Abstract
Pregnancy is a challenge to the immune system, which not only has to protect the mother and the fetus from invading pathogens but to also maintain immunological tolerance against the fetus. However, the mechanisms inhibiting local immune responses in the maternal decidual tissue are poorly understood. We have studied decidual CD14+ macrophages, which may be important in the maintenance of a tolerance against the developing fetus. Decidual macrophages expressed HLA-DR, but lower levels of costimulatory molecule CD86 than peripheral blood CD14+ monocytes from pregnant and non-pregnant women. Decidual macrophages produced spontaneously high levels of interleukin-10. Our findings suggest that decidual macrophages could represent an inhibitory type of APCs. Supporting this conclusion indoleamine 2,3-dioxygenase (IDO), suggested to have an immunosuppressive role in pregnancy, was expressed in decidual macrophages. Furthermore, decidual macrophages were not able to differentiate into dendritic cells under the influence of IL-4 + GM-CSF. These results suggest an immunoinhibitory function of decidual macrophages at the maternal–fetal interface.
Keywords: cytokines, decidual macrophages, IDO, phenotype
Introduction
Among the antigen-presenting cells (APCs), dendritic cells (DCs) have a central role in the initiation of adaptive immune responses [1,2]. In addition to their stimulatory properties, APCs have also been shown to have regulatory role in the immune system with their capacity to induce anergy or tolerance against the encountered antigen [3]. Furthermore, DCs induce T helper (Th) cell differentiation not only into Th1 but also into Th2 type of cells and the importance of DCs in the peripheral tolerance is evident [4–6].
An intriguing question is how the APCs regulate the immune system during pregnancy, when the maternal immune system is challenged with semi-allogenic fetal antigens. The invasion of the trophoblasts deep into the uterine tissue seems to occur without any apparent activation of the maternal immune system. Decidual tissue lining the uterus contains considerable amount of haematopoietic mononuclear cells throughout the pregnancy. Decidual macrophages comprise 20% of these cells, whereas only few T cells are present and B cells are virtually absent [7–9]. CD83+ DCs have been found in the decidual tissue in the early phase of pregnancy indicating the presence of immunostimulatory mature DCs [10]. NK-like cells constitute the main mononuclear cell population in the early pregnancy, but their number decreases significantly towards the term [9].
Several mechanisms have been suggested to explain the maternal tolerance against the fetus. Decidual tissue is known to be immunosuppressive and macrophages in decidua have been shown to mediate suppression in one-way mixed lymphocyte reaction [11–14]. The expression of MHC class I molecule HLA-G is considered to protect trophoblasts from the attack of maternal NK cells by activating the killer inhibitory receptors (KIRs) [15,16]. Placental cytotrophoblasts have been shown to produce anti-inflammatory cytokine interleukin (IL)-10 and express Fas ligand (FasL) [17,18]. It is believed generally that the predominance of Th2-type of responses favours successful pregnancy [19,20]. Progesterone has several immunosuppressive effects, including Th2-skewing [21,22]. Recently, tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) was shown to block the maternal T cell activation against the murine fetus [23]. Human placental and decidual cells have been shown to express IDO [24,25].
The mechanisms maintaining the immunosuppressive environment in the pregnant uterus has still remained unresolved. In the present study we show that the decidual macrophages express HLA-DR with low levels of costimulatory molecules CD80 and CD86. These decidual macrophages were not capable of differentiating into DCs. Furthermore, decidual macrophages produced spontaneously high levels of IL-10 and expressed IDO. These findings suggest that decidual CD14+ cells represent immunoinhibitory type of macrophages. We propose a central role for these cells in the local regulation of maternal immune responses against the fetus.
Materials and methods
Reagents and antibodies
In the isolation of decidual cells RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS; Bioproducts for Science, Indianapolis, IN, USA), penicillin and streptomycin (Biological Industries, Kibbutz beit Haemek, Israel) was used. Decidual cells were cultured in Iscove's modified Dulbecco's medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% FCS, 1 mmol/l HEPES (Gibco BRL), 0·1 mm 2-mercaptoethanol and 100 µg/ml gentamycin (Biological Industries, Kibbutz beit Haemek, Israel). Antibodies used in the flow cytometry are described in Table 1. Anti-CD14 conjugated microbeads for magnetic cell sorting (MACS) were from Miltenyi Biotec (Auburn, CA, USA). Purified recombinant human (rh) IL-4 and M-CSF were from R&D Systems (Minneapolis, MN, USA), rhGM-CSF (Leucomax) from Schering-Plough/Sandoz, interferon gamma (IFN-γ) was obtained from DNAX Research Institute (Palo Alto, CA, USA) and LPS (Escherichia coli serotype 0127:B8) from Sigma (St Louis, MO, USA). Enzymes DNase I, hyaluronidase and collagenase used for decidual cell isolation were obtained from Sigma (St Louis, MO, USA).
