Abstract
Natural killer (NK) cells have been implicated in the natural protection and healing of leishmaniasis by their ability to secrete the macrophage activating cytokine interferon (IFN)γ. Previous studies have demonstrated that early production of interleukin (IL)-12 triggers IFNγ secretion by NK cells. Here we report that live Leishmania promastigotes (the form that is injected by the vector) can directly induce human peripheral blood NK cells from healthy donors to IFNγ secretion in the absence of IL-12 and professional antigen presenting cells. Killing of promastigotes abolishes this property. This novel mechanism of activation of the innate immune response may be relevant for establishment of infection and thus also the design of vaccines against leishmaniasis.
Keywords: NK cells, Leishmania, IFNγ, LPG
Introduction
The development of successful vaccines against leishmaniasis seems to be achievable since recovery from the disease leads to protection against subsequent disease. The most efficient protection against cutaneous leishmaniasis is to date obtained by either natural infection or live vaccination using promastigotes (the form injected by the sandfly vector) in a procedure termed leishmanization [1]. Killed promastigotes in combination with BCG has been used as vaccine, but with less protective effect compared to live infection [2,3]. In mice, immunity against leishmaniasis is believed to be essentially through the induction of T-helper 1 (Th1) phenotype CD4+ cells [4]. The infection is controlled if adequate levels of IFN-γ are produced to activate macrophages to kill the parasite. The precise role of CD8+ cells and CD16+/56+, NK cells is not clarified, but these cells have been associated with protection in mice plus cure of cutaneous leishmaniasis in man [5,6]. We have shown repeatedly [6,7] that cells from healthy blood donors are able to respond to Leishmania antigens with the involvement of NK, CD8+ and CD4+ cells. Such reactivity in the absence of exposure could be fundamental in the outcome of subsequent infection.
Differential induction of immune responses by live and dead Leishmania could be part of the explanation why live promastigote vaccination induce better protection than vaccines based on killed promastigotes. In this context we have shown that live and dead parasites can differ in their ability to induce cellular responses in healthy donors as defined by IFNγ production and cell proliferation [8]. Live promastigotes (live-PROM) induced significantly more PBMC to secretion of IFNγ compared to heat killed promastigotes (hk-PROM). Stimulation with live-PROM had an inhibitory effect on the background IL-10 secretion, whereas hk-PROM had little or no inhibitory effect on IL-10 secretion. In these initial studies proliferative responses to live and dead promastigotes were variable. However, in the group of donors with the highest proliferative responses to Leishmania, killed promastigotes induced significantly more proliferative response than live-PROM [8].
In the present study we dissect the differences in cellular induction by examining the cell subsets activated by live vs. dead parasites. Furthermore, we made an attempt to find the structures/molecules on the parasite responsible for lymphocyte activation. We have used promastigotes of Leishmania aethiopica, Leishmania mexicana and Leishmania donovani to evaluate this in PBMC from healthy donors with no history of leishmaniasis. The data presented demonstrate that live Leishmania promastigotes have the capacity to directly activate purified NK cells to IFNγ secretion, while killed promastigotes preferentially activate CD4+ cells to proliferate. The relevance of these findings is discussed in the context of vaccine development.
Materials and methods
Cells
Blood was obtained from totally 14 healthy lab donors with no history of leishmaniasis after receiving their informed consent. Cell from some of these donors have previously been used to evaluate cell proliferation and cytokine induction [8]. Mononuclear cells separated from defibrinated peripheral blood on a ficoll gradient [9] were washed 3 times and eventually resuspended to the appropriate concentration in RPMI (Gibco BRL, Paisley, UK) containing 2 mm l-glutamine, 100 U penicillin (Gibco), 100 µg/ml streptomycin (Gibco) supplemented with 10% heat inactivated normal Swedish AB serum (NHS). The NK cell lines NKL (obtained from Dr M. Robertson, Indiana University School of Medicine, Indianapolis, IN, USA) and Nishi (generated from PBMC obtained from an NK-leukaemia patient) were cultured in IDMI (Gibco) medium supplemented with 2 mm l-glutamine, 100 U penicillin (Gibco), 100 µg/ml streptomycin (Gibco) and 10% heat inactivated normal Swedish AB serum.
Ethical approval (Dnr:00–246) for this study was given by the local ethical committee (KI forskningskommitté Nord).
Parasites and antigens
L. aethiopica promastigotes were propagated as previously described [10]. The promastigotes used to stimulate cells were at early stationary growth phase. Promastigotes were washed 3 times with sterile PBS pH 7·4 followed by centrifugation (2000 g, 10 min, 10°C). The parasites were resuspended in PBS, live parasite were then used directly for stimulation (live-PROM) or killed by boiling for 10 min (hk-PROM). Killing of parasites was also achieved by repeated freeze thawing (ft-LAg) as previously described [10]. The ft-LAg is our in-house antigen and was previously prepared using a mixture of other L. aethiopica isolates. The ft-LAg preparation was diluted in RPMI. If not otherwise stated the final promastigotes concentration used is 1·25 × 106 promastigotes/ml in all assays.
In some experiments promastigotes of Leishmania donovani and Leishmania mexicana prepared as above were used. Promastigotes of L.mexicana wild-type (WT) strain and derived mutants lacking proteophosphoglycans (PPGs) and/or lipophosphoglycans (LPG) were propagated in M199 medium supplemented with 5% FCS, 100 U penicillin and 100 µg/ml streptomycin (Gibco/Life Technologies). The LPG-deficient L. mexicana promastigotes used in this study were the gene deletion mutant Δlpg1, which lacks LPG only [11], and the Golgi GDP-Man transporter gene deletion mutant Δlpg2, which is deficient in phosphoglycan (PG) repeat synthesis resulting in the absence of LPG and complete lack of PG repeats on PPGs [12].
Recombinant LACK (Leishmania homologueue of activated C-kinase), a kind gift from Nicolas Gleichenhaus, was tested for purity and pretreated with polymyxin B as previously described [13]. Cells were stimulated with LACK at a final concentration of 12·5 or 25 µg/ml. PHA (Murex, Hartford, UK) and PPD (SBL, Stockholm, Sweden) were each used at 12·5 µg/ml.
