techniques for detecting intracellular cytokines in trophoblast cells [19]. There are reports of altered production of lymphocyte cytokines in a range of conditions [20,21] including normal pregnancy and pre-eclampsia [6]. There are also reports of increased monocyte production of IL-12 in immune deficient patients [22].
In this study we set out to investigate cell-specific Th1 (IL-12, IFN-γ, TNF-α) and Th2 (IL-4) type cytokine production in peripheral blood mononuclear cells isolated from normal pregnant women. The detection of intracellular cytokines in monocytes from pregnant women has not been reported previously.
Materials and methods
Subjects
Healthy non-pregnant women (n = 18) were recruited from hospital staff, and were of reproductive age (median 30, range 20–45 years), not on any medication and had no history of chronic inflammatory disease, allergy or blood transfusions. Similarly healthy pregnant women (n = 20) were recruited with informed consent from antenatal clinics (John Radcliffe Hospital and Quarry Surgery, Oxford), with a median age of 30·5 years (range 21–37). All were in the third trimester of pregnancy (median gestation 34 weeks, range 30–40) and not in labour at the time of blood sampling, and progressed normally to term. This study was approved by the Central Oxford Research Ethics Committee.
Preparation of peripheral blood mononuclear cells (PBMCs)
Fifteen ml of venous blood was obtained from the antecubital fossa using a syringe and 21-G needle, anticoagulated with preservative-free sodium heparin (10 IU/ml blood) (Sigma, St Louis, MO, USA), and added to a 50-ml Nunc tube (GibcoBRL, Life Technologies, Paisley, Scotland) containing 30 ml endotoxin-free phosphate buffered saline (PBS). Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation and resuspended in a standard concentration of 2 × 106 cells per ml.
PBMCs were suspended in RPMI culture medium with l-glutamine (GibcoBRL Life Technologies), containing 1 mm sodium pyruvate (Sigma), 2 × 10−5m beta-mercaptoethanol (Sigma), 105 IU/l penicillin (Glaxo-Wellcome, Greenford, UK), 5 mg/l streptomycin (Sigma) and 10% serum supreme (Biowhittacker, Wokingham, Berks, UK). A batch of RPMI medium with a very low level of endotoxin (0·0024 ng/ml) guaranteed by the supplier was reserved and used for all experiments. The sodium pyruvate and the antibiotics were solubilized in deionized, RNAse-free, endotoxin-free water (‘Milli-Q’, Millipore, Watford, UK) and, with the beta-mercaptoethanol, were filtered through a 0·2-µm filter before addition to the RPMI medium. Serum supreme, a fetal calf serum with minimal endotoxin contamination (<0·2 pg/ml), was heat-inactivated and freshly prepared as a 10% solution in RPMI medium for each experiment.
The stimulation of PBMCs in vitro
One-ml aliquots of 2 × 106 PBMCs were placed in flat-bottomed 24-well ‘cell suspension plates’ (Sarstedt, Leicester, UK), and incubated in 5% CO2 at 37°C with 2·4 µm monensin (Sigma), a Golgi apparatus inhibitor to increase cytokine accumulation within cells, together with leucocyte stimulant cocktails. Monocytes were stimulated with 0·04–40 ng/ml endotoxin (Sigma) and 5·5 ng/ml recombinant human interferon-γ (Pharmingen, Cambridge Bioscience, Cambridge, UK). Lymphocytes were stimulated with 39 ng/ml phorbol myristate acetate (Sigma) and 1 µm calcium ionophore A23187 (Sigma).
After incubation the PBMCs were resuspended gently using a pipette, and the contents of each well divided into two Eppendorf tubes (106 cells per tube) for antibody labelling. These aliquot pairs provided the test sample and a control for any changes in monocyte size, granularity and non-specific antibody binding (which could be considerable) for the different stimulation conditions in each well.