Table 1.
Monoclonal antibodies
| Antibody | Source |
|---|---|
| PE-conjugated antibodies | |
| HLA-DR | Becton Dickinson (San Jose, CA, USA) |
| CD14 | Becton Dickinson (San Jose, CA, USA) |
| CD80 | Becton Dickinson (San Jose, CA, USA) |
| CD86 | PharMingen (San Diego, CA, USA) |
| fitc-conjugated antibodies | |
| CD14 | Becton Dickinson (San Jose, CA, USA) |
| CD40 | PharMingen (San Diego, CA, USA) |
| Non-conjugated antibodies | |
| CD1a | PharMingen (San Diego, CA, USA) |
| CD40 | Schering-Plough Research Institute (Kenilworth, NJ, USA) |
| CD83 | Dr T.F. Tedder (Duke University Medical Center, Durham, NC, USA) |
| ILT-3 (ZM.4) | Dr M. Colonna (Basel Institute for Immunology, Basel, Switzerland) |
Peripheral blood and decidual cells
This study was approved by the joint ethical committee of Turku University Hospital and Turku University and informed consent was obtained from the women participating in this study. The peripheral blood samples were obtained from pregnant women visiting Turku University Hospital antenatal care unit and from healthy female controls. Buffy coats of blood were obtained from healthy human donors (Finnish Red Cross Blood Transfusion Service, Turku, Finland). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. The fetal membranes were obtained from women delivered by elective caesarean section at term (>37 weeks’ gestation) in Turku University Hospital. The isolation technique was a modification from a method described in detail by Vince et al. 1990 [9]. In brief, the decidual cells were isolated by scraping the decidual tissue from the maternal surface of the fetal membranes. The tissue was digested with DNase I (50 µg/ml), collagenase (300 U/ml) and hyaluronidase (2 mg/ml) in RPMI-1640 culture medium. Ten ml of this enzyme cocktail was used per 1 g wet weight of tissue, with pulsed digestion of 3 × 20 min at 37°C with stirring. After each incubation the tissue was allowed to settle and the supernatant containing released cells was removed. Finally, the dispersed cells were filtered through metal sieve and silk, and washed twice with Hanks's buffered solution (HBS). Mononuclear cells were then separated with Ficoll-Hypaque gradient centrifugation. In the flow cytometric analysis of the decidual cells, isolated as described above, 39% ± 8 (n = 9) of these cells were HLA-DR and 35% ± 9 (n = 9) CD14 positive. These frequencies were similar between individual samples. As CD14 is predominantly expressed on cells of myelomonocytic lineage the phenotypic characterization of decidual cells was performed using double staining, always including anti-CD14.
Flow cytometry and cell sorting
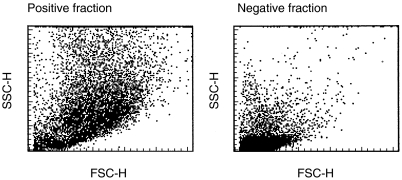
The isolated cells were incubated with FITC- or PE-conjugated MoAb for 30 min at 4°C followed by two washes. When non-conjugated MoAb was used, incubation of 30 min was followed by PE-conjugated goat antimouse IgM or IgG1 for another 30 min and 1% normal mouse serum for 15 min and finally incubation with FITC- or PE-conjugated MoAb for 30 min. Two washes were employed after each incubation. As negative controls, FITC- or PE-conjugated non-specific mouse IgG or Sp 2/0 (mouse hybridoma culture supernatant) was used. The cells were analysed using FACScan flow cytometer and Cellquest software (Becton Dickinson, San Jose, CA, USA). In the PB and decidual cell samples the CD14+ cells were gated and analysed for the expression of other surface antigens. The data are expressed as mean fluorescence intensity ratios (MFIRs) (mean fluorescence intensity with MoAb of interest/mean fluorescence intensity with control MoAb). The cells were sorted by MACS using CD14 microbeads (Miltenyi Biotec). The purity of the positive selection of CD14+ cells from decidua samples ranged from 72 to 94% (Fig. 1).