Depletion of cell subset from PBMC
PBMC were diluted to 10 × 106 cells/ml. T cells (CD4 and CD8) were depleted using antibody coated magnetic beads (Dynal, Oslo, Norway) according to the manufacture's instructions. NK cells were depleted by coating the cells with 15 µg/ml mouse-α- human CD56 antibodies (Pharmingen) in 2% FCS on ice for 15 min, followed by 2× washing with PBS, and finally incubation with sheep-α-mouse (SAM) coated magnetic beads in 2% FCS. The antibody coated cells were allowed to bind to the beads under slow rotation for 30 min, 4°C. The cells attached to the beads were removed and the supernatant containing the depleted cells was collected. As control for the depletion we used an isotype control antibody (IC) (Pharmingen). Plastic adherent cells (PAC) (i.e. mainly monocytes) were removed by allowing these cells to bind to plastic for one hour in RPMI containing 10% NHS at 37°C 5% CO2. Whole PBMC were diluted to a concentration of 2 × 105 cells/well. The depleted cells were used at a concentration that would corresponded to 2 × 105 whole PBMC/well before depletion (i.e. 2 × 105 cells minus specific cell subset). The percentage remaining cells after depletion were as follows PBMC minus IC: 92%, PBMC minus CD56: 90%, PBMC minus CD4: 50%, PBMC minus CD8: 73% (results from donor D7).
Isolation of NK cells
NK cells were purified from PBMC either using cell sorting or negative depletion. To obtain highly purified NK cells and exclude contamination of other cell subsets, NK cells were isolated by FACS. Purification of NK cells using negative depletion was used in subsequent assays due to convenience of preparation of such cells.
Cell sorting
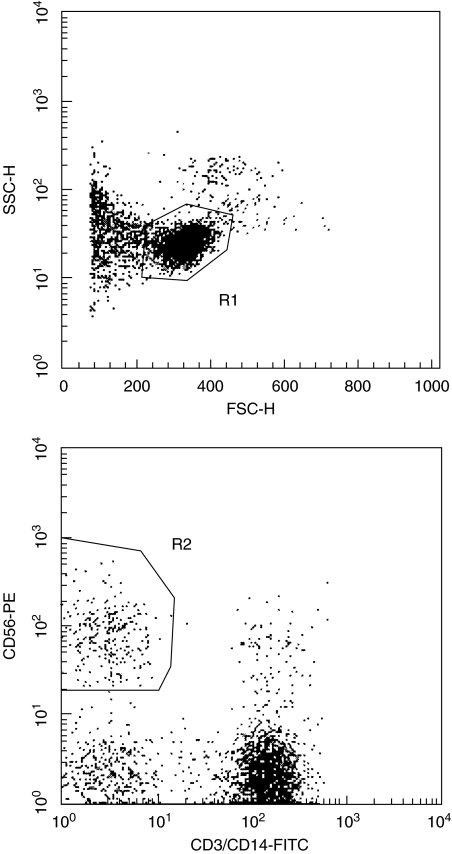
PBMC were depleted of plastic adherent cells by 1-h incubation in polystyrene cell culture flasks. PBMC depleted of plastic adherent cells were washed, diluted to a concentration of 10 × 106 cells/ml in 2% FCS-PBS and stained with α-CD3 (Pharmingen) and α-CD14* (DAKO) conjugated with flourescein isothiocyanate (FITC) and α-CD56 conjugated with phyco-erythrin (PE) (Pharmingen). 10 µl of each antibody was used per 106 cells for 30 min on ice. The stained cells were washed with 2% FCS-PBS and resuspended to 10 × 106 cells/ml in 2% FCS-PBS. PBMC were sorted on a FACS™ vantage SE (Becton Dickinson, Mountain View, CA, USA). From the cells gated as lymphocytes (R1), cells positive for CD56 and negative for CD3 and CD14 (CD56+, CD3-, CD14-*) were collected as NK cells (R2) (Fig. 1). (* CD14-FITC was only used on cells from one, D18, of two donors whose cells were sorted)
Fig. 1.
Purification of NK cells from PBMC by FACS™. The plots show the isolation performed on cells from D18. From the cell population gated in R1, which mainly contains viable lymphocytes, CD56+/CD3-, CD14- (NK) cells were collected. Approximately 75% of the plastic adherent cells (PAC) depleted PBMC were gated in R1 and out of these 9·5% were gated as CD56+/CD3-, CD14-. From 50 × 106 PAC depleted PBMC sorted, 2·85 × 106 were collected as pure NK cells.
Isolation by negative selection
Isolation of NK cells was performed according to the protocol supplied by the manufacturer (Dynal) [14,15], with slight modification. Briefly, PBMC were resuspended in 1–2 ml sterile water for approximately 20 s after the first washing, to reduce contamination by red blood cell (RBC), thereafter PBS was added and the cells washed two more times. The isolated PBMC were first incubated with CD19 coated beads (Dynal), with rotation for 30 min at +4°C. Beads were removed with a magnet and the supernatant containing the depleted cells was collected and further incubated 30 min with CD3 coated beads (Dynal) followed by 40 min incubation with CD14 coated beads (Dynal) as described above. To remove remaining antigen presenting cells, supernatants containing the depleted cells were transferred to a 250 ml cell culture flask and 10–15 ml cell culture medium (described above) was added. The cells were incubated at 37°C, 5% CO2 in air for 1–2 h. Unbound cells were collected as NK cells. To assess the efficacy of the depletion, the purified NK cells were stained with CD4; no cells were found to be CD4 positive. In the gate containing viable lymphocytes 90% were CD16/56 positive. The main contaminants using this assay are RBC, which are enriched for by this method.
Measurement of cytokine producing cells by ELISPOT
ELISPOT assays were performed using a commercial ELISPOT kit (Mabtech, Stockholm, Sweden). Briefly, mixed cellulose bottomed plates (MAHA S45 Millipore, Molsheim, France) were coated with monoclonal mouse anti human IL-10/IFNγ IgG (10 µg/ml/well) by overnight incubation at +4°C. The plates were washed 6x (200 µl/well) with filtered, sterile PBS pH 7·4. PBMC were isolated and prepared as described above. 2 × 105 PBMC or 2 × 105 PBMC minus a specific cell subset were plated per well with or without stimulation with live- or hk-PROM. Purified NK cells were plated at 1 × 105 cells/well (For D11 the isolated cells were tested at two concentrations 104 and 105 cells/well both stimulated with 1·25 × 106 p/ml. Due to extreme high frequency of IFNγ secreting cells in background, live- and hk-PROM counting/estimation was not possible at the higher cell concentration. There was however, a clear difference that could be detected visually between live and dead parasites. The T cell mitogen PHA was used at 12·5 µg/ml as positive control of the assay. Wells containing only cell culture medium or cell culture medium + live promastigotes, without cells were used as negative controls. The cells were cultured for 40 or 48 h in 5% CO2 in air at 37°C. Thereafter cells were discarded and the ELISPOT assay was further performed according to the manufacture's instructions. The wells were finally stained with NBT (4·4 µl/ml) and BCIP (3·3 µl/ml) (Gibco BRL, Gaithersburg, MD, USA) diluted in Tris-buffer pH 9·5. Spots were allowed to develop for 10–30 min in the dark at 20–22°C. The reaction was terminated by rinsing the wells with water. The plates were air-dried and spots forming units (SFU) were counted under a dissection microscope (Leica MZ6, Leica Microscope systems, Heerbrugg, Switzerland) or using computerized ELISPOT counting system (AID, Strassberg, Germany). One spot should theoretically correspond to one cytokine-producing cell.
