Abstract
A proportion of healthy neonates fail to produce protective levels of anti-HBs antibody following vaccination with recombinant hepatitis B vaccine. This study was undertaken to investigate contribution of Th1 and Th2 responses to anti-HBs antibody production and to explore the mechanism(s) of unresponsiveness to HBsAg in human neonates. Peripheral blood manonuclear cells (PBMCs) were isolated form 28 nonresponder (anti-HBs antibody <10 IU/l) and 25 responder neonates. The cells were stimulated in vitro with recombinant HBsAg and PHA mitogen and concentrations of IL-4, IL-10 and IFN-γ were quantified in culture supernatants by sandwich ELISA. Our results demonstrated significantly increased production of all cytokines, including IL-4 (P < 0·001), IL-10 (P < 0·002) and IFN-γ (P < 0·01) in responder compared to nonresponder vaccinees. No significant differences, however, were observed between the two groups of neonates in the levels of cytokines induced by PHA or secreted in absence of antigen and mitogen. Our findings suggest that unresponsiveness to recombinant HBsAg in healthy neonates is linked to inadequate secretion of both Th1 and Th2 cytokines.
Keywords: hepatitis B, vaccination, neonates, anti-HBs antibody, Th1/Th2 cytokines
Introduction
Hepatitis B virus (HBV) is an enveloped virus secreting and expressing three forms of overlapping surface proteins, including the small, middle and large proteins. These molecules are also known as s, pres2 and pres1 antigens, respectively. The ‘s’ antigen (HBsAg) is the predominant form of the surface antigens and constitutes the immunodominant ‘a’ determinant required for induction of protective antibody response in human [1].
Vaccination of neonates and healthy adults with recombinant HBsAg induces a protective immune response in 90–99% of vaccinees [2–4]. Administration of supplementary vaccine doses [5,6] and the use of new generation vaccines containing all three forms of the surface antigens [7,8] have significantly increased the rate of seroprotection. A proportion of healthy adult and neonate vaccinees, however, fail to produce protective levels of anti-HBs antibody, despite implementation of the above strategies.
Lack of response could be attributed to several mechanisms. Defect in antigen presentation due to expression of certain HLA antigens and haplotypes has been reported [9,10]. The HLA complex is central to the T-cell dependent antigen response. The expression profile of HLA antigens could regulate the immune response through cognate binding of the HLA antigen to the processed antigenic peptides or presentation of the HLA/antigenic peptide complex to T-cell receptors expressed on HBsAg-specific CD4+ T-cells. The latter event could induce either stimulatory or inhibitory signals, depending on the expressed haplotype of HLA. Defective HBsAg-specific T and/or B-cell repertoires have also been demonstrated [11–13]. This could either be a primary defect or secondary, successive to destruction of HBsAg- specific B-cells by cytotoxic T-cells [14]. Immunological tolerance [15,16] as well as functional defect in T-cell help necessary for production of anti-HBs antibody by B-cells [11, 17, 18] may also contribute to unresponsiveness to HBsAg.
Since HBsAg is a T-cell dependent glycoprotein, therefore defective T-helper (Th) cell function, either Th1 or Th2, could result in failure of immune response to this antigen.
In this study, Th1 and Th2 responses have been investigated in healthy responder and nonresponder neonates vaccinated with recombinant hepatitis B vaccine.