Fig 1.
Decidual cells after positive selection using anti-CD14 microbeads. The dotplots of FACS analysis of CD14 positive and negative fractions are shown.
Cell culture and cytokine measurement
The cells (0·5–2 × 106/well) were cultured in medium (IMDM, 10% FCS) with or without LPS (0·5 or 1 µg/ml) for 2 days. Cytometric bead array, human inflammation (BD Biosciences, CA, USA) was used to measure concentrations of IL-10, TNF-α, IL-1β and IL-12p70 from the culture supernatants. TGF-β (R&D Systems, Minneapolis, MN, USA) and also IL-10 (CLB, the Netherlands) concentrations were measured by specific ELISAs. In the case of DC culture the CD14+ cells (0·5–1 × 106/well) were cultured in flat-bottom 24-well plates (Costar, Cambridge, MA, USA) in medium alone or with IL-4 (500 U/ml) and GM-CSF (50 ng/ml) for 7 days [26,27]. To obtain mature DCs the cells were further cultured for additional 24–36 h with LPS (1 µg/ml) or TNF-α (10 ng/ml).
IDO expression by RT-PCR
Decidual CD14+ cells (1 × 106) (purity of positive cells ranged between 72 and 90%) were resuspended in Ultraspec (Biotecx, Houston, TX, USA) lysis buffer (n = 6). As positive control PB CD14+ cells (0·25 × 106/well) were cultured in the presence of M-CSF (200 U/ml) for five days and on the fifth day IFN-γ (10 ng/ml) was added for 24 h [28]. After culture the cells were resuspended in Ultraspec. PB CD14+ cells, immature DCs and mature DCs were also resuspended in Ultraspec. RNA isolation was done as described by the manufacturer. Oligo-p(dT)-primed cDNA were synthesized using avian myeloblastosis virus (AMV) reverse transcriptase in a 20-µl reaction volume (1st Strand cDNA Synthesis Kit for RT-PCR, Roche Diagnostics Corp., Indianapolis, IN, USA). Each reaction mixture was incubated at 42°C for 1 h using a DNA thermal cycler (Perkin-Elmer, Norwalk, CT, USA). A heat inactivation step at 99°C for 5 min was performed to denaturate reverse transcriptase, and this was followed by a cooling step to + 4°C for 5 min. Two microlitres of cDNA was then used for PCR amplification in 50 µl reaction mixtures containing 0·2 mm of each dNTP, 1 U of DyNAzyme™ DNA polymerase and 10× reaction buffer (Finnzymes OY, Espoo, Finland) and 15 pmol/ml of each primer set. The specific primers used for IDO were: sense (5′-AACTCCTGGACAATCAGTAAAG-3′) and antisense (5′-ATATATGCGAAGAACACTGAAAAA-3′). As a control constitutively expressed β-actin gene was amplified from the same pool of cDNA to normalize the cDNA level; sense primer (5′-GGGTCAGAAGGATTCCTATG-3′) and antisense (5′-CCTTAATGTCACGCACGATTT-3′). To exclude possible contamination of genomic DNA, AMV reverse transcriptase was omitted from control RT reactions. PCR amplification conditions were 94°C for 30 s, 57°C for 30 s, 72°C for 1 min for a total of 30 cycles. Finally, products were analysed by electrophoretic separation on a SeaKem 1·5% agarose gel (FMC Bioproducts, Rockland, ME, USA). Products of RT-PCR were transferred to Hybond™-N+ nucleic acid membrane (Amersham, Buckinghamshire, UK) for hybridization with an internal 32P-labelled oligonucleotide probe for IDO; 5′-ATTGATCATCTCA CAGACCACA-3′.
Statistical analysis
Independent samples Student's t-test was used in the statistical analysis of the phenotypic differences. Cytokine concentrations were analysed by Mann–Whitney U-test.