Measurement of proliferation
Cells were plated at 2 × 105 cells/well or for depleted cells 2 × 105 cells minus the specific cell subset in 96 well flat bottom tissue culture plates (Nunclon™, Denmark). Each stimulation was tested in triplicate as previously described [7]. Control cultures contained RPMI medium alone or live parasites alone.
Phenotype of proliferating cells
Mononuclear cells cultured for 6 days were prepared for surface marker staining as previously described [6]. Phenotype analysis for CD4+ and CD8+ (T-helper and cytotoxic), CD 3+ and CD16+/56+ (pan-T and NK cells, respectively) stained with FITC and PE, respectively, and FITC stained γδ-TCR were carried out. Control IgG1 and IgG2 double stained with FITC and PE respectively, was used to determine the negative population. All antibodies were obtained from Becton Dickinson. Cells were analysed on a FACScan™ (Becton Dickinson).
Proliferation was determined by the enrichment of large blast cells after stimulation, as described previously [6]. Determined by forward light scatter, smaller cells with the least light scatter as seen in most unstimulated cultures were gated in region (R) 1, and the area outside this was gated R2. In response to antigens, blast cells undergoing activation were scattered forward most away from the R1 cell population into the R2.
The proportional increase (PI) of specific cell subsets was calculated using the following formula as previously described [6]:
The correction factor + 1/+1 was necessary since in some instances the percentage of large cells of a particular phenotype in the unstimulated cultures was zero.
Non-specific cytotoxicity assay
NK cells purified by depletion from PBMC were stimulated for 24 h with live- or hk-PROM and used as effector cells. Cytotoxicity against 721·221, a MHC class I negative B lymphoblastoid cell was measured using a standard 4-h 51Cr-release assay.
Results
Leishmania induced IFNγ and IL-10 secreting cells after depletion of cell subsets
To determine the cellular source of IFNγ and IL-10 secretion after in vitro exposure to Leishmania promastigotes, specific cell subsets were depleted from 2 × 105 PBMC before stimulation.
Depletion of CD56+ cells clearly reduced the number of IFNγ secreting cells in cultures stimulated with live-PROM in all four donors tested using the ELISPOT assay ( Table 1). Depletion of CD8+ cells also reduced the number of IFNγ secreting cells (in 3 out of 4 donors), although to a lesser extent. Depletion of CD4+ cells had no appreciable effect on the number of IFNγ secreting cells. However, the depletion of CD4+ cells revealed that these cells rather than the plastic adherent cells, consisting mainly of monocytes/macrophages, were responsible for the background IL-10 secretion (Table 1).
Table 1.
Number of Leishmania induced IFNγ spot forming units (SFU) after depletion of specific cell subsets. Tables show mean of triplicate culture ± SD for individual donors/2 × 105 PBMC**after 48 h stimulation with live- or hk-PROM. (a) IFNγ (b) IL-10
| D6 | D7 | D10 | D15 | |
|---|---|---|---|---|
| Background IFNγ SFU | ||||
| Control | 20 ± 5 | 0 | 21 ± 4 | 75 ± 14 |
| minus PAC | 15 ± 12 | 3 ± 5 | 0 | 210 ± 75 |
| minus IC | 12 ± 11 | 0 | 8 ± 5 | 112 ± 49 |
| minus CD56 | 3 ± 1 | 0 | 3 ± 3 | 32 ± 13 |
| minus CD4 | 21 ± 17 | 0 | 10 ± 5 | 133 ± 31 |
| minus CD8 | 34 ± 5 | 0 | 1 ± 1 | 45 ± 10 |
| live-PROM induced IFNγ SFU | ||||
| Control | 341 ± 17 | 138 ± 6 | 49 ± 12 | 250 ± 48 |
| minus PAC | 220 ± 52 | 70 ± 5 | 33 ± 8 | 285 ± 114 |
| minus IC | 227 ± 58 | 84 ± 37 | 59 ± 5 | 260 ± 112 |
| minus CD56 | 76 ± 21 | 36 ± 14 | 8 ± 4 | 67 ± 4 |
| minus CD4 | 308 ± 23 | 74 ± 31 | 54 ± 21 | 120 ± 20 |
| minus CD8 | 353 ± 39 | 51 ± 21 | 25 ± 1 | 62 ± 7 |
| hk-PROM induced IFNγ SFU | ||||
| Control | 87 ± 25 | 33 ± 10 | 10 ± 4 | 86 ± 13 |
| minus PAC | 77 ± 41 | 6 ± 6 | 2 ± 4 | 128 ± 24 |
| minus IC | 18 ± 6 | 3 ± 2 | 5 ± 3 | 118 ± 40 |
| minus CD56 | 16 ± 6 | 9 ± 4 | 3 ± 2 | 26 ± 6 |
| minus CD4 | 21 ± 3 | 2 ± 2 | 5 ± 5 | 65 ± 23 |
| minus CD8 | 128 ± 58 | 3 ± 3 | 4 ± 2 | 92 ± 63 |
| background IL-10 SFU | ||||
| Control | 143 ± 31 | 25 ± 14 | 274 ± 54 | 181 ± 5 |
| minus PAC | 32 ± 9 | 37 ± 18 | 31 ± 11 | 57 ± 13 |
| minus IC | 90 ± 50 | 17 ± 3 | 135 ± 25 | 54 ± 13 |
| minus CD56 | 112 ± 7 | 23 ± 4 | 58 ± 7 | 106 ± 19 |
| minus CD4 | 6 ± 3 | 3 ± 3 | 11 ± 4 | 11 ± 4 |
| minus CD8 | 105 ± 17 | 27 ± 10 | 90 ± 25 | 104 ± 8 |
| live-PROM induced IL-10 SFU | ||||
| Control | 94 ± 19 | 8 ± 3 | 49 ± 12 | 95 ± 15 |
| minus PAC | 79 ± 6 | 0 | 29 ± 5 | 19 ± 7 |
| minus IC | 99 ± 3 | 0 | 68 ± 18 | 36 ± 11 |
| minus CD56 | 98 ± 4 | 4 ± 2 | 82 ± 29 | 36 ± 6 |
| minus CD4 | 8 ± 1 | 6 ± 5 | 11 ± 4 | 16 ± 8 |
| minus CD8 | 122 ± 7 | 11 ± 8 | 86 ± 18 | 71 ± 28 |
| hk-PROM induced IL-10 SFU | ||||
| Control | 144 ± 35 | 29 ± 9 | 319 ± 74 | 231 ± 68 |
| minus PAC | 58 ± 2 | 21 ± 11 | 58 ± 8 | 61 ± 11 |
| minus IC | 130 ± 44 | 19 ± 13 | 130 ± 39 | 96 ± 9 |
| minus CD56 | 165 ± 24 | 23 ± 4 | 77 ± 19 | 121 ± 38 |
| minus CD4 | 16 ± 2 | 3 ± 3 | 7 ± 7 | 6 ± 3 |
| minus CD8 | 166 ± 36 | 27 ± 10 | 121 ± 15 | 177 ± 17 |
IC, isotype control; PAC, plastic adherent cells.