Materials and methods
Subjects and vaccination scheme
Triple 10 microgram doses of a recombinant hepatitis B vaccine (Heberbiovac, Heberbiotec Co., Cuba) were administered i.m. to a large cohort of healthy Iranian neonates at 0, 1·5 and 9 months intervals. Vaccination was carried out in two cities of Iran (Kerman and Uromia) following the regulations and guidelines set up by the National Vaccination Committee of Iran and the study was approaved by the Ethical Committee of the Undersecretary for Research and Technology of the Ministry of Health, Treatment and Medical Education of Iran. The first dose was given 24–48 h after delivery in five local maternity hospitals (Kashani and Davazdah Emam Hospitals in Kerman; Kowsar, Tamin Ejtemaee and Azarbaijan Hospitals in Uromia), and subsequent doses were administered in selected local health centres. Two to four weeks after completion of the vaccination course, peripheral blood was collected and anti-HBs antibody was quantified in serum by sandwich ELISA. A total of 721 neonates were enrolled into the study. Collectively, 30 nonresponders (anti-HBs <10 IU/l) were identified of whom 2 were positive for HBsAg and excluded from the study. Of the high-responder vaccinees (anti-HBs >10 000 IU/l) (n = 186 neonates), who were arbitrarily distributed in 25 groups, each consisting of 7 subjects, 25 subjects were randomly selected from all groups.
Measurement of anti-HBs antibody in serum
Anti-HBs antibody was detected in serum by a sandwich ELISA using a commercial kit (Boehring, Marburg, Germany). The concentration of the antibody was extrapolated from a standard curve constructed from know concentrations of a standard sample provided by the manufacturer.
In vitro stimulation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were separated from heparinized peripheral blood by Ficoll (Pharmacia, Uppsala, Sweden) gradient centrifugation. After washing in RPMI-1640 medium (Gibco Life Techologies Ltd, Paisley, UK), PBMCs were resuspended in complete culture medium containing RPMI-1640 supplemented with 10% heat inactivated fetal calf serum (Seromed, Berlin, Germany) and antibiotics, including penicillin (100 U/ml) and streptomycin (100 µg/ml). Cells were then seeded at 1 × 106 cells/ml in a 24-well sterile tissue culture plate (Nalge Nunc International, Roskilde, Denmark) in presence or absence of 10 µg/ml of purified rHBsAg without vaccine additives (Heberbiovac, Cuba), or 10 µg/ml of PHA (Sigma-Aldrich Corporation, St Louis, MO, USA). Following 72 h incubation at 37°C in a humidified CO2 (5%) incubator, culture supernatants were collected and stored in −70°C until use.
Quantification of cytokines
All cytokines including IL-4, IL-10 and IFN-γ were measured by sandwich ELISA using commercial kits (Biosource International, Camarillo, CA, USA). The assay for IL-4 and IL-10 was optimized by titration of the paired capture and detection antibodies as suggested by the manufacturer to determine the optimum concentrations of both antibodies. Accordingly, the capture antibodies were coated in polystyrene ELISA plates (Maxisorp, Nunc) at 1 µg/ml and the biotinylated detection antibody was employed at 0·4 µg/ml for both cytokines.
Statistical analysis
Levels of cytokine production between the responders and nonresponders and within each group of subjects were compared using two tailed Mann–Whitney U-test. In addition, Wilcoxon Signed Rank Test was applied to compare cytokine levels and serum antibody levels in nonresponder neonates under different stimulation conditions. P-values of less than 0·05 were considered significant.
Results
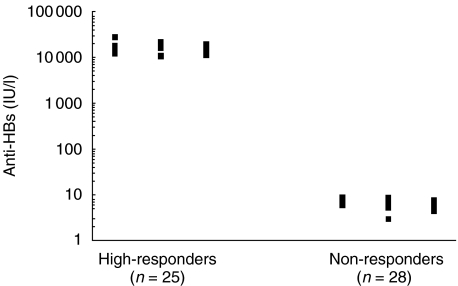
The concentration of anti-HBs antibody in serum of responder and nonresponder neonates was found to be 16740 ± 7555 and 7 ± 1·4 (mean ± SD), respectively (Fig. 1).
Fig. 1.
Distribution of serum levels of anti-HBs antibody in responder and nonresponder neonates.