Antibodies
Directly conjugated monoclonal antibodies were used to label surface antigen markers for monocytes (CD14) and natural killer (NK) cells (CD56) (Table 1). T-helper cells were labelled with an unconjugated anti-CD4 antibody and then a secondary goat antimouse antibody directly conjugated to the fluorescent dye allophycocyanin (APC). Mouse isotypic control antibodies were FITC-conjugated IgG2b (Coulter, Luton, UK), PE-conjugated IgG1 (Serotec) and unconjugated IgG1 (Serotec, Kidlington, UK). Intracellular cytokine production was detected with directly conjugated antibodies to: TNF-α and IL-12 in monocytes, IL-4 in CD4+ T-cells, and IFN-γ in both CD4+ T-cells and CD56+ NK cells. Mouse isotypic control antibodies were all IgG1 (PE or FITC conjugated) and obtained from Pharmingen. All antibodies were titrated with stimulated PBMCs to determine saturating concentrations, and isotypic control antibodies were then used at equivalent immunoglobulin concentrations.
Table 1.
Antibodies for labelling leucocytes
| Antibody | ||||
|---|---|---|---|---|
| Antigen | Location | Subtype | Dilution | Conjugate label |
| Monocytes | ||||
| CD14 | Surface | IgG2b | 1 : 4 | FITC* |
| TNF-α | Intracellular | IgG1 | 1 : 4 | PE† |
| IL-12 | Intracellular | IgG1 | 1 : 8 | PE† |
| Lymphocytes | ||||
| CD4 | Surface | IgG1 | Neat | Unconjugated‡ |
| CD56 | Surface | IgG1 | Neat | PE‡ |
| IFN-γ | Intracellular | IgG1 | 1 : 40 | FITC† |
| IL-4 | Intracellular | IgG1 | 1 : 4 | PE† |
Coulter, Luton, UK (My4 epitope).
Pharmingen, Cambridge Bioscience, Cambridge, UK
Serotec, Kidlington, UK
PBMC antibody labelling
The cells were spun down in a microcentrifuge (13 000 g for 7 s), the supernatant was removed, and the cells were resuspended in 50 µl ‘staining buffer’ [PBS 0·5% sodium azide and 0·5% serum supreme (Biowhittacker)] containing monoclonal antibodies to leucocyte surface antigens. Samples were incubated on ice in the dark for 30 min, washed by adding 400 µl staining buffer and spun down. Samples labelled with directly conjugated antibodies were then fixed and permeabilized. All buffers had their pH adjusted to 7·4–7·6 before being passed through a 0·2-µm filter and stored at 4°C.
Samples labelled with unconjugated anti-CD4 antibody were resuspended in 50 µl staining buffer with a secondary goat antimouse allophycocyanin (APC)-conjugated antibody (Molecular Probes, Cambridge Bioscience, Cambridge, UK) and kept on ice in the dark for 30 min. After two washes with staining buffer, the cells were resuspended for 15 min in 150 µl staining buffer containing mouse IgG immunoglobulin (Sigma) to block non-specific binding. The samples were centrifuged and the pelleted cells fixed and permeabilized by resuspending in 100 µl ‘fixation buffer’ (PBS, 4% paraformaldehyde). After 20 min on ice in the dark, 400 µl staining buffer was added, the samples gently vortexed, and spun down in a microcentrifuge. The samples were resuspended in 50 µl ‘permeabilization buffer’ (PBS, 0·5% sodium azide, 0·5% serum supreme and 0·1% saponin) containing either one or two monoclonal antibodies to intracellular cytokines. The samples were incubated for 30 min on ice in the dark, and washed by adding 400 µl permeabilization buffer, gently vortexed, and spun down. The samples were finally resuspended in 250 µl staining buffer and analysed by flow cytometry.
Flow cytometry
Samples were analysed on a Coulter Epics Elite flow cytometer using an Argon-ion (488 nm) and Helium–Neon (633 nm) laser. The lasers were aligned using Immunocheck beads (Coulter). Photomultiplier tubes (PMTs) collected fluorescent light at 535 nm (FITC), 575 nm (PE) and 675 nm (APC). Due to spectral overlap, fluorescence colour compensation was performed using single-labelled positive controls (either monocytes or lymphocytes). This was done before each experiment, although there were consistent compensations of approximately 18% for FITC fluorescence overlap on PE, 1% for PE overlap on FITC, 10% for PE on APC and 1% for APC on PE.
Up to 70 000 PBMC events were collected, and all data were saved in ‘listmode’ for subsequent analysis using Coulter Elite and Reallist software. Monocytes and lymphocytes were distinguished by forward and side scatter characteristics, and gated initially on these physical characteristics. Then, within each gate, monocytes and lymphocytes were analysed for surface antigen markers and intracellular cytokines.