the number of PBMC were 2×105 before depletion.
Leishmania induced IFNγ secretion in FACS purified cells
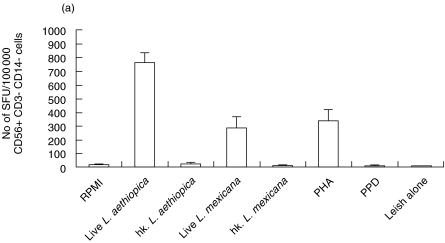
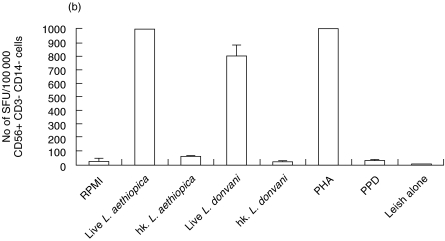
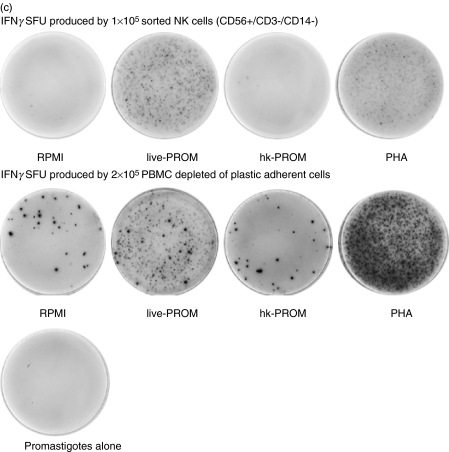
CD56+, CD3- (CD14-) cells were isolated from two donors by FACS™. The purified cells were stimulated with either live- or hk-PROM to assess their ability to induce IFNγ secretion. As control for the assay PBMC (depleted of plastic adherent cells) were stimulated in the same way as the FACS isolated. Live Leishmania promastigotes could, without involvement of other cell subsets, directly activate sorted NK cells to IFNγ secretion (Fig. 2a,b,c). The NK cells that are isolated by FACS should be 100% pure, thus we can exclude that other cells are involved in this activation. This activation was clearly inhibited when the promastigotes were heat killed. This direct effect of live Leishmania promastigotes was obtained using L. aethiopica, L. mexicana or L. donovani.
Fig. 2.
Leishmania induced IFNγ secretion in NK cells purified from human PBMC by FACS (two donors tested D18 and D6). Graphs show IFNγ spot forming units (SFU) in cells (a) D18 cells sorted for CD56+/CD3-, CD14- after 40 h stimulation and (b) D6 cells sorted for CD56+/CD3– after 48 h stimulation. Bars show mean SFU of three wells counted + SD. Spot frequencies greater than ≥1000 SFU/well are represented as 1000 SFU. (c)Representative picture of Leishmania aethiopica induced IFNγ SFU produced by FACS purified NK cells (D18), pictures of SFU produced by PBMC are shown as comparison.
Leishmania induced IFNγ secretion in negatively selected cells
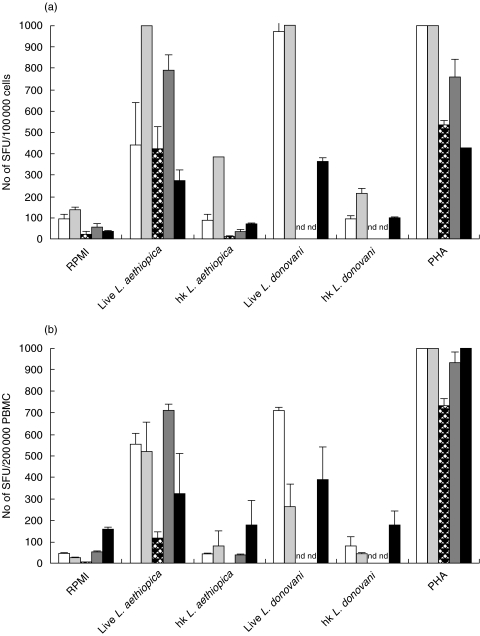
Knowing that sorted NK cells could be directly activated further experiments were for convenience performed using PBMC depleted of CD19+, CD3+, CD14+ and plastic adherent cells. The results showed the same trend as sorted cells, although the background was higher in most donors. A marked increase in the frequency of IFNγ secreting cells was observed in NK cells from 5 different donors tested when stimulated with Live-PROM (Fig. 3a), the increase of IFNγ secreting cells in response to live promastigotes was also detectable in the whole PBMC population prior to depletion (Fig. 3b).
Fig. 3.
Leishmania induced IFNγ secretion in (a) 1 × 105 NK cells obtained by depleting PBMC of CD3+, CD14+, CD19+ and plastic adherent cells (b) 2 × 105 PBMC before depletion of cells; each bar represents one donor. The bars show the mean SFU of triplicate wells + SD after 48 h stimulation. Spot frequencies greater than ≥1000 SFU/well are represented as 1000 SFU. D1 D6 D12 D17D11*(*No of SFU/10 000 cells), nd, not done. □ D1, ( ) D6, (
) D6, (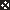 ) D12, (
) D12, ( ) D17, ▪ D11.