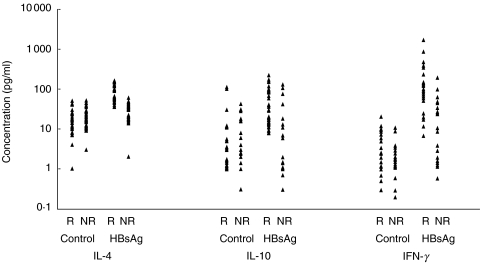
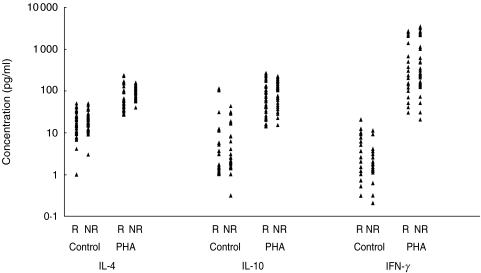
The levels of secreted IL-4, IL-10 and IFN-γ following in vitro stimulation with HBsAg and PHA are illustrated in Figs 2 and 3. A significantly increased production of all cytokines was observed following stimulation of PBMCs from responder vaccinees with HBsAg, compared to non responders (P < 0·01–P < 0·001) ( Table 1). Contrary to HBsAg, no significant differences were found in cytokine profile between the two groups of vaccinees following stimulation with PHA or in absence of stimulation. Comparison of secreted cytokines within each group of vaccinees revealed significant differences between the levels of all cytokines induced by HBsAg and in absence of antigen (control) only in responder vaccinees ( Table 2). However, when the levels of cytokines induced by PHA and HBsAg were compared, PHA induced cytokines in both groups similarly, whereas HBsAg induced significantly higher cytokine levels in responders.
Fig. 2.
Distribution of cytokines production in presence and absence of HBsAg in responder (R) and nonresponder (NR) neonates.
Fig. 3.
Distribution of cytokines production in presence and absence of PHA in responder (R) and nonresponder (NR) neonates.
Table 1.
Levels of cytokines secreted in vitro from PBMCs of responder and nonresponder neonates following stimulation with HBsAg, PHA or without stimulation
| Cytokine | Stimulator | Responders | Non-responders | P -value |
|---|---|---|---|---|
| IL- 4 | HBsAg | 76·5±35·5 | 29·8±15·3 | <0·001 |
| (59·0±7·1) | (33·0±2·9) | |||
| PHA | 88·4±52 | 82·5±20 | ns | |
| (64·0±9·9) | (79·0±3·9) | |||
| Control | 18·7±12·9 | 21·9±11·9 | ns | |
| (17·0±2·7) | (21·0±2·1) | |||
| IL-10 | HBsAg | 59·9±59 | 15·7±32·9 | <0·005 |
| (40·0±11·37) | (6·2±1·5) | |||
| PHA | 115·7±89·2 | 122·4±69 | ns | |
| (90·0±16·76) | (145·0±13) | |||
| Control | 12·2±28·6 | 5·2±9·6 | ns | |
| IFN-γ | HBsAg | 230·0±385 | 21·6±42·4 | <0·01 |
| (90·0±77·1) | (3·0±8·1) | |||
| PHA | 825·0±950 | 981·0±1249 | ns | |
| (370·0±190) | (310·0±238·3) | |||
| Control | 4·2±4·6 | 2·9±3·1 | ns | |
| (2·5±0·93) | (1·7±0·59) |
ns, not significant. The results represent mean±SD (median±SEM) values (pg/ml).
Table 2.
Statistical comparisons of cytokines secreted in the presence or absence of HBsAg and PHA within each vaccinated group
| Comparsion groups | ||||
|---|---|---|---|---|
| Vaccinees | Cytokine | HBsAg versus Cont | PHA versus Cont | HBsAg versus PHA |
| Responders | ||||
| IL-4 | 0·009* | 0·002 | 0·35 | |
| IL-10 | 0·05 | 0·0025 | 0·12 | |
| IFN-γ | 0·001 | 0·005 | 0·09 | |
| Non-responders | ||||
| IL-4 | 0·22 | 0·006 | 0·001 | |
| IL-10 | 0·21 | 0·005 | 0·009 | |
| IFN-γ | 0·14 | 0·0001 | 0·001 | |
the figures represents p; Cont: control culture in absense of HBsAg or PHA
Discussion
T-helper (Th) cells can be functionally distinguished based on the profile of cytokine production. Th1 cells induce cell- mediated immune response by secreting cytokines such as IFN-γ, IL-2 and TGF-β. Secretion of Th2 cytokines such as IL-4, IL-5, IL-6, IL-10 and IL-13 seems to be essential for antibody production [19]. Humoral and cellular immune responses against HBV antigens are responsible for clearance of the virus and destruction or cure of infected hepatocytes [20,21].