Intracellular TNF-α and IL-12 were detected in cells within the ‘monocyte gate’ which were double-labelled with anticytokine-PE and CD14-FITC antibodies. Positively labelled populations were determined by quadrant gates, which were set to include ≤1% of cells of the negative control (Fig. 1a). In addition, the brightness of fluorescence of the positive cells was a semiquantitative measure of the amount of antigen (surface or intracellular) detected. For direct comparison between different experiments, standard Flow Set Beads (Coulter) were analysed and the ratio of the mean channel brightness (mean fluorescence intensity) of positive cells to the mean channel brightness of the beads was recorded as the ‘brightness ratio’.
Fig. 1.
Flow cytometry analysis. (a) Monocytes: monocytes were gated on size and granularity and analysed for cytokine production by double labelling with anti-CD14-FITC and either anti-TNF-α-PE or anti IL-12-PE antibodies. (b) Th lymphocytes: lymphocytes were gated on size and granularity and CD4+ lymphocyte production of IFN-γ and IL-4 was analysed by triple labelling with anti-CD4-APC, anti-IFN-γ-FITC and anti IL4-PE. Single colour plots were gated for CD4+ lymphocytes and two colour plots of these cells labelled for IL-4 and IFN-γ are shown. (c) NK cells: lymphocytes were gated on size and granularity and NK cell production of IFN-γ was analysed by double-labelling with anti CD56-PE and anti IFN-γ-FITC.
In the lymphocyte gate, triple labelling enabled CD4+ T helper cells to be analysed for both IFN-γ and IL-4 simultaneously (Fig. 1b) and CD56+ NK cells were analysed for IFN-γ production (Fig. 1c).
In order to validate the stimulation of leucocyte intracellular cytokines a series of preliminary experiments on non-pregnant individuals (not presented here) used similar stimulation protocols except for the absence of monensin. In these, the supernatants were collected and tested for TNF-α, IL-12, IFN-γ and IL-4 with standard commercial ELISA kits (Genzyme, West Malling, Kent, UK; Lifescreen, Watford, UK). Dose and time response profiles were identical whether cytokines were detected intracellularly by flow cytometry or extracellularly by ELISA.
Six hours’ incubation was chosen empirically as a time when all cytokines were detectable intracellularly, most leucocytes were viable and surface antigen markers enabled specific cell-types to be analysed by flow cytometry. This also meant that a single blood sample could be used for the analysis of more than one cytokine, including controls for fluorescence compensation.
Leucocyte viability
Cell viability was monitored by labelling with propidium iodide (Clontech, Cambridge Bioscience, Cambridge, UK). In the presence of monensin and after stimulation with endotoxin and IFN-γ viability was consistently over 95%, although viability was reduced to approximately 80% when lymphocytes were stimulated with the PMA and ionophore cocktail.
Statistics
Comparisons between groups of non-pregnant and normal pregnant women were analysed by the Mann–Witney U-test (GraphPad Prism Software, San Diego, CA, USA), and differences were considered statistically significant for P-values < 0·05.
Results
Monocyte production of TNF-α
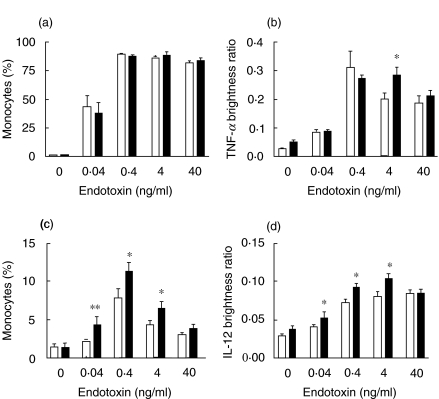
There were no differences in the percentages of CD14+ monocytes from non-pregnant and normal pregnant women producing TNF-α after stimulation with a wide range of concentrations of endotoxin (Fig. 2a). There was no difference in the intensity of staining for TNF-α between non-pregnant and pregnant monocytes apart from at 4 ng/ml endotoxin (Fig. 2b). In both groups, the TNF-α brightness ratio was significantly less for an endotoxin concentration of 40 ng/ml, compared with 0·4 ng/ml (P < 0·05).
Fig. 2.