Results
Inhibitory phenotype of decidual macrophages
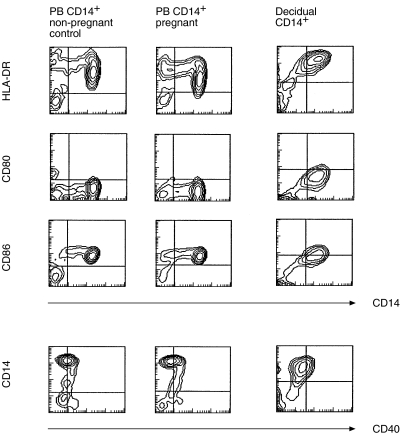
First we analysed the expression of cell surface molecules involved in the antigen presentation on decidual macrophages in comparison with PB monocytes isolated from pregnant and nonpregnant control women. No significant differences between the phenotype of PB CD14+ monocytes from pregnant and non-pregnant women were observed ( Table 2, Fig. 2). HLA-DR was expressed at higher levels on decidual macrophages than on PB monocytes. However, decidual macrophages expressed significantly lower levels of CD86. The expression of CD80 was low in both cell types. No difference in the expression of CD40 was observed. Low expression of costimulatory molecules CD80 and CD86 in relation to HLA-DR expression suggests that decidual macrophages could rather induce anergy than activate T cells. ITIM-containing receptors inhibiting leucocyte activation have been characterized in DCs and monocytes/macrophages. ILT3 negatively regulates the activation of APCs and is involved in the induction of the anergy in T helper cells [29,30]. Decidual macrophages were observed to express ILT3 as did PB monocytes (MFIR 5·8 ± 2·4 (n = 5), MFIR 4·2 ± 1·6 (n = 6), respectively).
Table 2.
The phenotype of decidual macrophages
| Monocytes | |||
|---|---|---|---|
| Decidual macrophages§ | pregnant‡ | control† | |
| HLA-DR | 126·0 ± 52·7** | 54·7 ± 26·7 | 56·5 ± 30·3 |
| CD80 | 2·1 ± 0·4** | 0·9 ± 0·1 | 1·0 ± 0·2 |
| CD86 | 6·5 ± 1·6** | 16·2 ± 4·8 | 13·9 ± 4·9 |
| CD40 | 2·3 ± 0·6 | 2·9 ± 0·6 | 2·8 ± 1·4 |
The mean fluorescence intensity ratios (MFIRs, see Materials and Methods) of different cells surface markers on CD14+ decidual cells and CD14+ PB monocytes from pregnant and nonpregnant controls are shown (mean ± s.d.).
P < 0·01 when compared with control.
n = 10,
n = 11,
n = 12.
Fig 2.
The phenotype of decidual macrophages. The expression of HLA-DR, CD80, CD86 and CD40 on CD14+ cells from decidua and PB from pregnant and non-pregnant controls was analysed by double staining and using flow cytometry. Representative contour plots from nine individual experiments are shown.
Decidual macrophages express IDO, an inhibitor of T cell proliferation
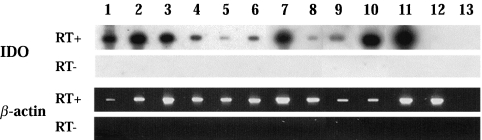
We determined the expression of the IDO mRNA in decidual CD14+ macrophages using RT-PCR. As a positive control, we used monocytes cultured for five days with M-CSF and then stimulated with IFN-γ [28]. Jurkat cells were studied as a negative control (Fig. 3, lane 12). IDO transcripts were detected in all decidual samples indicating that decidual macrophages spontaneously express IDO (Fig. 3, lanes 1–6). Furthermore, we found that both immature (CD1a+) and mature (CD83+) monocyte-derived DCs express IDO mRNA. Similar findings have been recently published [31,32].
Fig 3.
RT-PCR analysis of IDO expression in decidual macrophages. Lanes 1–6, decidual CD14+ cells; lanes 7 and 8, PB CD14+ cells; lane 9, immature CD1a+ DCs; lane 10, mature CD83+ DCs; lane 11, PB CD14+ cells cultured with M-CSF for 5 days and 1 day with IFN-γ; lane 12, Jurkat cells; lane 13, aqua control.