Vaccination with HBsAg induces protective immunity through Th cell dependent production of anti-HBs antibody [17]. Several studies have recently been conducted to investigate the precise role of Th1 and Th2 derived cytokines in the immune response to hepatitis B vaccination and to get further insights in to the cellular basis of unresponsiveness to HBsAg. Controversial results, however, have been reported. Analyses of in vitro HBsAg-induced cytokine production have revealed defects in:
Th2 response in both responder and nonresponder groups [18];
Different patterns of cytokine production have been observed in T-cell clones isolated from responder subjects, with either predominant Th0 or Th2 response [24,25], or Th1 and Th2 responses in high and low responders, respectively [26].
Insufficient production of both types of cytokines in healthy nonresponder individuals has recently been demonstrated [22,23]. However, these studies and all other similar studies reported so far in the literature have been performed on adult subjects. There is some evidence which suggests that the mechanisms underlying unresponsiveness to a given T-cell dependent antigen may be different in adults and neonates. The most important differences are:
neonatal CD4+ T-cells seem to be phenotypically and functionally more immature than adult counterparts [27];
neonatal responses to T-cell dependent antigens are biased towards a Th2 phenotype [28];
dendritic cells (DC) from neonates express very low levels of MHC class 2 and other costimulatory molecules such as B 7·1, B 7·2 and CD11c and are defective in presentation of antigen [29] and IL-12 synthesis [30];
GM-CSF accelerates maturation of neonatal DC resulting in prevention of neonatal tolerance [31];
double positive CD4+ CD8+ T-cells are severely depleted in the human neonatal thymus [32];
the number of T-cells and antigen presenting cells are several folds higher in adults than neonates [33].
These inherent immune defects in neonates could deviate the immune response to HBV infection leading to establishment of a chronic state in 70–90% of infected neonates, as compared to 5–10% of infected adults [21]. Similarly, the antibody response to ABsAg may also be different in neonate and adult vaccinees [34]. The precise cellular and molecular basis of such differences, however, are not well understood. Different profiles of Th1 and Th2 responses have been shown to operate in neonate and adult mice immunized with a T-cell dependent antigen [35]. Our recent data and those of others obtained in healthy adult HBsAg nonresponder vaccinees indicate suppression of both Th1 and Th2 responses [22,23]. The results presented in this paper suggest involvement of a similar mechanism in nonresponder neonates, as evidenced by production of decreased levels of Th1 and Th2 cytokines. This assumption is supported by our recent findings indicating expression of similar HLA antigens and haplotypes, particularly DR7 and DQ2, in both adult and neonate nonresponders [9,36]. The number of subjects included in this study, particularly nonresponder neonates (n = 28), is significantly higher than other studies. This minimizes variability of the results and allows comprehensive and clear-cut statistical comparisons. Statistical analysis has not been performed in most of previous studies, due to the small sample size and variability of the results between the subjects [11, 17, 18, 22].