Monocyte production of intracellular TNF-α and IL-12 PBMCs from 12 healthy non-pregnant and 12 normal pregnant women were incubated for 6 h with endotoxin (0·04–40 ng/ml), IFN-γ (5·5 ng/ml) and monensin (2·4 µm), or with monensin only. The cells were double-labelled for CD14 and intracellular TNF-α or IL-12, and 60 000 events were analysed by flow cytometry. The percentage of CD14+ cells which were TNF-α positive (a) and the brightness of TNF-α positive monocytes (b) were calculated. Significant differences are shown between non-pregnant and pregnant groups (*P < 0·05).Monocyte production of IL-12 was analysed in the same way (c, d). Significant differences are shown between non-pregnant and pregnant groups (*P < 0·05, **P < 0·01). □, Non-pregnant control (n = 12); ▪, normal pregnant subjects (n = 12).
Monocyte production of IL-12
Fewer monocytes were stimulated to produce IL-12 than TNF-α, and monocytes from non-pregnant women were not stimulated at all with endotoxin levels of 0·04 ng/ml (Fig. 2c). With endotoxin levels of 0·04, 0·4 and 4 ng/ml, significantly more CD14+ monocytes from pregnant women were stimulated to produce IL-12 than non-pregnant women, although there was no difference at 40 ng/ml. The pregnant group also had higher IL-12 brightness ratios than the non-pregnant group with endotoxin levels of 0·04, 0·4 and 4 ng/ml (Fig. 2d). Again, there was no significant difference with 40 ng/ml endotoxin.
T-helper cell production of IL-4 and IFN-γ
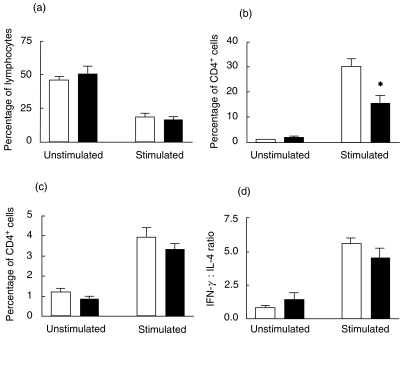
The percentage of CD4+ cells in the lymphocyte gate was reduced after stimulation (Fig. 3a) due to down-regulation of CD4 expression. Analysis of CD4- cells in the lymphocyte gate was similar to CD4+ cells and is not presented here. There were no differences on the percentages of CD4+ cells in the lymphocyte gate for non-pregnant and pregnant women with or without stimulation (Fig. 3a). However, significantly fewer CD4+ T-cells from pregnant women were stimulated to produce IFN-γ than CD4+ T-cells from non-pregnant control women (Fig. 3b). Less than 5% of CD4+ cells produced detectable levels of intracellular IL-4 after stimulation for 6 h, and there was no difference between the pregnant and control groups (Fig. 3c). For each stimulated PBMC sample, the ratio of CD4+ cells that were IFN-γ positive (i.e. Th1) to IL-4 positive (i.e. Th2) was calculated. The decrease in the IFN-γ : IL-4 ratio in pregnancy failed to reach statistical significance (Fig. 3d).
Fig. 3.
CD4+ T-helper cell production of intracellular IFN-γ and IL-4 PBMCs from six healthy non-pregnant and six normal pregnant women were incubated for 6 h with monensin (2·3 µm) alone, or with the additional presence of stimulants PMA (40 ng/ml) and calcium ionophore A23187 (1 µm). Cells were triple-labelled for CD4 and intracellular IFN-γ and IL-4, and 60 000 events analysed by flow cytometry. The percentage of CD4+ cells in the lymphocyte gate is shown for non-pregnant and pregnant women (a) The percentage of CD14+ cells which were IFN-γ positive (b) and IL-4 positive (c) was calculated. Significant differences are shown between non-pregnant and pregnant groups (*P < 0·05). The ratio of IFN-γ : IL-4 producing cells (d) was not significantly different between the two groups. □, Non-pregnant control (n = 6); ▪, normal pregnant subjects (n = 6).
Natural killer (NK) cell production of IFN-γ
The percentage of CD56+ NK cells producing IFN-γ in response to PMA/calcium ionophore was significantly lower in pregnant women compared to non-pregnant women. No IFN-γ production was seen in unstimulated cells from either group (Fig. 4).
Fig. 4.