Differentiation of decidual CD14+ cells into DCs is compromised
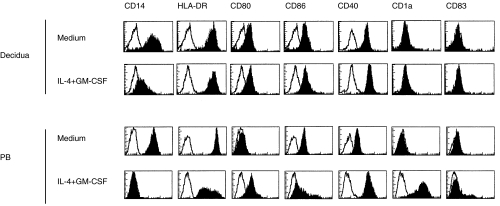
If the decidual CD14+ cells have the capacity to differentiate into DCs they could initiate T cell responses against the fetal alloantigens. Therefore, we cultured decidual CD14+ macrophages in the presence of IL-4+ GM-CSF [26, 27, 33]. After 7 days of cell culture, decidual macrophages remained partially CD14 positive and did not gain CD1a expression, whereas control PB monocyte-derived immature DCs expressed high levels of CD1a and became CD14 negative (Fig. 4). Furthermore, only slight up-regulation of the expression of HLA-DR, CD80, CD86 and CD40 was observed in decidual macrophages cultured with IL-4 + GM-CSF compared with cells cultured in medium alone ( Table 3). PB monocyte-derived immature DCs up-regulated the expression of CD80, CD86 and CD40. Our results show that decidual macrophages are unable to differentiate into immature DCs under the influence of IL-4 + GM-CSF. This finding was unexpected, as normally CD14+ monocytes/macrophages either from PB or synovial fluid differentiate into functional DCs [27, 33, 34].
Fig 4.
Decidual CD14+ cells are impaired to differentiate into DCs. Decidual and PB CD14+ cells were cultured in medium alone or in the presence of IL-4 + GM-CSF. The cells were stained for various cell surface markers after seven days of culture and analysed using flow cytometer. Representative histograms from three individual experiments are shown.
Table 3.
The phenotype of decidual and PB CD14+ cells after 7 days of culture with IL-4 + GM-CSF
| MFIR | CD14 | HLA-DR | CD80 | CD86 | CD40 | CD1a | CD83 | |
|---|---|---|---|---|---|---|---|---|
| Decidua | Medium | 31 ± 17 | 94 ± 43 | 3 ± 1 | 6 ± 1 | 15 ± 5 | 1 ± 0 | 2 ± 1 |
| IL-4 + GM-CSF | 2 ± 1 | 102 ± 20 | 5 ± 1 | 9 ± 2 | 33 ± 12 | 1 ± 0 | 2 ± 1 | |
| PB | Medium | 47 ± 2 | 181 ± 46 | 2 ± 0 | 6 ± 1 | 4 ± 1 | 1 ± 0 | 1 ± 0 |
| IL-4 + GM-CSF | 1 ± 0 | 74 ± 38 | 12 ± 5 | 15 ± 12 | 61 ± 16 | 70 ± 39 | 2 ± 1 |
MFIRs of the cell surface antigens on cultured cells are shown (mean ± s.d.). Three individual experiments were performed.
Decidual macrophages produce spontaneously high levels of IL-10
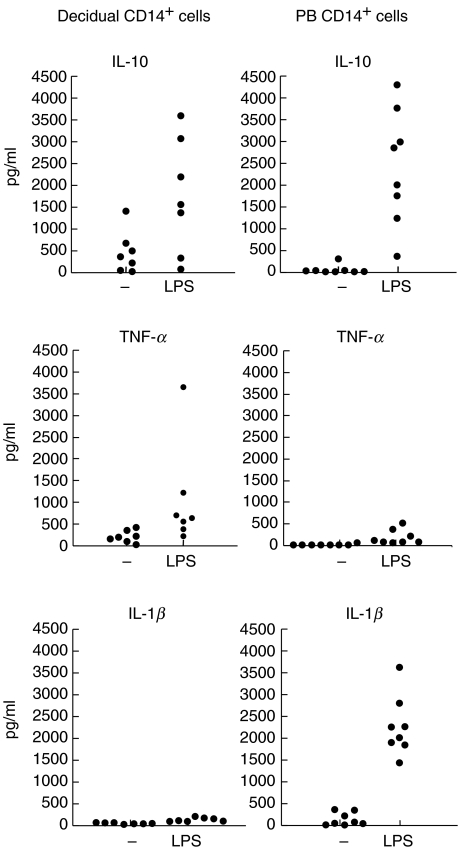
We studied the production of IL-10, TNF-α, IL-1β, TGF-β and IL-12p70 by decidual and normal PB CD14+ cells. Spontaneous production of IL-10 was significantly higher in decidual macrophages (mean 459 pg/ml, P < 0·05) when compared with normal PB monocytes (mean 59 pg/ml) (Fig. 5). Decidual macrophages also produced higher levels of TNF-α (mean 210 pg/ml, P < 0·01) when compared with PB monocytes (mean 23 pg/ml). IL-10 and TNF-α production was up-regulated in both cell types after LPS stimulation (Fig. 5). However, decidual macrophages were observed to produce much lower levels of IL-1β (mean 50 pg/ml) than control cells (mean 141 pg/ml). Interestingly, decidual macrophages hardly up-regulated IL-1β production after LPS stimulation (134 ± 47 pg/ml, mean ± s.d.), whereas a considerable up-regulation was observed in control cells (2267 ± 680 pg/ml, P < 0·01). To confirm the high spontaneous IL-10 production we used also a classic ELISA method and the results were similar with those obtained with the cytometric bead method (decidual macrophages: IL-10 mean 525 pg/ml; PB monocytes: IL-10 mean 75 pg/ml). No production of the active form of TGF-β was observed in decidual macrophages (data not shown). In addition, neither decidual nor PB CD14+ cells produced IL-12p70 spontaneously or after LPS stimulation. Neither IL-10 nor TNF-α production was detected in the CD14 negative fraction of decidual cells, suggesting that CD14+ macrophages are the main cytokine producing cells in the maternal decidua.