) D17, ▪ D11.
IFNγ secretion in cell lines
The high frequency of background IFNγ secreting cells observed in the two cell lines tested, NKL-1 and Nishi, made it impossible for us to evaluate the effect of the various antigens tested (data not shown).
Stimulation with LPG deficient promastigotes and purified LPG
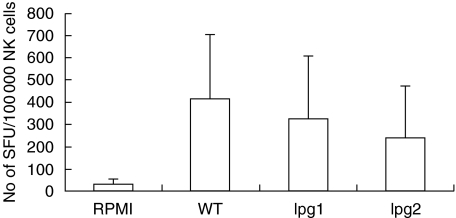
It has previously been speculated that carbohydrate structures may have the ability to activate NK cells [16]. To test if the LPGs were involved in the activation of NK cells we stimulated PBMC and NK cells isolated by negative depletion with promastigotes of two different L. mexicana gene deletion mutants (Δlpg1 and Δlpg2) that are deficient in LPG expression, and compared the response with stimulation with WT L. mexicana. The data show that the frequency of IFNγ secreting cells was slightly, but not significantly lower when LPG deficient promastigotes were used as stimuli compared to the WT promastigotes, indicating that the LPGs may contribute to the activation of IFNγ secreting cells, but are not required for the direct activation of human primary NK cells (Fig. 4). Furthermore purified LPGs did not have the capacity to activate purified NK cells to IFNγ secretion (data not shown).
Fig. 4.
IFNγ secretion induced by L. mexicana wild type (WT) and L. mexicana mutants deficient in LPG, Δlpg1 (lpg1) and Δlpg2 (lpg2), after 40 h stimulation. The bars show the mean result of four donors tested + SD.
Stimulation with LACK
We have previously reported that LACK can activate NK cells to IFNγ production in PBMC cultures prepared from donors with no previous history of leishmaniasis [13]. Although the frequency of IFNγ secreting cells increased in whole PBMC cultures after LACK stimulation in the two donors tested in the ELISPOT assay (RPMI: 122 + 23 SFU/200 000 cells and LACK: 217 + 70 SFU/200 000 cells). Isolated NK cells were not induced to IFNγ secretion by LACK (RPMI: 170 + 126 SFU/100 000 cells and LACK: 193 ± 147 SFU/100 000 cells).
Cytotoxicity
We evaluated the effect of the promastigotes on NK cells further, by testing if stimulation by promastigotes enhanced the nonspecific cytotoxic capacity of NK cells towards susceptible target cells. Stimulation with neither live- nor hk-PROM affected NK cell cytotoxicity against NK sensitive target cells (data not shown).
Phenotype of proliferating cells
To evaluate the phenotype of proliferating cells, PBMC from 6 donors were analysed by FACS™ using antibodies to specific cell surface markers. Stimulation with either live or dead parasites induced an appreciable proportional increase in large CD3–/CD16/56+, NK, and CD8+ cells. However, induction of CD4+ blast cells was mainly a feature of stimulation with killed promastigotes. Neither live nor dead promastigotes induced γδ-T cells proliferation. One donor (D11) had low responses to all the Leishmania preparations as well as to PPD (Table 2). Representative scattergrams of unstimulated and stimulated cells are shown in Fig. 5.
Table 2.
Phenotype of proliferating cells in response to live- or hk-PROM after six days stimulation.
| D1 | D6 | D9 | D10 | D11 | D12 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PI | % | PI | % | PI | % | PI | % | PI | % | PI | % | |
| CD56+ | ||||||||||||
| RPMI | 4·8 | 1·9 | 17·9 | 3·8 | 2·7 | 2·9 | ||||||
| live-PROM | 29·9 | 16·8 | 18·4 | 17·9 | 0·6 | 1·3 | 65·9 | 17·5 | 5·7 | 2·6 | 38·5 | 13 |
| hk-PROM | 20·9 | 4·2 | 138 | 17·2 | 15·2 | 16·9 | 66·4 | 11·7 | 1·9 | 2·2 | 23·1 | 5·3 |
| ft-LAg | 37·8 | 7·4 | 106 | 14·8 | 24·6 | 25·1 | 126 | 21 | 1·9 | 1·7 | 14·4 | 8·2 |
| PPD | nd | nd | 206 | 14·7 | 40·8 | 24·1 | 259 | 19·6 | 4·1 | 3·9 | 46 | 5 |
| CD8+ | ||||||||||||
| RPMI | 9·3 | 5·6 | 8·7 | 26·2 | 6·1 | 15·2 | ||||||
| live-PROM | 18·8 | 26·8 | 9·5 | 20·1 | 11·5 | 15·4 | 29·3 | 32·5 | 3·6 | 5·2 | 9·5 | 14·7 |
| hk-PROM | 10 | 5·5 | 3·2 | 8·6 | 23·9 | 12·1 | 14·3 | 11·2 | 1·4 | 5·5 | 3·2 | 3·2 |
| ft-LAg | 19·7 | 10·3 | 3·7 | 6·8 | 46·6 | 18·5 | 32·5 | 21·6 | 2·3 | 5·4 | 3·7 | 6·8 |
| PPD | nd | nd | 7·1 | 3·9 | 69·4 | 19·1 | 51·5 | 15·4 | 3·9 | 4·1 | 7·1 | 3·9 |
| CD4+ | ||||||||||||
| RPMI | 49·2 | 38·9 | 38·9 | 14·9 | 65·7 | 46·4 | ||||||
| live-PROM | 5 | 34·8 | 5·7 | 37·1 | 8·8 | 45·5 | 37·1 | 23·7 | 4·8 | 70·4 | 9·3 | 42·5 |
| hk-PROM | 29·8 | 72·3 | 50 | 60·9 | 33·9 | 66·1 | 136 | 62·3 | 1·6 | 67·2 | 23·7 | 70·2 |
| ft-LAg | 22 | 56·4 | 72·7 | 59·3 | 37·8 | 57·4 | 121 | 47 | 2·6 | 63·3 | 9·7 | 52·3 |
| PPD | nd | nd | 129 | 70 | 48·9 | 51·5 | 310 | 54 | 6·2 | 65·8 | 45·5 | 73·6 |
| γδ-T cells | ||||||||||||
| RPMI | 11·9 | 12·5 | 4·6 | 4·4 | 4·3 | 4·4 | ||||||
| live-PROM | 3·6 | 3·8 | 1·6 | 2·9 | 1·7 | 4·2 | 12·6 | 3·4 | 6 | 4·6 | 5·4 | 4·9 |
| hk-PROM | 10·1 | 3·9 | 0·8 | 0·4 | 2·2 | 1·8 | 14·7 | 2·6 | 1·9 | 1 | 2·8 | 4·7 |
| ft-LAg | 45·6 | 14·6 | 2·6 | 3·6 | 3·3 | 2·1 | 18·3 | 2·85 | 2·6 | 3·6 | 2·6 | 3·6 |
| PPD | nd | nd | 3·9 | 1·9 | 11 | 4·3 | 21·4 | 1·64 | 3·9 | 1·9 | 9·2 | 2·3 |
Data represents the percentage (%) and proportional increase (PI) of large cells gated in R2 expressing CD4, CD8, CD16/56 or γδ-T cell surface markers following stimulation, evaluated by FACS. nd, not done
Fig. 5.