Despite the statistically significant differences observed for all the cytokines between the two groups of vaccinees, two important issues deserve further consideration. First, the level of cytokines induced by HBsAg in nonresponders, particulary IFN-γ, varies between different individuals; in some subjects it reaches that of the responder group (Fig. 2). This is also reflected in the statistical significance obtained for the cytokines. The difference between responders and nonresponders in HBsAg-specific IFN-γ response was modest (P < 0·01) compared to IL-4 (P < 0·001) or IL-10 (P < 0·005) ( Table 1). The variation in cytokine levels, however, has not been significantly correlated with the titre of anti-HBs antibody in the nonresponder neonates (data not presented), suggesting involvement of some other unidentified immunological parameters in the cytokine response to HBsAg. The second point which worths mentioning is the variation observed within the nonresponder group regarding the levels of spontaneously secreted cytokines (control), particularly IL-10 ( Table 2). Again, no significant correlation or difference was found between the HBsAg-induced and background cytokine levels in these neonates (data not presented), implying presence of insignificant difference between different individuals. The decreased levels of both Th1 (IFN-γ) and Th2 (IL-10 and IL-4) cytokines observed in our nonresponder neonates seem to be HBsAg-specific, since PHA-induced cytokine secretion was similarly represented in both groups of neonates ( Table 1). Spontaneously secreted cytokines in absence of antigen or mitogen were also quantitatively similar, in responder and nonresponder vaccinees. These findings assure precision of our results and exclude the possibility of involvement of a generalized immune dysfunction in the nonresponder subjects or technical shortcomings with regard to in vitro stimulation and culture condition.
Our novel findings of significantly increased production of all cytokines in response to HBsAg as compared to control cultures without stimulation in responder vaccinees, together with significantly higher secretion of these cytokines induced by PHA compared to HBsAg in nonresponder, but not responder neonates ( Table 2), may have important implications. First of all these results suggest that in addition to the serum levels of anti-HBs antibody, the profile of cytokine secretion could also be used as an objective criterium and distinctive parameter to identify hepatitis B vaccine responder and nonresponder individuals. Furthermore, our data could also be taken to imply dysfunction of APC in nonresponders. The latter assumption is supported by our recent observation in adult vaccinees demonstrating lack of proliferative response of PBMCs from nonresponders to HBsAg [23]. Although, due to ethical restrictions, the volume of peripheral blood samples taken from the neonates were not enough to perform proliferation assay, we assume that similar to our findings in adults, diminished Th1 and Th2 responses in neonates could be taken as an indication of lack of proliferation of these cells, presumably due to specific APC dysfunction. Our recent findings of decreased production of IL-12 which is produced by activated APC, in nonresponder neonates supports our assumption (unpublished data).
In summary, we have demonstrated that both Th1 and Th2 responses are defective in human nonresponder neonates to recombinant HBsAg vaccine. This could be due to defect in either the HBsAg-specific T-cell repertoire or antigen presentation. Both issues are currently being investigated in our laboratory.
Acknowledgments
The authors are grateful to Gholam Ali Kardar, Azam Roohi and Jalal Khoshnoodi for their invaluable technical help. Thanks are also due to Dr Mahmood J Tehrani from the AveSina Research Center, Tehran, for critical review of the article. The skilful technical assistance of Mina Mirzababoo is also appreciated. This study was supported in part by a grant from the Undersecretary for Research and Technology, Ministry of Health and Medical Education of Iran.