Fig 5.
The production of IL-10, TNF-α and IL-β in CD14+ cells isolated from decidua. The cells were cultured for 2 days in medium alone or with LPS (decidua n = 7, PB n = 8).
Discussion
One of the most intriguing questions in biology is how an allogenic pregnancy is maintained in a potentially immunologically hostile environment. As reproduction is crucial to all species, these mechanisms must be very effective. Human semi-allogeneic fetal tissues survive several months in a very close contact with a biologically active and well-circulated maternal decidua. One clue to this question could be the exceptional characteristics of decidual macrophages, which are found in considerable numbers locally adjacent to fetal tissues.
We show that these cells have several immunosuppressive properties. The decidual macrophages expressed HLA-DR, but low levels of the costimulatory molecules CD80 and CD86. CD86 has been considered more important than CD80 in the human T cell activation [35,36]. Blockade of CD40 signalling, and especially CD86–CD28 interaction, seems to generate antigen-specific immunoregulatory T cells able to suppress alloreactive T cells [36]. It is also known that blockade of the function of CD86 and CD40 enhance the graft survival [37]. Low expression of CD80 and CD86 suggests that decidual macrophages may rather induce tolerance of maternal T cells by failing to give a sufficient costimulatory signal [38,39]. The phenotype of decidual macrophages, which we have here characterized, could explain the earlier findings that decidual macrophages possess suppressive capacity in mixed lymphocyte reactions [13,14]. It is also well known that decidual tissue as such is immunosuppressive [11]. We show here that decidual macrophages express IDO, which has been shown to be involved in the protection of the allogenic fetus by preventing maternal T cell activation [23]. It has been shown that there are distinct subsets of DCs and macrophages expressing IDO [32]. Based on our results we propose that there could be a subset of decidual macrophages expressing IDO in at the maternal–fetal interface but further studies are needed to assess more clearly which subset of mononuclear cells represents IDO expressing immunoregulatory cells in decidua. Recently it was shown that CD200 (OX-2), which is also expressed by trophoblast cells and in decidua, might trigger in subset of antigen presenting macrophages an increase in IDO activity [40–42].
IL-10 has been shown to be important to the successful pregnancy. IL-10 was demonstrated to reverse the experimental fetal growth restriction in mice [43]. By immunohistochemical studies, the production of IL-10 was shown to be reduced in the placental tissue from patients with pre-eclampsia [44]. Here we show, that decidual macrophages produce spontaneously high levels of IL-10. As IL-10 is known to inhibit the expression of HLA-DR and the costimulatory molecules on APCs [45,46], maternal IL-10 could be able to reduce the capacity of APCs to activate T cells acting against fetal tissues. We propose that the effects of IL-10 on the maternal APC function explain the inhibitory role of IL-10 during pregnancy. We also observed that decidual macrophages produced lower levels of IL-1β. In contrast to decidual macrophages, inflammatory macrophages typically produce high levels of proinflammatory cytokines such as IL-1β and TNF-α. Reduced production of IL-1β by decidual macrophages further supports the special properties of decidual macrophages. The profile of cytokines produced by decidual macrophages suggests that in the case of an intrauterine infection, these cells may be less potent promoters of inflammation than other tissue macrophages. These findings might also explain, why intrauterine infections usually remain without clinical symptoms of inflammation for a prolonged time [47]. Obviously, it is important to sustain the tolerogenic environment to maintain successful pregnancy, even at the expense of reduced resistance to infections.