Representative scattergram (D6) of proliferation determined by enrichment of blast cells after stimulation with live- or hk-PROM. Dot-blots shown from left to right are: Determination of small cells (R1) and blast cells (R2) by forward scatter following stimulation; cells gated in R1 expressing the CD4 and the CD8 surface markers; cells gated in R2 expressing the CD4 and CD8 surface marker.
Proliferative response after depletion of cell subsets
To further dissect the phenotype of cells proliferating to Leishmania promastigotes we measured the proliferative response after depletion of specific cell subsets in 4 donors.
In all four donors the presence of CD4+ cells was required to measure proliferation by 3H-thymidine incorporation. Depletion of CD4+ cells almost completely removed the proliferative response ( Table 3). The effect was most prominent in the cultures stimulated with hk-PROM. Even the low levels of proliferation induced by live-PROM were significantly reduced by depletion of CD4+ cells in 3 donors. Depletion of neither CD56+ nor CD8+ cells affected the proliferative response to promastigotes.
Table 3.
Six days proliferative response to live- and hk-PROM after depletion of cell subsets
| D6 | D7 | D10 | D15 | |
|---|---|---|---|---|
| Background (RPMI) proliferative response | ||||
| Control | 697 ± 160 | 1030 ± 246 | 2711 ± 2151 | 559 ± 27 |
| minus PAC | 759 ± 231 | nd | nd | 508 ± 61 |
| minus IC | 2705 ± 1313 | 792 ± 48 | 1335 ± 475 | 435 ± 33 |
| minus CD56 | 636 ± 48 | 1037 ± 35 | 1697 ± 1156 | 441 ± 36 |
| minus CD4 | 305 ± 6 | 473 ± 1 | 806 ± 195 | 253 ± 29 |
| minus CD8 | 648 ± 62 | 1133 ± 123 | 1311 ± 360 | 487 ± 69 |
| live-PROM induced proliferative response | ||||
| Control | 3145 ± 1221 | 1137 ± 125 | 2102 ± 184 | 8088 ± 1158 |
| minus PAC | 1888 ± 107 | nd | nd | 1745 ± 33 |
| minus IC | 2920 ± 260 | 1015 ± 30 | 1648 ± 1229 | 2123 ± 627 |
| minus CD56 | 2967 ± 904 | 1025 ± 47 | 1533 ± 143 | 1734 ± 185 |
| minus CD4 | 570 ± 124 | 824 ± 23 | 2521 ± 88 | 308 ± 8 |
| minus CD8 | 4098 ± 1454 | 1204 ± 181 | 1779 ± 96 | 3646 ± 348 |
| hk-PROM induced proliferative response | ||||
| Control | 23963 ± 2199 | 1444 ± 82 | 14300 ± 373 | 30530 ± 734 |
| minus PAC | 23577 ± 2707 | nd | nd | 15544 ± 3501 |
| minus IC | 21801 ± 1803 | 960 ± 93 | 16827 ± 2408 | 23022 ± 3762 |
| minus CD56 | 22614 ± 1699 | 1018 ± 325 | 8197 ± 2210 | 15096 ± 2496 |
| minus CD4 | 326 ± 52 | 552 ± 3 | 669 ± 42 | 386 ± 137 |
| minus CD8 | 26049 ± 2208 | 1204 ± 181 | 13479 ± 1207 | 20445 ± 702 |
The Table shows incorporation of 3H-thymidine as counts per minute (CPM), mean of duplicates or triplicates ± SD for individual donors/200 000 PBMC. nd, not done; minus indicates depletion. IC, isotype control, PAC, plastic adherent cells
Discussion
Leishmaniasis remains a deadly disease in areas of the World where visceral leishmaniasis exists, and a socially excluding disease in areas where the cutaneous form is prevalent. Many candidate vaccines have shown efficacy in mouse Leishmania infection models, however, to date the best protection against human leishmaniasis has been obtained with live vaccination/natural infection. Killed promastigote vaccines have been tested in several endemic areas with variable results [2, 3, 17]. We speculated that these differences in the ability to induce immune responses between live and dead promastigotes may influence the induction of protection against leishmaniasis. Previously we have shown that mononuclear cells from healthy individuals with no history of leishmaniasis differentially respond to live and dead Leishmania promastigotes [8, 17, 18]. Live promastigotes had the potential to dramatically induce numerous cells to IFNγ secretion, a property, which the dead promastigotes lacked. In this study we have further dissected the cellular responses induced by live and dead promastigotes. The data shows that live and dead parasites stimulate different cell subsets, with live showing a preference to activate CD56+ and CD8+ cells to IFNγ secretion while dead parasites tend to induce CD4+ cell proliferation. Depleting PBMC of CD56+ cells, i.e. NK cells, clearly shows that these cells are required for the observed induction of IFNγ. Furthermore we demonstrate a novel finding, that live Leishmania promastigotes have the ability to directly activate primary NK cells to IFNγ secretion in the absence of other cell types. The observation was seen in all donors tested and with three different Leishmania species tested. This suggests that Leishmania promastigotes can activate NK cells by at least two separate mechanisms. The direct activation of NK cells mediated by live, but not dead Leishmania, described here, and the previously described indirect activation of NK cells requiring the interplay between antigen presenting cells, T-cells and cytokines [19]. LACK which we have previously shown to induce IFNγ production by NK cells in a mixed PBMC population, probably through an indirect mechanism [13] did not activate the purified NK cells to secrete IFNγ.