References
- 1.Shokrgozar MA, Shokri F. Subtype specificity of anti-HBs antibodies produced by human B-cell lines isolated from normal individuals vaccinated with recombinant hepatitis B vaccine. Vaccine. 2002;20:2215–20. doi: 10.1016/s0264-410x(02)00116-0. [DOI] [PubMed] [Google Scholar]
- 2.Shokri F, Jafarzadeh A. High seroprotection rate induced by low doses of a recombinant hepatitis B vaccine in healthy Iranian neonates. Vaccine. 2001;19:4544–8. doi: 10.1016/s0264-410x(01)00183-9. [DOI] [PubMed] [Google Scholar]
- 3.Amani A, Shokri F. Immunogenicity of recombinant hepatitis B vaccine in Iranian neonates: High frequency of unresponsiveness independent of the carrier state of mothers. Irn J Med Sci. 1995;20:87–92. [Google Scholar]
- 4.Zannoli R, Morgese G. Hepatitis B vaccine: current issues. Ann Pharmacother. 1997;31:1059–67. doi: 10.1177/106002809703100916. [DOI] [PubMed] [Google Scholar]
- 5.Shokri F, Amani A. High rate of seroconversion following administration of a single supplementary dose of recombinant hepatitis B vaccine in Iranian healthy non-responder neonates. Med Microbiol Immunol. 1997;185:231–5. doi: 10.1007/s004300050035. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman JN, Zuckerman AJ. Is there a need for boosters of hepatitis B vaccine. Viral Hepatitis. 1998;4:43–6. [Google Scholar]
- 7.McDermott AB, Madrigal JA, Sabin CA, et al. The influence of host factors and immunogenetics on lymphocyte responses to Hepagene vaccination. Vaccine. 1999;17:1329–37. doi: 10.1016/s0264-410x(98)00389-2. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman JN, Zuckerman AJ, Syminaton I, et al. Evaluation of a new hepatitis B triple-antigen vaccine in inadequate responders to current vaccines. Hepatology. 2001;34:798–802. doi: 10.1053/jhep.2001.27564. [DOI] [PubMed] [Google Scholar]
- 9.Shokrgozar MA, Shokri F. HLA-associated antibody response to recombinant hepatitis B vaccine in healthy Iranian adults. Irn J Med Sce. 1999;24:98–103. [Google Scholar]
- 10.McDermott AB, Cohen SB, Zuckerman JN, Madrigal JA. Human Leukocyte antigens influence the immune response to a pre-S/S hepatitis B vaccine. Vaccine. 1999;17:330–9. doi: 10.1016/s0264-410x(98)00203-5. [DOI] [PubMed] [Google Scholar]
- 11.Chedid MG, Deulofeut H, Yunis DE, et al. Defect in Th1-like cells of non-responders to hepatitis B vaccine. Hum Immunol. 1997;58:42–51. doi: 10.1016/s0198-8859(97)00209-7. [DOI] [PubMed] [Google Scholar]
- 12.Holer T, Meyer CU, Notghi A. The influence of major histocompatibility complex class II genes and T-cell repertoire on response to immunization with HBsAg. Hum Immunol. 1998;59:212–8. doi: 10.1016/s0198-8859(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 13.Shokrgozar MA, Shokri F. Enumeration of HBsAg-specific B-lymphocytes in Iranian healthy adult responders and non-responders vaccinated with recombinant hepatitis B vaccine. Immunology. 2001;104:75–9. doi: 10.1046/j.0019-2805.2001.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnaba V, Franco A, Alberti A. Selective killing of hepatitis B envelope antigen specific B-cells by calss I restricted exogenous antigen-specific T-lymphocytes. Nature. 1990;345:258–60. doi: 10.1038/345258a0. [DOI] [PubMed] [Google Scholar]
- 15.Lazizi Y, Badur S, Perk Y. Selective unresponsiveness to HBsAg vaccine in newborns related with an in utero passage of hepatitis B virus DNA. Vaccine. 1997;15:1095–100. doi: 10.1016/s0264-410x(97)00005-4. [DOI] [PubMed] [Google Scholar]
- 16.Milich DR, Jones JE, Hughes JI. Is a function of the secreted HBe antigen to induce immunological tolerance in utero? Proc Natl Acid Sci USA. 1990;87:6599–603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livingston BD, Alexander J, Crimi C, et al. Altered helper T-lymphocyte function associated with chronic hepatitis B virus infection and its role in response to therapeutic vaccination in human. J Immunol. 1999;162:3088–95. [PubMed] [Google Scholar]