Natural killer (NK) cell production of intracellular IFN-γ PBMCs from healthy six non-pregnant and six normal pregnant women were incubated for 6 h with monensin (2·3 µm) alone, or with the additional presence of stimulants PMA (40 ng/ml) and calcium ionophore A23187 (1 µm). Cells were double-labelled for CD56 and intracellular IFN-γ, and 60 000 events analysed by flow cytometry. Significant differences between non-pregnant and pregnant groups in the percentages of IFN-γ producing cells in the lymphocyte gate are shown (**P < 0·01). □, Non-pregnant control (n = 6); ▪, normal pregnant subjects (n = 6).
Discussion
To the best of our knowledge, this is the first report of intracellular cytokine expression by monocytes in pregnancy. Cell-specific methods were used to show that, in response to stimulation in vitro, expression of TNF-α is not suppressed, while monocytes are primed to express the critical Th1 type cytokine IL-12. These results are independent of the increased monocyte numbers [8] and expression of CD14 [7] in pregnancy. As a percentage, there were more CD14+ monocytes stimulated to produce IL-12, and more IL-12 was detected intracellularly in those stimulated cells. The cytokines detected intracellularly are not necessarily secreted, although similar profiles of cytokine release were demonstrated, and others have correlated intracellular cytokine detection with cytokine release [15]. In pregnant women in vivo there may be other regulatory mechanisms (e.g. anergy, regulatory T cells, HLA-G) which might influence cytokine secretion. Nevertheless, we have shown that the ability of PBMCs to produce cytokines is not Th2 biased.
Relatively few studies of intracellular cytokine detection in monocytes have been published [17,18]. This study is consistent with others in that TNF-α was detected in up to 70% of monocytes [17], while IL-12 was detected in fewer cells (up to 20%) in identical conditions. This was not owing to inadequate stimulation, fixation or permeabilization because a TNF-α ‘positive control’ was used in each experiment. Moreover, preliminary experiments confirmed that the IL-12 antibody was used at saturating concentrations and also that other potential IL-12-producing cell-types (e.g. CD19+ B cells) as well as T and NK cells were not significant producers of IL-12 in these stimulation conditions.
Varying doses of endotoxin produced similar monocyte IL-12 and TNF-α response profiles, and it was interesting that higher doses of endotoxin produced less, rather than more intracellular cytokines. It is possible that different stimuli could result in different time courses of cytokine production, with higher doses producing an earlier peak. Other studies have found a variable monocyte response to endotoxin depending on the dose and time of incubation [23]. However, differences in the production of IL-12 between non-pregnant and pregnant groups of women were found at both high and low concentrations of endotoxin.
The priming of monocyte IL-12 production in pregnancy appears to be a specific effect as TNF-α production is not primed. The significance of this activated phenotype is not known, although it has also recently been reported in patients with common variable immunodeficiency [22]. In pregnancy the findings are consistent with a report that PBMC expression of TNF-α mRNA expression is not altered [24]. There are no reports on IL-12 mRNA expression in pregnancy. However, there is an apparent inconsistency with a recent report that production of IL-12 by cultured PBMCs was reduced in normal pregnancy [25]. In that study, however, not only were there significant methodological differences, but IL-12 was detected extracellularly by ELISA, and therefore was not a specific measure of monocyte activity.
IL-12 activates NK cells, facilitates the induction of cytotoxic T cell responses, stimulates T cell and to a lesser extent NK cell proliferation, stimulates IFN-γ production from both T and NK cells and specifically regulates the induction of Th1 cells [11]. Thus, in the event of IL-12 production, Th2 type responses may, to some extent, be switched to Th1 or Th0 type responses.
As far as stimulated lymphocytes are concerned, our findings are consistent with other reports demonstrating a pregnancy Th2 bias [6]. The stimulation and detection of IFN-γ and IL-4 in CD4+ cells (up to 30% and 4% of CD4+ cells, respectively) demonstrated in this study were in general agreement with other investigators [14, 15, 16,20,21]. Fewer CD4+ T helper cells from pregnant women were stimulated to produce the Th1 cytokine IFN-γ. However, this study failed to show a specific increase in production of the Th2 type cytokine IL-4 in pregnancy, possibly because the incubation time of 6 h was optimized for other cytokines (TNF-α and IFN-γ) rather than IL-4. IL-4 is a notoriously difficult cytokine to detect by flow cytometry [14, 15, 16,20,21] or ELISA [5], and perhaps clearer differences would have emerged if conditions were specifically optimized for it. In preliminary experiments PBMCs were primed by incubating them for 3 days with IL-4, IL-2 and anti-CD3 before stimulation, and over 30% of cells were found to be IL-4 positive in those conditions.