We have previously discussed the potential role of NK cells in human leishmaniasis and shown that patients with active cutaneous leishmaniasis tend to have reduced NK responses, compared to cured individuals [6]. Furthermore, activated NK cells are apparently present in unstimulated cultures of endemic controls that resist infection by Leishmania, suggesting a role for NK cells in protection against cutaneous leishmaniasis [6]. While the implications of these findings remain unclear, we show in the present study that NK cells are central in the early induction of IFNγ in response to live promastigotes. Whether the early activation of NK cells is beneficial or not is yet to be elucidated. The downside may be that live parasites by avoiding activation of CD4+ cells can more easily establish an infection. It is possible that the NK-responses induced by live-PROM are associated with NK cell death or anergy, which could explain the low levels of NK cells detected in leishmaniasis patients with active disease [6].
How the live promastigotes activate the NK cells to IFNγ secretion is unclear, but it is apparently not molecules secreted by the live promastigotes [8]. It has been speculated that polysaccharides might have the ability to activate NK cells directly [16] and NK cells are known to have receptors recognizing carbohydrate structures [20,21]. LPG is the major cell surface glycoconjugate of Leishmania parasites. The abundance, location and the unique structure of this molecule implies that LPG has one or more important functions for the parasite. However when tested in our assay the direct activation of NK cells was found not to be LPG dependent. Purified promastigotes lacking LPG still had the capacity to induce NK cells to IFNγ secretion. The mutated promastigotes tended to induce fewer cells to IFNγ secretion than the wild type, suggesting that LPG may contribute to the IFNγ secretion. Direct activation of NK cells to IFNγ secretion has previously been achieved by stimulating cells with bacterial DNA, CpGs [22]. Eukaryotic DNA has not been described to have this capacity. The effect of leishmanial CpGs remains to be tested. It is worth noting that PHA stimulated IFNγ secretion in purified NK cells.
Neither live nor dead promastigotes enhanced the non-specific cytotoxic capacity of NK cells toward susceptible target cells. One could speculate that this implies that the mechanism of IFNγ production induced directly by Leishmania act distinct from the mechanism that triggers cytotoxicity. However, further studies need to be preformed using target cells of various sensitivity before categorically excluding some induction of cytotoxicity by Leishmania.
The NK receptor KIR2DL4 has the property of induction of IFNγ production but not cytotoxicity in resting NK cells [23]. It remains to be tested if the Leishmania promastigotes act through this receptor.
The mechanisms of induction of proliferation in response to Leishmania implicate CD4+ cells as central. FACS analysis of the phenotype of proliferating cells showed that stimulation with hk-PROM resulted in a higher percentage of large CD4+cells over that induced by live-PROM. The central role of CD4+ cells in the proliferation to heat killed Leishmania antigens was confirmed when depletion of CD4+ cells before stimulation almost completely inhibited the Leishmania induced proliferative response. Removal of plastic adherent cells did not appreciably affect the Leishmania induced proliferative response in PBMC. This could indicate that very few residual antigen presenting cells (APC) are necessary for the response, or that the APC needed for this early response are not highly plastic adherent, or that plastic adherent cells are not necessary for the early cellular response observed. Our present results do not allow us to predict which of these possibilities are correct. γδ-T cells have been implicated in innate immune responses. These cells were not induced to proliferate by either live or killed Leishmania promastigotes.
CD4+ cells were mainly responsible for the numerous IL-10 secreting cells observed in unstimulated cultures. The significance of such background cytokines is unknown, but may have a regulatory function. Stimulation of cells with live, but not heat-killed promastigotes tended to have an inhibitory effect on this number of IL-10 secreting cells, a phenomenon which appears to be a reflection of the increase in the number of IFNγ secreting cells induced by live promastigotes. The precise role of IL-10 in this system is still to be elucidated.
In mouse experiments it was shown that subcutaneous vaccination with killed promastigotes without addition of adjuvant tended to exacerbate the disease rather than induce protection [24]. However, when administered with adjuvant, such as BCG [25], Corynebacterium parvum [26] or IL-12 [27] protection was induced. This was interpreted as non-antigen specific polyclonal activation of mononuclear cells being required to direct the immune system to induce protective type of immune responses [27–29]. In other words the adjuvant may dictate the induction of a Th1 phenotype. Our data suggest that the killed promastigotes, in the absence of a Th1 directing agent, may direct the response away from a Th1 and thus allow the stimulation of a Th2 type response a mechanism that does not appear to operate when live parasites are used. PPD, the purified protein derivate of Mycobacterium bovis has been shown to activate amongst other cell types NK cells [30] (and own unpublished observations). PPD however, did not activate NK cells directly.
These finding may have relevance for the clinical outcome of leishmaniasis. The differential induction of cell subsets by live and dead promastigotes could be a part of the explanation why heat killed vaccines do not induce protective responses. The role of the direct activation may be to prevent the establishment of infection by rapidly activating macrophages to kill the invading parasite, or creating a milieu for Th1 cells development. Alternatively, such strong IFNγ induction may be involved in the tissue damage observed during cutaneous leishmaniasis. Whichever way, such live parasite induced IFNγ can affect the subsequent immune response to a natural infection and must be taken into consideration in the design of vaccines against leishmaniasis.
Acknowledgments
This work was supported by Infection & Vaccinology Research Program (I & V 2401/98), Foundation for Strategic Research Sweden and funds from Swedish International Development Agency (Sida/SAREC) and Swedish Medical Research Council (MFR).