We observed also that decidual macrophages were incapable to differentiate into DCs under the influence of IL-4 + GM-CSF, indicating active inhibitory mechanisms preventing DC differentiation. It is known that the maturation of CD14+ monocytes into DCs can be inhibited by IL-10 [48], TGF-β [49] and 1α,25-dihydroxyvitamin D3 [50]. While inhibiting the differentiation of CD14+ monocytes into DCs, IL-10 directs their maturation into macrophages [51]. Based on our results, we propose, that the high endogenous IL-10 production in decidual macrophages is one of the mechanisms inhibiting their differentiation into DCs.
In conclusion, we have characterized the phenotype of CD14+ decidual macrophages and provide several lines of evidence that these cells could have an immunoinhibitory function, probably important in maintaining maternal tolerance against the developing fetus. Based on our findings, decidua may provide a special environment to activate regulatory T cells, which could directly inhibit the activation of naive T cells. Decidual macrophages may represent a special type of APCs able to induce regulatory T cells [52–54].
Acknowledgments
We thank Anna Karvonen for excellent technical assistance. Dr M. Colonna is acknowledged for providing the anti-ILT3 MoAb. We thank Drs Kohonen, Koskela, Liippo, Nera, Nieminen, Salminen and Veistinen for their critical comments of the manuscript. This study was supported by the Academy of Finland Life 2000 Programme, the special funds for Turku University Hospital and Turku University Foundation. Dr Jenni Heikkinen is a recipient of a training grant from Turku Graduate School of Biomedical Sciences.
References
- 1.Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells? Nature. 1988;334:253–5. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins MK, Pardoll DM, Mizuguchi J, et al. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci USA. 1987;84:5409–13. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YJ, Kanzler H, Soumelis V, et al. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 5.Langenkamp A, Messi M, Lanzavecchia A, et al. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus. the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulmer JN, Johnson PM. Macrophage populations in the human placenta and amniochorion. Clin Exp Immunol. 1984;57:393–403. [PMC free article] [PubMed] [Google Scholar]
- 8.Lessin DL, Hunt JS, King CR, et al. Antigen expression by cells near the maternal–fetal interface. Am J Reprod Immunol Microbiol. 1988;16:1–7. doi: 10.1111/j.1600-0897.1988.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 9.Vince GS, Starkey PM, Jackson MC, et al. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Meth. 1990;132:181–9. doi: 10.1016/0022-1759(90)90028-t. [DOI] [PubMed] [Google Scholar]
- 10.Kämmerer U, Schoppet M, McLellan AD, et al. Human decidua contains potent immunostimulatory CD83 (+) dendritic cells. Am J Pathol. 2000;157:159–69. doi: 10.1016/S0002-9440(10)64527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daya S, Clark DA, Devlin C, et al. Suppressor cells in human decidua. Am J Obstet Gynecol. 1985;151:267–70. doi: 10.1016/0002-9378(85)90024-9. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama E, Asano S, Kodo H, et al. Suppression of mixed lymphocyte reaction by cells of human first trimester pregnancy endometrium. J Reprod Immunol. 1985;8:25–31. doi: 10.1016/0165-0378(85)90075-0. [DOI] [PubMed] [Google Scholar]
- 13.Parhar RS, Kennedy TG, Lala PK. Suppression of lymphocyte alloreactivity by early gestational human decidua. I. Characterization of suppressor cells and suppressor molecules. Cell Immunol. 1988;116:392–410. doi: 10.1016/0008-8749(88)90240-7. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno M, Aoki K, Kimbara T. Functions of macrophages in human decidual tissue in early pregnancy. Am J Reprod Immunol. 1994;31:180–8. doi: 10.1111/j.1600-0897.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 15.Carosella ED, Rouas-Freiss N, Paul P, et al. HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol Today. 1999;20:60–2. doi: 10.1016/s0167-5699(98)01387-5. [DOI] [PubMed] [Google Scholar]
- 16.Rouas-Freiss N, Goncalves RM, Menier C, et al. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci USA. 1997;94:11520–5. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth I, Corry DB, Locksley RM, et al. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–48. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bamberger AM, Schulte HM, Thuneke I, et al. Expression of the apoptosis-inducing Fas ligand (FasL) in human first and third trimester placenta and choriocarcinoma cells. J Clin Endocrinol Metab. 1997;82:3173–5. doi: 10.1210/jcem.82.9.4360. [DOI] [PubMed] [Google Scholar]