In pregnancy, not only were there fewer CD56+ NK cells in PBMC suspensions, but production of IFN-γ was suppressed (as it was in CD4+ cells). These data are consistent with reports of specific suppression of NK cell numbers [26] and activity [27] from the first trimester of normal pregnancy.
Thus this study demonstrates the previously recognized suppression of some elements of maternal adaptive immunity (T cells, NK cells) in normal pregnancy. It is therefore highly significant that, using similar techniques on the same blood samples, monocyte cytokine expression of IL-12 is enhanced. We cannot conclude that IL-12 is a constant feature of the maternal immune system in vivo but there appears to be a more complex alteration of overall maternal immune function than that proposed by Wegmann et al. [3]. We have proposed that components of innate immunity are activated [28].
The factors that activate monocytes in pregnancy are not known. We have recently reported that trophoblast cells produce IL-4 [19] which, after 24 h incubation in vitro, primes the production of IL-12 (both mRNA and protein release) [29]. Alternatively, whole cells (e.g. trophoblast cells, fetal red and white blood cells) as well as subcellular particles (e.g. syncytiotrophoblast microvillous membrane fragments) are detectable in the maternal circulation [30] and we hypothesize that such material is likely to be phagocytosed by circulating monocytes and result in their activation [31]. We have, moreover, proposed that this represents an important signalling mechanism for pregnancy [28].
This paper analyses systemic priming which is not necessarily reflective of local cytokine production at the maternal–fetal interface. Such studies are more technically challenging but are necessary to define the cytokine environment of the invading trophoblast. However, two recent studies of decidual cell-types in spontaneous abortion also challenge predictions from the Th1/Th2 hypothesis [32,33].
The model used in this study (i.e. the effect of endotoxin on monocytes) may have significant clinical implications. Monocyte expression of the main endotoxin receptor (CD14) is increased significantly in the third trimester of normal pregnancy [7], and this could mediate the enhanced monocyte cytokine response to endotoxin. Increased sensitivity of pregnant women to endotoxin in vivo is not proven, although there is evidence that some bacterial infections have more severe clinical presentations [34], and such sensitivity is well recognized in pregnant animals [35]. In particular, the experimental Shwartzman reaction, to which pregnant animals are uniquely primed, is a fatal inflammatory response mediated by IL-12 [36], and we have hypothesized that the inflammatory sequences underlying the reaction have their human equivalent in the pathogenesis of pre-eclampsia [37]. Indeed, there is a report that women with severe or complicated pre-eclampsia have elevated serum levels of IL-12 [12]. We propose that the source of production of this cytokine is circulating monocytes, which are already primed to produce IL-12 by the state of pregnancy.
Other pregnancy complications may also be mediated by abnormal IL-12 production resulting from increased sensitivity to endotoxin. IL-12 has been shown to be abortifacient in mice [38], and increased levels are found in the amniotic fluid of women with preterm labour [39].
In conclusion, in normal human pregnancy the relative suppression of Th1 type cytokine lymphocyte responses (producing a ‘Th2 bias’ as proposed by Wegmann et al. [3]) is accompanied by a sparing or even enhancement of monocyte pro-inflammatory cytokine responses. Although not described previously in pregnancy, a similar pattern of changes has been reported in other conditions, notably in allergic asthma [40], giving credence to the hypothesis that such modulation of the immune system is not only possible but also more common than believed hitherto. We believe it signals a critical role of innate immunity in the maternal adaptation to pregnancy.
Acknowledgments
This study was supported by Action Research. The authors would like to thank Dr Sarah Germain for here expert assistance with the preparation of this manuscript.