References
- 1.Nadim A, Javadin E, Birundi G, Ghorbani M. The effectivness of leishmanisation in control of cutaneous leishmaniasis. Bull Soc Path Ex. 1983;76:377–83. [PubMed] [Google Scholar]
- 2.Sharifi I, FeKri A, Aflatonian M-R, Khamesipour A, Nadim A, Mousavi M-R, Momeni A, Dowlati Y, Godal T, Zicker F, Smith P, Modabber F. Randomised vaccine trial of a single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 1998;351:1540–3. doi: 10.1016/S0140-6736(98)09552-X. [DOI] [PubMed] [Google Scholar]
- 3.Khalil E, El Hassan A, Zijlstra E, Mukhtar M, Ghalib H, Musa B, Ibrahim M, Kamil A, Elsheikh M, Babiker A, Modabber F. Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet. 2000;356:1565–9. doi: 10.1016/s0140-6736(00)03128-7. [DOI] [PubMed] [Google Scholar]
- 4.Scott P, Pearce E, Cheever AW, Coffman LR, Sher A. Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev. 1989;112:161–82. doi: 10.1111/j.1600-065x.1989.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 5.Da-Cruz AM, Conceicao-Silva F, Bertho AL, Coutinho SG. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect Immun. 1994;62:2614–8. doi: 10.1128/iai.62.6.2614-2618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maasho K, Sanchez F, Schurr E, Hailu A, Akuffo H. Indication of the protective role of Natural Killer cells in human cutaneous leishmaniasis in an area of endemicity. Infect Immun. 1998;66:2698–704. doi: 10.1128/iai.66.6.2698-2704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akuffo H, Maasho K, Howe R. Natural and acquired resistance to Leishmania: Cellular activation of Leishmania aethiopica of mononuclear cells from unexposed individuals is through the stimulation of NK cells. Clin Exp Immunol. 1993;94:516–21. doi: 10.1111/j.1365-2249.1993.tb08227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nylén S, Mörtberg U, Kovalenko D, Satti I, Engström K, Bakheit M, Akuffo H. Differential induction of cellular responses by live and dead Leishmania promastigotes in healthy donors. Clin Exp Immunol. 2001;124:43–53. doi: 10.1046/j.1365-2249.2001.01501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bøyum A. Separation of lymphocytes from blood and bone marrow. Scand J Clin Laboratory Invest. 1969;21:77–89. [Google Scholar]
- 10.Maasho K, Akuffo HO. Cells from healthy non-exposed individuals produce cytokines to selected fractions of Leishmania promastigotes. Scand J Immunol. 1992;36:179–84. doi: 10.1111/j.1365-3083.1992.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 11.Ilg T. Lipophosphglycan is not required for infection of macrophages. EMBO J. 2000;19:1953–62. doi: 10.1093/emboj/19.9.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilg T, Demar M, Harbecke D. Phosphoglycan repeat deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J Biol Chem. 2001;276:4988–97. doi: 10.1074/jbc.M008030200. [DOI] [PubMed] [Google Scholar]
- 13.Maasho K, Satti I, Nylén S, Guzman G, Koning F, Akuffo H. A Leishmania homologue of receptors for activated C-kinase (LACK) induces both interferon-gamma and interleukin-10 in natural killer cells of healthy blood donors. J Infect Dis. 2000;182:570–8. doi: 10.1086/315725. [DOI] [PubMed] [Google Scholar]
- 14.Maisel AS, Fowler P, Rearden A, Motulsky HJ, Michel MC. A new method for isolation of human lymphocyte subsets reveals differential regulation of B-adrenergic receptors by terbutaline treatment. Clin Pharmacolo Ther. 1989;46:429–39. doi: 10.1038/clpt.1989.161. [DOI] [PubMed] [Google Scholar]
- 15.Matikainen S, Paananen A, Miettinen M, Kurimoto M, Timonen T, Julkunen I, Sareneva T. IFN-α and IL-18 synergistically enhance IFN-g production in human NK cells: differential regulation of Stat4 activation and IFN-g gene expression by IFN-α and IL-12. Eur J Immunol. 2001;31:2236–45. [PubMed] [Google Scholar]
- 16.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independen antigens. Curr Opin Immunol. 1995;7:349–54. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 17.Armijos R, Weigel M, Aviles H, Maidonado R, Racines R. Fiel trial of a vaccine against new world cutaneous leishmaniasis in an at-risk population: Safety, immunogenicity, and efficacy during the first 12 months of follow-up. JID. 1998;177:1352–7. doi: 10.1086/515265. [DOI] [PubMed] [Google Scholar]
- 18.Akuffo HO, Britton SFF. Contribution of non-Leishmania-specific immunity to resistance to Leishmania infection in humans. Clin Exp Immunol. 1992;87:58–64. doi: 10.1111/j.1365-2249.1992.tb06413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharton-Kersten T, Afonso LC, Wysocka M, Trinchieri G, Scott P. IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol. 1995;154:5320–30. [PubMed] [Google Scholar]
- 20.Daniels BF, Nakamura MC, Rosen SD, Yokoyama VM, Seaman WE. Ly-49A, a receptor for H-2Dd, has a functional carbohydrate recognition domain. Immunity. 1994;1:785–92. doi: 10.1016/s1074-7613(94)80020-0. [DOI] [PubMed] [Google Scholar]
- 21.Duan X, Ackerly M, Vivier E, Anderson P. Evidence for involvment of B-glucan-binding cell surface lectins in hunam natural killer cells. Cell Immunol. 1994;157:393–402. doi: 10.1006/cimm.1994.1236. [DOI] [PubMed] [Google Scholar]
- 22.Verthelyi D, Ishii KJ, Gursel M, Takerhita F, Klinman DM. Human Peripheral BLood Cells Differntialy Recognize and Respond to Two Distinct CpG Motifs. J Immunol. 2001;166:2372–7. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 23.Rajagopalan S, Fu J, Long EO. Induction of IFN-γ Production but Not Cytotoxicity by the Killer Cell Ig-Like Receptor KIR2DL4 (CD158d) in Resting NK cells. J Immunol. 2001;167:1877–81. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 24.Liew FY, Hale C, Howard JG. Prophylactic immunization against experimental leishmaniasis IV. Subcutaneous immunization prevents the induction of protective immunity against fatal Leishmania major infection. J Immunol. 1985;135:2095–101. [PubMed] [Google Scholar]
- 25.Convit J, Castellanos PL, Rondon A, Pinardi ME, Ulrich M, Castes M, Bloom B, Garcia L. Immunotherapy versus chemotherapy in localised cutaneous leishmaniasis. Lancet. 1987;1:401–5. doi: 10.1016/s0140-6736(87)90116-4. [DOI] [PubMed] [Google Scholar]
- 26.Scott P, Pearce E, Natovitz P, Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987;139:221–7. [PubMed] [Google Scholar]
- 27.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–7. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 28.Rivier D, Bovay P, Shah R, Didishheim S, Mauel J. Vaccination against Leishmania major in a CBA mouse model infection. role of adjuvants and mechanisms of protection. Parasite Immunol. 1999;9:461–73. doi: 10.1046/j.1365-3024.1999.00244.x. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 30.Batoni G, Esin S, Pardini M, Bottai D, Wigzell H, Campa M. Identification of distinct lymphocyte subsets responding to subcellular fractions of Mycobacterium bovis bacille calmette-Guerin (BCG) Clin Exp Immunol. 2000;119:270–9. doi: 10.1046/j.1365-2249.2000.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]