- 18.Vingerhoets J, Vanham G, Kestens L, et al. Deficient T-cell response in non-responders to hepatitis B vaccination. absence of TH1 cytokine production. Immunol Lett. 1994;39:163–8. doi: 10.1016/0165-2478(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 20.Bertoletti A, Maini MK. Protection or damage. a dual role for the virus specific cytotoxic T-lymphocyte response in hepatitis B and C infections. Cur Opin Immunol. 2000;12:403–8. doi: 10.1016/s0952-7915(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 21.Chisari FV, Ferrari C. Hepatitis B virus immunophathogenesis. Ann Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 22.Larsen CG, Xu J, Lee S, et al. Complex cytokine response to hepatitis B surface antigen and tetanus toxoid in responder, nonresponders and subjects naive to hepatitis B surface antigen. Vaccine. 2000;18:3021–30. doi: 10.1016/s0264-410x(00)00084-0. [DOI] [PubMed] [Google Scholar]
- 23.Kardar GA, Jeddi-Tehrani M, Shokri F. Diminished Th1 and Th2 cytokine production in healthy adult non-responders to recombinant hepatitis B vaccine. Scand J Immunol. 2002;55:311–4. doi: 10.1046/j.1365-3083.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsui H, Mizoguchi Y, Morisawa S. There is no correlation between function and lymphokine production of HBsAg-specific human CD4+ cloned T-cells. Scand J Immunol. 1991;34:433–44. doi: 10.1111/j.1365-3083.1991.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 25.Honorati MC, Dolzani P, Mariani E, et al. Epitope specificity of Th0/Th2 CD4+ T-lymphocyte clones induced by vaccination with rHBsAg vaccine. Gastroenterology. 1997;112:2017–27. doi: 10.1053/gast.1997.v112.pm9178695. [DOI] [PubMed] [Google Scholar]
- 26.Wataya M, Sano T, Kamikawaji N, et al. Comparative analysis of HLA restriction and cytokine production in hepatitis B surface antigen-specific T-cells from low- and high-antibody responders in vaccinated humans. J Hum Genet. 2001;46:197–206. doi: 10.1007/s100380170089. [DOI] [PubMed] [Google Scholar]
- 27.Delespesse G, Yang LP, Ohshima Y, Demeure C, Shu U, Byun DG, Sarfati M. Maturation of human neonatal CD4+ and CD8+ T-lymphocytes in to Th1/Th2 effectors. Vaccine. 1998;16:1415–9. doi: 10.1016/s0264-410x(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 28.Barrios C, Brawand P, Berney M, Brandt C, Lambert PH, Siegrist CA. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–96. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 29.Muthukkumar S, Goldstein J, Stein KE. The ability of B-cells and dendritic cells to present antigen increases during ontogeny. J Immunol. 2000;165:4803–13. doi: 10.4049/jimmunol.165.9.4803. [DOI] [PubMed] [Google Scholar]
- 30.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL-12 (P35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 31.Ishii KJ, Weiss WR, Klinman DM. Prevention of neonatal tolerance by a plasmid encoding GM-CSF. Vaccine. 1999;18:703–10. doi: 10.1016/s0264-410x(99)00267-4. [DOI] [PubMed] [Google Scholar]
- 32.Varas A, Jimenez E, Sacedon R, Rodrigues-Mahou M, Maroto E, Zapata AG, Vicente A. Analysis of the human neonatal thymus: evidence for a transient thymic involution. J Immunol. 2000;164:6260–7. doi: 10.4049/jimmunol.164.12.6260. [DOI] [PubMed] [Google Scholar]
- 33.Fadel S, Sarzotti M. Cellular immune responses in neonates. Int Rev Immunol. 2000;19:173–93. doi: 10.3109/08830180009088504. [DOI] [PubMed] [Google Scholar]
- 34.Shokrgozar MA, Shokri F. Antibody response to recombinant hepatitis B surface antigen in healthy adults following primary and supplementary vaccination. Irn J Med Sci. 2001;26:10–5. [Google Scholar]
- 35.Forsthuber T, Yip HC, Lehmann PV. Induction of Th1 and Th2 immunity in neonatal mice. Science. 1996;271:1728–30. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 36.Jafarzadeh A, Shokri F. Association between HLA antigens and lack of antibody response to recombinant hepatitis B vaccine in healthy Iranian neonates(Abstract) Scand J Immunol. 2001;54:45. [Google Scholar]