References
- 1.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–38. [Google Scholar]
- 2.Sargent IL. Maternal and fetal immune responses during pregnancy. Exp Clin Immunogenet. 1993;10:85–102. [PubMed] [Google Scholar]
- 3.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 4.Raghupathy R. Pregnancy: success and failure within the Th1/Th2/Th3 paradigm. Semin Immunol. 2001;13:219–27. doi: 10.1006/smim.2001.0316. [DOI] [PubMed] [Google Scholar]
- 5.Marzi M, Vigano A, Trabattoni D, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996;106:127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Quantitative analysis of peripheral blood Th0, Th1, Th2 and the Th1 : Th2 cell ratio during normal human pregnancy and preeclampsia. Clin Exp Immunol. 1999;117:550–5. doi: 10.1046/j.1365-2249.1999.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks GP, Studena K, Sargent IL, Redman CWG. Normal pregnancy and pre-eclampsia both produce inflammatory changes in peripheral blood leucocytes akin to sepsis. Am J Obstet Gynecol. 1998;179:80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 8.Smarason AK, Gunnarsson A, Alfredsson JH, Valdimarsson H. Monocytosis and monocytic infiltration of decidua in early pregnancy. J Clin Laboratoryimmunol. 1986;21:1–5. [PubMed] [Google Scholar]
- 9.Shibuya T, Izuchi K, Kuroiwa A, Okabe N, Shirakawa K. Study on nonspecific immunity in pregnant women: increased chemiluminescence response of peripheral blood phagocytes. Am J Reprod Immunol Microbiol. 1987;15:19–23. doi: 10.1111/j.1600-0897.1987.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Hanlon AM, Jang S, Salgame P. Signaling from cytokine receptors that affect Th1 responses. Front Biosci. 2002;7:1247–54. doi: 10.2741/hanlon. [DOI] [PubMed] [Google Scholar]
- 11.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 12.Dudley DJ, Hunter C, Mitchell MD, Varner MW, Gately M. Elevations of serum interleukin-12 concentrations in women with severe pre-eclampsia and HELLP syndrome. J Reprod Immunol. 1996;31:97–107. doi: 10.1016/0165-0378(96)00976-x. [DOI] [PubMed] [Google Scholar]
- 13.Kupferminc MJ, Peaceman AM, Wigton TR, Tamura RK, Rehnberg KA, Socol ML. Immunoreactive tumor necrosis factor-alpha is elevated in maternal plasma but undetected in amniotic fluid in the second trimester. Am J Obstet Gynecol. 1994;171:976–9. doi: 10.1016/0002-9378(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 14.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Meth. 1995;188:117–28. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 15.Elson LH, Nutman TB, Metcalfe DD, Prussin C. Flow cytometric analysis for cytokine production identifies T helper 1, T helper 2, and T helper 0 cells within the human CD4+CD27- lymphocyte subpopulation. J Immunol. 1995;154:4294–301. [PubMed] [Google Scholar]
- 16.Openshaw P, Murphy EE, Hosken NA, et al. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Caestecker MP, Telfer BA, Hutchinson IV, Ballardie FW. The detection of intracytoplasmic interleukin-1 alpha, interleukin-1 beta and tumour necrosis factor alpha expression in human monocytes using two colour immunofluorescence flow cytometry. J Immunol Meth. 1992;154:11–20. doi: 10.1016/0022-1759(92)90207-a. [DOI] [PubMed] [Google Scholar]
- 18.Fujishima S, Nakamura H, Waki Y, et al. Cell-associated IL-8 in human blood monocytes: analysis by flow cytometry. Cytometry. 1996;24:382–9. doi: 10.1002/(SICI)1097-0320(19960801)24:4<382::AID-CYTO10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Sacks GP, Clover LM, Bainbridge DR, Redman CW, Sargent IL. Flow cytometric measurement of intracellular Th1 and Th2 cytokine production by human villous and extravillous cytotrophoblast. Placenta. 2001;22:550–9. doi: 10.1053/plac.2001.0686. [DOI] [PubMed] [Google Scholar]
- 20.North ME, Ivory K, Funauchi M, Webster AD, Lane AC, Farrant J. Intracellular cytokine production by human CD4+ and CD8+ T cells from normal and immunodeficient donors using directly conjugated anti-cytokine antibodies and three-colour flow cytometry. Clin Exp Immunol. 1996;105:517–22. doi: 10.1046/j.1365-2249.1996.d01-795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakazawa M, Sugi N, Kawaguchi H, Ishii N, Nakajima H, Minami M. Predominance of type 2 cytokine-producing CD4+ and CD8+ cells in patients with atopic dermatitis. J Allergy Clin Immunol. 1997;99:673–82. doi: 10.1016/s0091-6749(97)70030-7. [DOI] [PubMed] [Google Scholar]