- 19.Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 20.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997;18:478–82. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 21.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]
- 22.Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–94. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- 23.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 24.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 25.Sedlmayr P, Blaschitz A, Wintersteiger R, et al. Localization of indoleamine 2,3-dioxygenase in human female reproductive organs and the placenta. Mol Hum Reprod. 2002;8:385–91. doi: 10.1093/molehr/8.4.385. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95:2875–82. [PubMed] [Google Scholar]
- 28.Munn DH, Pressey J, Beall AC, et al. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages. A potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol. 1996;156:523–32. [PubMed] [Google Scholar]
- 29.Cella M, Dohring C, Samaridis J, et al. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med. 1997;185:1743–51. doi: 10.1084/jem.185.10.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by TS cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 31.Hwu PMX, Du MX, Lapointe R, et al. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 32.Munn DH, Sharma MD, Lee JR, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 33.Mottonen M, Isomaki P, Saario R, et al. Interleukin-10 inhibits the capacity of synovial macrophages to function as antigen-presenting cells. Br J Rheumatol. 1998;37:1207–14. [PubMed] [Google Scholar]
- 34.Komi J, Mottonen M, Luukkainen R, et al. Non-steroidal anti-oestrogens inhibit the differentiation of synovial macrophages into dendritic cells. Rheumatology (Oxford) 2001;40:185–91. doi: 10.1093/rheumatology/40.2.185. [DOI] [PubMed] [Google Scholar]
- 35.Hathcock KS, Hodes RJ. Role of the CD28-B7 costimulatory pathways in T cell-dependent B cell responses. Adv Immunol. 1996;62:131–66. doi: 10.1016/s0065-2776(08)60429-0. [DOI] [PubMed] [Google Scholar]
- 36.Koenen HJ, Joosten I. Blockade of CD86 and CD40 induces alloantigen-specific immunoregulatory T cells that remain anergic even after reversal of hyporesponsiveness. Blood. 2000;95:3153–61. [PubMed] [Google Scholar]
- 37.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 38.Gimmi CD, Freeman GJ, Gribben JG, et al. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90:6586–90. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 40.Gorczynski RMYuK, Clark D. Receptor engagement on cells expressing a ligand for the tolerance-inducing molecule OX2 induces an immunoregulatory population that inhibits alloreactivity in vitro and in vivo. J Immunol. 2000;165:4854–60. doi: 10.4049/jimmunol.165.9.4854. [DOI] [PubMed] [Google Scholar]
- 41.Gorczynski RM, Hadidi S, et al. The same immunoregulatory molecules contribute to successful pregnancy and transplantation. Am J Reprod Immunol. 2002;48:18–26. doi: 10.1034/j.1600-0897.2002.01094.x. [DOI] [PubMed] [Google Scholar]
- 42.Barclay AN, Wright GJ, Brooke G, et al. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–90. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 43.Rivera DL, Olister SM, Liu X, et al. Interleukin-10 attenuates experimental fetal growth restriction and demise. FASEB J. 1998;12:189–97. doi: 10.1096/fasebj.12.2.189. [DOI] [PubMed] [Google Scholar]
- 44.Hennessy A, Pilmore HL, Simmons LA, et al. A deficiency of placental IL-10 in preeclampsia. J Immunol. 1999;163:3491–5. [PubMed] [Google Scholar]
- 45.Ding L, Linsley PS, Huang LY, et al. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 46.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 48.Buelens C, Verhasselt V, De Groote D, et al. Interleukin-10 prevents the generation of dendritic cells from human peripheral blood mononuclear cells cultured with interleukin-4 and granulocyte/macrophage-colony-stimulating factor. Eur J Immunol. 1997;27:756–62. doi: 10.1002/eji.1830270326. [DOI] [PubMed] [Google Scholar]
- 49.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 50.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 51.Allavena P, Piemonti L, Longoni D, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 52.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 53.Roncarolo MG, Levings MK, Traversari C. Differentiation of T regulatory cells by immature dendritic cells. J Exp Med. 2001;193:F5–9. doi: 10.1084/jem.193.2.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Read S, Powrie F. CD4 (+) regulatory T cells. Curr Opin Immunol. 2001;13:644–9. doi: 10.1016/s0952-7915(01)00273-4. [DOI] [PubMed] [Google Scholar]