- 22.Cambronero R, Sewell WA, North ME, Webster AD, Farrant J. Up-regulation of IL-12 in monocytes: a fundamental defect in common variable immunodeficiency. J Immunol. 2000;164:488–94. doi: 10.4049/jimmunol.164.1.488. [DOI] [PubMed] [Google Scholar]
- 23.Wittmann M, Larsson VA, Schmidt P, Begemann G, Kapp A, Werfel T. Suppression of interleukin-12 production by human monocytes after preincubation with lipopolysaccharide. Blood. 1999;94:1717–26. [PubMed] [Google Scholar]
- 24.Chen GR, Wilson Wang SH, Zheng HZ, Walker JJ, McKillop JH. Tumour necrosis factor-alpha (TNF-alpha) gene polymorphism and expression in pre-eclampsia. Clin Exp Immunol. 1996;104:154–9. doi: 10.1046/j.1365-2249.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai M, Tsuda H, Tanebe K, Sasaki Y, Saito S. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Am J Reprod Immunol. 2002;47:91–7. doi: 10.1034/j.1600-0897.2002.1o020.x. [DOI] [PubMed] [Google Scholar]
- 26.Matthiesen L, Berg G, Ernerudh J, Hakansson L. Lymphocyte subsets and mitogen stimulation of blood lymphocytes in normal pregnancy. Am J Reprod Immunol. 1996;35:70–9. doi: 10.1111/j.1600-0897.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Gregory CD, Rees GB, Scott IV, Golding PR. Cytotoxic activity and phenotypic analysis of natural killer cells in early normal human pregnancy. J Reprod Immunol. 1987;12:35–47. doi: 10.1016/0165-0378(87)90079-9. [DOI] [PubMed] [Google Scholar]
- 28.Sacks GP, Sargent IL, Redman CWG. An innate view of human pregnancy. Immunol Today. 1999;20:114–8. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 29.D’Andrea A, Ma X, Aste Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor alpha production. J Exp Med. 1995;181:537–46. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight M, Redman CWG, Linton EA, Sargent IL. Syncytiotrophoblast microvilli are shed into the maternal circulation in increased amounts in pre-eclamptic pregnancy. Br J Obstet Gynaecol. 1998;105:632–40. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 31.Redman CWG, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- 32.Plevyak M, Hanna N, Mayer S et al. Deficiency of decidual IL-10 in first trimester missed abortion: a lack of correlation with the decidual immune cell profile. Am J Reprod Immunol. 2002;47:242–50. doi: 10.1034/j.1600-0897.2002.01060.x. [DOI] [PubMed] [Google Scholar]
- 33.Michimata T, Ogasawara MS, Tsuda H, et al. Distributions of endometrial NK cells, B cells, T cells an Th1/Th2 cells fail to predict pregnancy outcome following recurrent abortion. Am J Reprod Immunol. 2002;47:196–202. doi: 10.1034/j.1600-0897.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 34.Brabin BJ. Epidemiology of infection in pregnancy. Rev Infect Dis. 1985;7:579–603. doi: 10.1093/clinids/7.5.579. [DOI] [PubMed] [Google Scholar]
- 35.Mori W. The Shwartzman reaction: a review including clinical manifestations and proposal for a univisceral or single organ third type. Histopathology. 1981;5:113–26. doi: 10.1111/j.1365-2559.1981.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 36.Ozmen L, Pericin M, Hakimi J, et al. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–15. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redman CWG, Sacks GP, Sargent IL. Pre-eclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 38.Shiraishi H, Hayakawa S, Satoh K. Murine experimental abortion by IL-12 administration is caused by activation of cytotoxic T lymphocytes and placental apoptosis. Clin Laboratory Immunol. 1996;48:93–108. [PubMed] [Google Scholar]
- 39.Lemancewicz A, Urban R, Skotnicki M, Kretowska M, Sierakowski S. Evaluation of interleukin concentrations in amniotic fluid in preterm and term parturution and in oligohydramnios. Med Sci Monit. 1997;7:924–7. [PubMed] [Google Scholar]
- 40.Wong CK, Ho CY, Ko FW, et al. Proinflammatory cytokines (IL-17, IL-6, IL-18 and IL-12) and Th cytokines (IFN-gamma, IL-4, IL-10 and IL-13) in patients with allergic asthma. Clin Exp Immunol. 2001;125:177–83. doi: 10.1046/j.1365-2249.2001.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]